Beruflich Dokumente
Kultur Dokumente
Howto Companion April2011
Hochgeladen von
lybrakissCopyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Howto Companion April2011
Hochgeladen von
lybrakissCopyright:
Verfügbare Formate
14
|
companion
HOW TO
How to
How to utilise blood
products in small animals
Gillian Gibson, clinical
pathologist at Axiom
Veterinary Laboratories,
helps us make the
most of this precious
resource
Blood component therapy
Initial blood collection, using a suitable
anticoagulant and collection system,
yields whole blood containing red blood
cells (RBCs), white blood cells (WBCs)
and platelets suspended in the liquid
plasma portion. Historically whole blood
has been most often administered to small
animal patients, often collected at the time
of need. However, blood components
can be separated using variable-speed
temperature-controlled centrifuges,
according to standard blood banking
protocols.
Advantages of component therapy include:
Specific replacement therapy only
giving the patient what is needed and
reducing the risk of transfusion reactions
One unit of donated blood can be used
to help more than one patient
Maximises the storage life of blood
products.
The production of components is
limited by the availability of specialist
equipment and skills (e.g. referral hospitals
with in-house blood banks) but, following
recent legislative changes, licensed UK
pet blood banks are now able to provide
components for more widespread use.
The components of whole blood most
often used in veterinary transfusion
medicine are RBCs and plasma products.
RBCs contain haemoglobin, which is
necessary for oxygen transport from the
lungs to the rest of the body. RBC
transfusions provide the recipient with an
additional red cell mass and consequently
increased oxygen-carrying capacity.
Plasma products are a source of
coagulation factors and various plasma
proteins that may be used to replace
them in states of deficiency. Platelet
products are not readily available and
will not be discussed here. Table 1
summarises the properties and uses of
different blood products.
Blood product preparation
To prevent microbial contamination of any
of the component products, a closed
collection system must be used, and the
transfer of the components from the
collection bag to the individual satellite
storage bags is achieved by a system of
integrated tubing (Figure 1). In these
closed systems there is no exposure of the
Figure 1: Closed collection system used for blood component processing
collection bag or its contents to air prior to
administration, other than when the needle
is uncapped to perform venepuncture at
collection.
The collection and processing of blood
components following appropriate
guidelines provides a supply of products
with little risk of microbial contamination
and maximal storage times. Plasma is
separated from the RBCs by
centrifugation; the red cells separate to the
bottom of the collection unit and the
plasma can be removed and stored
separately (Figure 2). The prepared units
are sealed with a hand held clip sealer or
heat sealer prior to storage.
If blood is collected into an open
system, one in which there is one or more
additional sites of potential bacterial
contamination during blood collection or
processing, all components must be used
within 24 hours. Using syringes with added
anticoagulant to collect blood is an
example of an open system.
14-19 How To.indd 14 21/03/2011 13:42
companion
|
15
HOW TO
How to utilise blood
products in small animals
Figure 2: Separation of plasma and PRBCs. (A) Collection bags in a specialised variable-speed
temperature-controlled centrifuge. (B) Following centrifugation, the plasma component is gently separated
from the RBCs by gentle pressure. The plasma is transferred to a separate empty storage bag that is
connected to the original blood collection bag by integral tubing. (C) Plasma and PRBCs are stored
separately and may be used for different patients
A B C
Blood component Constituents/properties Storage Indications
Red blood cell products
Fresh whole blood
(FWB)
RBCs, platelets, coagulation
factors and plasma proteins all
present and functional
Must be used within 8 hours of
collection
Thrombocytopenia or
thrombocytopathia causing severe
uncontrolled or life-threatening
haemorrhage; anaemia with
concurrent coagulopathy
Stored whole blood
(SWB)
RBCs and plasma proteins, but not
functional platelets or coagulation
factors
Stored at 16C for approximately 28
days; depends on anticoagulant used
Anaemia with concurrent
hypoproteinaemia
Packed red blood
cells (PRBCs)
Red blood cells RBCs separated from plasma, stored at
16C for 20 days; or extended to 35
days with use of appropriate
preservative
Clinically symptomatic euvolaemic
anaemia (e.g. IMHA, non-regenerative
anaemia)
Plasma products
Fresh frozen plasma
(FFP)
