Beruflich Dokumente
Kultur Dokumente
Chemical Lab 3
Hochgeladen von
sagarchawla13Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Chemical Lab 3
Hochgeladen von
sagarchawla13Copyright:
Verfügbare Formate
CL-251: Chemical Engineering Lab-1 Experiment: Vapor Liquid Equilibrium K.
Abhishek 12110002 Abstract: The purpose of this experiment is to study binary liquid-vapor equilibrium (in this case, benzene and acetone). The set-up includes a flask into which the mixture is poured. There is a heater for heating the flask. A collector is setup for collecting the condensed vapor. A temperature sensor is also present to measure temperature. A refract-o-meter and a measuring cylinder, apart from the set-up itself, are required for the experiment. Introduction: In this experiment we are going to study the vapor-liquid equilibrium of a binary mixture (acetone and benzene). The experiment is used to determine the effect of composition of a mixture on its boiling point. Unlike the pure compounds in which the vapor and liquid have the same composition, boiling mixtures will have a different composition in the liquid phase than in the vapor phase. In this case, acetone is more volatile than benzene. In real world, this process is applied when separation of two different liquids (based on their boiling point) is needed. Procedure: The mixture is made such that the lesser volatile component is taken in a relatively larger proportion. Hence, initially, a mixture of 100 ml of acetone and 400 ml of benzene is taken (the resultant volume need not necessarily be 500 ml; hence we take each component separately to avoid confusion). This mixture is poured into the flask (500 ml makes up to 2/3rd of the flask) and it is heated. It is left untouched until it starts boiling. The vapor goes up into the condenser and is collected in the collector. After a considerable amount of time, when the temperature is observed to not/infinitesimally change, the temperature is noted. The power supply is then turned off. A sample of the mixture in the flask and a sample of the condensed vapor from the collector are taken and each is put in the refract-o-meter to get the refractive index. The same is repeated for different volumes of each component in the mixture. Results: The properties of pure components are stated below: Table 1: Molecular Weight (g/mole) Boiling Point (oC) Refractive Index at 30oC Benzene 78 80 1.5 Acetone 58 56 1.35
The results obtained from the experiment are as follows: Table 2: Refractive indices calculated using refract-o-meter Volume of Acetone (ml) 100 250 300 Volume of Benzene (ml) 400 250 200 Temperature (oC) 67.8 64.2 57.4 Refractive Index of condensed vapor 1.4757 1.4418 1.4005 Refractive index of the liquid 1.4935 1.4736 1.4060
The mole fractions of individual components are calculated by using: y = -0.1397x + 1.4995
CL-251: Chemical Engineering Lab-1 Experiment: Vapor Liquid Equilibrium K.Abhishek 12110002 Where x is the mole fraction and y is the refractive index value. Hence the mole fractions are as follows: Table 3: Temperature (oC) Refractive Index of liquid XA : Mole Fraction of Acetone (liquid) 0.043 0.19 0.67 XB: Mole fraction of Benzene (liquid) 0.957 0.81 0.33 Refractive index of condensed vapor YA : Mole fraction of Acetone (vapor) 0.17 0.41 0.71 YB: Mole fraction of Benzene (vapor) 0.83 0.59 0.29 KA = YA/XA KB = YB/XB
67.8 64.2 57.4
1.4935 1.4736 1.4060
1.4757 1.4418 1.4005
3.95 2.16 1.06
0.87 0.72 0.88
Using Antoine equation, we get PA which then can be used to calculate A. Antoine Equation: ln P = A + (B/(T+c)) Table 4: Values of A, B and C for acetone and benzene Acetone A 14.3145 B 2756.22 C 228.06 Benzene 13.7819 2726.81 217.572
Table 5: Values of A and ln A (A= Acetone) and B and ln B (B= Benzene) Temperature (oC) 67.8 64.2 57.4 ln PA 4.99 4.88 4.66 PA (atm) 1.46 1.299 1.04 A 2.7 1.66 1.02 ln A 0.99 0.50 0.02 ln PB 4.22 4.10 3.87 PB (atm) 0.67 0.59 0.47 B 1.29 1.22 1.87 ln B 0.25 0.19 0.63 ln (A/B) 0.7432 0.3168 -0.61
CL-251: Chemical Engineering Lab-1 Experiment: Vapor Liquid Equilibrium K.Abhishek 12110002
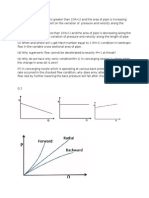
Figure 1: ln A vs XB and ln B vs XB
Table 6: Calculating Van Laar Constants, Temperature (oC) a 67.8 64.2 57.4 b
2.07 0.25 2.43 0.49 2.44 0.32
CL-251: Chemical Engineering Lab-1 Experiment: Vapor Liquid Equilibrium K.Abhishek 12110002 Discussions and Conclusions: There was a leak in the apparatus, but in no way did it disturb the experiment. On increasing the concentration of the more volatile component in the binary mixture, boiling point goes on decreasing.