All plasma coagulation factors,
including labile coagulation factors
V and VIII, and plasma proteins
Separated from PRBCs and frozen
within 8 hours of collection; stored at
20C for up to 1 year*
Acquired or inherited coagulopathies
(e.g. inherited factor deficiencies,
vitamin K deficiency, vitamin K
antagonist intoxication, DIC, severe
liver disease)
Stored frozen
plasma
Some vitamin-K dependent factors,
albumin and globulin
FFP >1 year of age, or plasma not
frozen within 8 hours, or FFP that has
been thawed and refrozen. Many useful
clotting factors and anti-inflammatory
proteins will have been lost. Stored at
20C for 5 years*
Vitamin K deficiency, vitamin K
antagonist intoxication,
hypoproteinaemia (colloidal support)
Cryoprecipitate
(CPP)
von Willebrand factor, factor VIII,
factor XIII, fibrinogen and
fibronectin
Extracted from FFP. Stored at 20C for
up to 1 year*
Replacement therapy for deficiencies
of von Willebrand factor, factor VIII
(haemophilia A) or fibrinogen
Cryosupernatant/
Cryo-poor plasma
Many clotting factors, including
vitamin K-dependent factors II, VII,
IX and X, other anticoagulant and
fibrinolytic factors, albumin and
globulin
Produced from a unit of FFP by
separation from CPP. Stored at 20C
for up to 1 year*
Coagulopathies or hypoproteinaemias
not requiring supplementation of the
CPP components
Table 1: Summary of blood component properties, storage requirements and indications for use. (*from date
of collection)
14-19 How To.indd 15 21/03/2011 13:42
16
|
companion
HOW TO
Utilise blood products
in small animals
Storage of blood products
RBCs are refrigerated.
Temperature maintained at 16C,
checked daily with a refrigerator
thermometer.
A dedicated household refrigerator is
often suitable, as long as there is low in
and out traffic (e.g. it is not used for
storing frequently used products such
as vaccinations).
Store upright.
Plasma products are frozen.
Temperature maintained at 20C or
below, checked daily with a
thermometer to ensure maintenance of
adequate storage conditions.
A dedicated household freezer may
suffice, but the temperature within the
freezer may vary depending on the
storage compartment and it is therefore
important to check the temperature in
each compartment.
When the plasma is still liquid, an
elastic band is placed around the
middle of the bag to create an
indentation (waist) during freezing. The
band is removed once the unit is frozen
hard. Loss of this waist in a stored
frozen plasma unit suggests that the
unit has thawed and refrozen, which
could compromise the plasma quality.
Care should be taken not to drop the
frozen plasma units as they are
vulnerable to cracking.
Choosing the appropriate blood
product for the patient
RBC products
RBC products may be indicated in any
anaemic patient, regardless of the cause
of anaemia (haemorrhage, haemolysis or
impaired erythropoiesis) if they will
benefit from an additional red cell mass
and hence increased oxygen-carrying
capacity.
Tachypnoea
Tachycardia
Bounding or poor peripheral pulses
Pallor
Collapse
Lethargy or weakness
Decreased appetite.
Plasma products
Plasma products are most commonly used
to treat inherited or acquired
coagulopathies. Plasma is of little benefit in
hypoproteinaemic patients, and other
modes of therapy (synthetic colloids,
nutritional support) are recommended.
Administration of blood
products
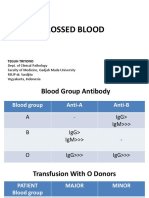
Blood types are determined by species-
specific inherited cell surface antigens.
Incompatibility between species or
individuals may result in transfusion
reactions or neonatal isoerythrolysis. A
more in-depth discussion regarding blood
types and cross-matching may be found in
the BSAVA Manual of Canine and Feline
Emergency and Critical Care, 2nd edn.
However, these general guidelines should
be followed:
Canine
Blood type of donor and recipient
should be assessed prior to
transfusion.
If it is not possible to type the recipient,
ideally a DEA 1.1-negative donor
should be used.
DEA 1.1-negative recipients should
only receive DEA 1.1-negative RBCs.
DEA 1.1-positive recipients may receive
either DEA 1.1-negative or DEA
1.1-positive RBCs.
Feline
Incompatibility transfusion reactions
can be fatal.
There is no precise packed cell volume
(PCV) below which RBC transfusion should
be administered. Consideration of a
number of factors, including volume status,
rate of onset of anaemia, ongoing losses
and patient clinical condition, must
influence the decision to transfuse. General
guidelines are offered below.
PCV
Almost all patients with a PCV 12%
would benefit from a transfusion.
Any patient with a PCV 20% should
be considered a transfusion candidate.
Some patients with a PCV >20% may
benefit from a RBC transfusion (e.g.
acute haemorrhage with ongoing
blood loss).
Chronic anaemia is typically better
tolerated than acute anaemia due to
compensatory mechanisms.