Appendix: Least count of measuring cylinder = 5 ml y = -0.1397x + 1.4995 (y is the refractive index value and x is the mole fraction of the component). KI= YI / XI ( I= A or B) KA = (PA/A )A (a = total pressure = 1 atm, ln PI = AI + BI/ (T+cI) [Antoine Equation, T is temp in C, values of A, B, c can be obtained from literature.] Van Laar equation: log a = a / [1 + (a* Xa)/(b* Xb)]2 log B = b / [1 + (b* XB)/(a* XA)]2 References: http://en.wikipedia.org/wiki/Van_Laar_equation http://www.thermopedia.com/content/4290/?tid=110&sn=26 Chemical Lab Manual
Das könnte Ihnen auch gefallen
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryVon EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryBewertung: 3.5 von 5 Sternen3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Von EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Bewertung: 4.5 von 5 Sternen4.5/5 (119)
- Never Split the Difference: Negotiating As If Your Life Depended On ItVon EverandNever Split the Difference: Negotiating As If Your Life Depended On ItBewertung: 4.5 von 5 Sternen4.5/5 (838)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaVon EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaBewertung: 4.5 von 5 Sternen4.5/5 (265)
- The Little Book of Hygge: Danish Secrets to Happy LivingVon EverandThe Little Book of Hygge: Danish Secrets to Happy LivingBewertung: 3.5 von 5 Sternen3.5/5 (399)
- Grit: The Power of Passion and PerseveranceVon EverandGrit: The Power of Passion and PerseveranceBewertung: 4 von 5 Sternen4/5 (587)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyVon EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyBewertung: 3.5 von 5 Sternen3.5/5 (2219)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeVon EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeBewertung: 4 von 5 Sternen4/5 (5794)
- Team of Rivals: The Political Genius of Abraham LincolnVon EverandTeam of Rivals: The Political Genius of Abraham LincolnBewertung: 4.5 von 5 Sternen4.5/5 (234)
- Shoe Dog: A Memoir by the Creator of NikeVon EverandShoe Dog: A Memoir by the Creator of NikeBewertung: 4.5 von 5 Sternen4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerVon EverandThe Emperor of All Maladies: A Biography of CancerBewertung: 4.5 von 5 Sternen4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreVon EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreBewertung: 4 von 5 Sternen4/5 (1090)
- Her Body and Other Parties: StoriesVon EverandHer Body and Other Parties: StoriesBewertung: 4 von 5 Sternen4/5 (821)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersVon EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersBewertung: 4.5 von 5 Sternen4.5/5 (344)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceVon EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceBewertung: 4 von 5 Sternen4/5 (890)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureVon EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureBewertung: 4.5 von 5 Sternen4.5/5 (474)
- The Unwinding: An Inner History of the New AmericaVon EverandThe Unwinding: An Inner History of the New AmericaBewertung: 4 von 5 Sternen4/5 (45)
- The Yellow House: A Memoir (2019 National Book Award Winner)Von EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Bewertung: 4 von 5 Sternen4/5 (98)
- How To Be Happy All The Time - NLP AnchorsDokument9 SeitenHow To Be Happy All The Time - NLP Anchorsmramakrishna919Noch keine Bewertungen