Volume status
Animals with a reduced red cell mass
but a normal intravascular volume
(euvolaemic anaemia) may benefit from
a RBC transfusion but not require the
volume of accompanying plasma;
therefore a PRBCs transfusion would
be recommended. Examples would be
animals with immune-mediated
haemolytic anaemia (IMHA) or a
non-regenerative anaemia.
In animals with volume depletion as a
consequence of acute haemorrhage
(hypovolaemic anaemia), a whole
blood transfusion or the combination of
appropriate components (e.g. PRBCs
plus plasma) would be recommended.
Clinical signs
Clinical signs are the most important factor
in deciding when to administer a RBC
transfusion. The following signs suggest
that the patient may benefit from additional
oxygen-carrying support:
14-19 How To.indd 16 21/03/2011 13:42
companion
|
17
HOW TO
Blood type of donor and recipient must
ALWAYS be assessed prior to
transfusion.
Type A cats should only receive type A
blood or plasma.
Type B cats should only receive type B
blood or plasma.
Type AB cats may receive either type
AB (preferable) or type A (acceptable)
blood and ideally only type AB plasma.
Cross-match recommendations
Cross-matching should be carried out:
Prior to first transfusion in cats
When the recipient has previously been
transfused (>4 days prior)
When there is history of a transfusion
reaction
If the patients transfusion history is
unknown.
Figure 3 provides a description of an
abbreviated in-house slide cross-match
method that may be used in an emergency
situation if laboratory services are not
available.
Route of administration
Blood products are usually
administered intravenously.
If venous access is not possible (e.g.
neonatal patient) the intraosseous route
may be used.
Blood products should not be given
intraperitoneally.
Filter
An in-line filter is required for all products
with a maximum size of 170260 m; this is
usually incorporated within a standard
blood infusion set.
Filtration removes any small blood clots
and particles that could be harmful if
infused into the recipient.
Microaggregate filters of 1840 m are
Figure 4:
Example of a
paediatric
blood filter that
may be used in
line with small
blood
transfusions
Figure 5: Formula for calculating the volume of whole blood required for transfusion
Volume (ml) =
85 (dog) or 60 (cat) x bodyweight (kg) x [ (desired PCV actual PCV) / donor PCV ]
available and can be placed in line
when infusing small volumes of
product or blood collected in syringes
(Figure 4).
Dose
The amount of product to be given greatly
depends on the product type, indication
1. Collect blood into an EDTA tube from recipient and donor.
2. Centrifuge tubes to settle the RBCs, remove the supernatant and transfer to a
clean, labelled glass or plastic tube.
3. For each donor prepare 3 slides labelled as major, minor and recipient control.
4. Place 1 drop of RBCs and 2 drops of plasma on to each slide according to the
following:
Major cross-match = donor RBCs + recipient plasma
Minor cross-match = recipient RBCs + donor plasma
Recipient control = recipient RBCs + recipient plasma
5. Gently rock the slides to mix the plasma and red cells and examine for
haemagglutination after 15 minutes (presence of agglutination indicates
incompatibility); recipient control agglutination will invalidate results.
Figure 3: Abbreviated slide cross-match procedure
for administration, and desired response in
the patient. A useful formula to calculate
the amount of whole blood required for
transfusion is noted in Figure 5. In general
most patients will receive:
Whole blood: 1022 ml/kg
PRBCs or FFP: 612 ml/kg
14-19 How To.indd 17 21/03/2011 13:42
18
|
companion
HOW TO
Utilise blood products
in small animals
Product quality inspection
Stored RBC products should be
examined for any discoloration of the
cells or suspension fluid (e.g. brown
or purple) and the presence of clots, as
these changes may indicate bacterial
contamination, haemolysis or other
storage lesions.
Plasma bags should be examined for
evidence of thawing and refreezing
(disappearance of the waist), and
cracking or tearing of the bag.
Preparation
Stored RBC products need not be
warmed prior to use, unless they are
being given to very small animals or
neonates.
PRBCs stored without an added
preservative may be resuspended or
co-administered with 100 ml of
physiological saline to decrease
viscosity and improve flow (Figure 6).
Frozen plasma products are gently
thawed in a warm water bath. The
plasma bag should be placed inside a
sealed plastic bag prior to immersing in
the water bath to protect the injection
ports from possible microbial
contamination.
Method and rate of administration
Blood products are most commonly
infused by gravity flow, although
infusion pumps validated for the
administration of products may also be
used (see Figure 6).