- On Fire: The (Burning) Case for a Green New DealVon EverandOn Fire: The (Burning) Case for a Green New DealBewertung: 4 von 5 Sternen4/5 (73)
- Milankovitch Cycles - Earth ClimateDokument11 SeitenMilankovitch Cycles - Earth ClimateJohn MarkakisNoch keine Bewertungen
- Quiz - Waste Heat RecoveryDokument3 SeitenQuiz - Waste Heat Recoverysagarchawla13100% (1)
- Introduction of MechatronicsDokument23 SeitenIntroduction of MechatronicsSathish SatNoch keine Bewertungen
- Introduction of Catalytic Reforming ProcessDokument10 SeitenIntroduction of Catalytic Reforming Processsagarchawla13100% (1)
- Solutions To Concepts: Chapter - 1Dokument4 SeitenSolutions To Concepts: Chapter - 1Jayanth VgNoch keine Bewertungen
- RTD in CSTRRDokument14 SeitenRTD in CSTRRsagarchawla13Noch keine Bewertungen
- Chem Lab2Dokument8 SeitenChem Lab2sagarchawla13100% (1)
- Thermo WellDokument4 SeitenThermo Wellsagarchawla13Noch keine Bewertungen
- Des AlterDokument5 SeitenDes Altersagarchawla13Noch keine Bewertungen
- QDokument2 SeitenQsagarchawla13Noch keine Bewertungen
- A Summer Internship Report On Image Processing of 2 D Bubble Column atDokument8 SeitenA Summer Internship Report On Image Processing of 2 D Bubble Column atsagarchawla13Noch keine Bewertungen
- AppendixDokument4 SeitenAppendixsagarchawla13Noch keine Bewertungen
- Lab 04Dokument8 SeitenLab 04sagarchawla13Noch keine Bewertungen
- ThermometerDokument3 SeitenThermometersagarchawla13Noch keine Bewertungen
- Lab 04Dokument8 SeitenLab 04sagarchawla13Noch keine Bewertungen
- Numofc 2&compnames 1&comp1 8&comp2 4&comp3 2&vlemode Isobaric&Vletype Xy& Numberforvle 1Dokument3 SeitenNumofc 2&compnames 1&comp1 8&comp2 4&comp3 2&vlemode Isobaric&Vletype Xy& Numberforvle 1sagarchawla13Noch keine Bewertungen
- Lab 4Dokument3 SeitenLab 4sagarchawla13Noch keine Bewertungen
- Observations and CalculationsDokument14 SeitenObservations and Calculationssagarchawla13Noch keine Bewertungen
- IdacDokument7 SeitenIdacsagarchawla13Noch keine Bewertungen
- Roasting PlantDokument20 SeitenRoasting PlantsagarchawlaNoch keine Bewertungen
- ME-495 Laboratory Exercise - Number 5 Me Dept, Sdsu - KassegneDokument5 SeitenME-495 Laboratory Exercise - Number 5 Me Dept, Sdsu - Kassegnesagarchawla13Noch keine Bewertungen
- Flaring Are Important Safety Devices Used in Refineries and Petrochemical FacilitiesDokument3 SeitenFlaring Are Important Safety Devices Used in Refineries and Petrochemical Facilitiessagarchawla13Noch keine Bewertungen
- Vocational Training Report at IOCL Gujarat RefineryDokument45 SeitenVocational Training Report at IOCL Gujarat Refinerysagarchawla13Noch keine Bewertungen
- CL 351: Chemical Engineering Lab IIDokument9 SeitenCL 351: Chemical Engineering Lab IIsagarchawla13Noch keine Bewertungen
- Lab ReportDokument1 SeiteLab Reportsagarchawla13Noch keine Bewertungen
- Assign#1Dokument3 SeitenAssign#1suryaNoch keine Bewertungen
- ME-495 Laboratory Exercise - Number 5 Me Dept, Sdsu - KassegneDokument5 SeitenME-495 Laboratory Exercise - Number 5 Me Dept, Sdsu - Kassegnesagarchawla13Noch keine Bewertungen
- 2 DDokument8 Seiten2 Dsagarchawla13Noch keine Bewertungen
- ClinicalKey - Supporting Healthcare ProfessionalsDokument51 SeitenClinicalKey - Supporting Healthcare ProfessionalsrsbhyNoch keine Bewertungen
- Ijser: Failure Modes of RC Columns Under LoadingDokument22 SeitenIjser: Failure Modes of RC Columns Under LoadingSidharth KambleNoch keine Bewertungen
- Lattitude and Longitude PDF ProblemDokument2 SeitenLattitude and Longitude PDF ProblemSatyendranath KarNoch keine Bewertungen
- Accelerating research insightsDokument13 SeitenAccelerating research insightsViệt Dũng NgôNoch keine Bewertungen
- 1st Bay Area Mathematical Olympiad February 23, 1999Dokument2 Seiten1st Bay Area Mathematical Olympiad February 23, 1999Karn KumarNoch keine Bewertungen
- Endcarriage - KZL-S 315Dokument116 SeitenEndcarriage - KZL-S 315Josip Nuno CoricNoch keine Bewertungen
- NetAct Plan Editor 4.9-4 CNDokument4 SeitenNetAct Plan Editor 4.9-4 CNAshraf JarjeesNoch keine Bewertungen
- For Mail 2023 24 Middle Senior Textbooks Notebooks List Class 12Dokument3 SeitenFor Mail 2023 24 Middle Senior Textbooks Notebooks List Class 12YatinNoch keine Bewertungen
- Identity Collage RubricDokument1 SeiteIdentity Collage Rubricapi-709145254Noch keine Bewertungen
- Munsat, S. - ProcessDokument6 SeitenMunsat, S. - ProcessBen FortisNoch keine Bewertungen
- Q4-Hinge Theorem-ActivityDokument2 SeitenQ4-Hinge Theorem-ActivityEmelie HernandezNoch keine Bewertungen
- Centre For Political Studies: End-Semester Examination Time-Table Monsoon Semester 2019 ExaminationDokument2 SeitenCentre For Political Studies: End-Semester Examination Time-Table Monsoon Semester 2019 ExaminationAbhijeet JhaNoch keine Bewertungen
- CRC CDokument13 SeitenCRC Cjosei_telspecNoch keine Bewertungen
- My Journey in PharmacologyDokument30 SeitenMy Journey in PharmacologysureshNoch keine Bewertungen
- NAHRIM - Institut Penyelidikan Hidraulik Kebangsaan Malaysia - Rainwater Harvesting SystemDokument4 SeitenNAHRIM - Institut Penyelidikan Hidraulik Kebangsaan Malaysia - Rainwater Harvesting SystemAnonymous e1j2F5Ge0Noch keine Bewertungen
- Ang Alamat NG Gubat Bob OngDokument3 SeitenAng Alamat NG Gubat Bob OngSunshine SungaNoch keine Bewertungen
- Ignou Assignment 2018 BA III YearDokument6 SeitenIgnou Assignment 2018 BA III YearTelika RamuNoch keine Bewertungen
- TPB QuestionnaireDokument9 SeitenTPB QuestionnaireAhmad FazullahNoch keine Bewertungen
- Benefits of Audio-Visual Aids and Visual Learning Highlighted in ResearchDokument6 SeitenBenefits of Audio-Visual Aids and Visual Learning Highlighted in ResearchAngelo Joshua FajamolinNoch keine Bewertungen
- How To Install Icinga and Icinga Web On Ubuntu 16Dokument9 SeitenHow To Install Icinga and Icinga Web On Ubuntu 16aracostamNoch keine Bewertungen
- Food Chemistry: Analytical MethodsDokument7 SeitenFood Chemistry: Analytical Methodswildan ariefNoch keine Bewertungen
- DOO OBR Usage v3Dokument73 SeitenDOO OBR Usage v3Kirti ThakurNoch keine Bewertungen
- Five Necessairy Condition For Project SuccessDokument2 SeitenFive Necessairy Condition For Project SuccessSimeon Petos100% (1)
- Introduction To Hse Benchmarking: Symposium Series No. 156 Hazards Xxii # 2011 IchemeDokument8 SeitenIntroduction To Hse Benchmarking: Symposium Series No. 156 Hazards Xxii # 2011 IchemeLegend AnbuNoch keine Bewertungen
- Housekeeping & Etiquette BibliographyDokument92 SeitenHousekeeping & Etiquette BibliographyDouglas CavalheiroNoch keine Bewertungen
- Aneka Cloud IntroductionDokument36 SeitenAneka Cloud IntroductionPradeep Kumar Reddy ReddyNoch keine Bewertungen
- Dark Mode Log0Dokument2 SeitenDark Mode Log0picochulo2381Noch keine Bewertungen