Small volumes (<100 ml) are often
delivered by syringe driver or slow,
intermittent, small-volume bolus
injections (Figure 4).
Animals should not receive any food or
medications during a transfusion.
To prevent incompatibility reactions,
only 0.9% saline may be
simultaneously administered through
the same catheter as a blood product.
All infusions should be completed
within 4 hours.
The rate of administration is dependent
on the cardiovascular status of the patient,
and care should be taken when infusing
patients with risk of volume overload (e.g.
cardiovascular disease, renal failure).
During the first 20 minutes use an
infusion rate of 0.251.0 ml/kg/h.
If the transfusion is well tolerated, the
rate is increased to deliver the
remaining product over 4 hours.
NOTE: If the patient is at risk of volume
overload, do not exceed 34 ml/kg/h.
Monitoring the transfusion
patient
The patients vital signs should be
measured prior to (baseline) and every
1530 minutes during, as well as 1, 12
and 24 hours following the transfusion.
These include:
Attitude
Rectal temperature
Pulse rate and quality
Respiratory rate and character
Mucous membrane colour and
capillary refill time.
Any change in plasma or urine colour
indicative of haemolysis may be significant
and should be noted. Blood samples to
reassess PCV or clotting times are usually
obtained at some time point after
completion of the transfusion, unless
deterioration of the patient requires
otherwise. Repeating these parameters
provides an assessment of efficacy as well
as helps to determine whether a further
transfusion is required.
Transfusion reactions
Any undesired side effect noted as a
consequence of a blood product
transfusion is considered a transfusion
reaction. Reactions are classified as
immunological, non-immunological and
delayed. Prompt recognition and
treatment of potential adverse transfusion
reactions is essential.
Figure 6: PRBC transfusion being
administered to a dog. Note the use of a
specialised blood infusion set that allows
co-administration of physiological saline.
When a preservative has been added to
the PRBCs prior to storage,
co-administration with saline is not
required. An intravenous fluid pump
validated for the administration of red
cells is being used in this case
14-19 How To.indd 18 21/03/2011 13:42
companion
|
19
HOW TO
REGISTERS AND BANKS
The Animal Blood Register (www.animalbloodregister.com) is a national donor
database for UK pets, and Pet Blood Bank (www.petbloodbankuk.org) is a not for
profit charitable organisation offering a full range of transfusion products. These
organisations give veterinary surgeons in practice previously unattainable access to
lists of matched donors and to blood products respectively. This freedom
from the necessity for individual practices to health screen and cross
match local donors and be proficient in blood collection allows the use
of blood products more widely than has previously been possible.
See the BSAVA Manual of Canine and Feline Haematology and
Transfusion Medicine and BSAVA Manual of Canine and Feline
Emergency and Critical Care for useful information.
RBC incompatibility reactions
(immunological) may cause acute
haemolysis and can be life-threatening.
Signs may include:
Pyrexia
Tachycardia
Tachypnoea
Weakness
Salivation/vomiting
Diarrhoea
Haemoglobinaemia
Haemoglobinuria.
Non-haemolytic immunological
reactions are acute allergic/anaphylactic
reactions, signs of which include:
Oedema
Erythema
Pruritus
Urticaria
Vomiting
Dyspnoea.
Non-immunological transfusion
reactions may often be avoided by
rigorous donor screening and adherence
to blood banking protocols. Such
reactions include:
Anaphylactoid (often a consequence
of rapid infusion rate)
Volume overload
Transmission of infectious disease
Hypocalcaemia (secondary to
excessive citrate anticoagulant)
Polycythaemia and hyperproteinaemia
(excessive volume of blood product
administered)
Dilutional coagulopathy
Microbial contamination (signs may be
similar to acute haemolytic reaction)
Hyperkalaemia, acidosis,
hyperammonaemia,
hypophosphataemia (storage lesions)
Air embolus.
Treatment of acute transfusion
reactions
1. Stop transfusion:
a In case of allergic/anaphylactic
reaction, if reaction subsides may
restart transfusion at 2550% of
previous rate
b If signs of acute haemolytic
reaction, do not continue with
transfusion.
2. Donor and recipient blood type,
product type and expiration date
should be confirmed, and a cross-
match may be performed (if not done
prior to transfusion).
3. Treat any clinical signs of shock,
including fluid therapy.
4. Antihistamines:
a Chlorpheniramine: Maximum
recommended dose 0.5 mg/kg
q12h (dogs and cats)
i. Dogs: small to medium,
2.55 mg/dog i.m. q12h
ii. Dogs: medium to large, 510
mg/dog i.m. q12h
iii. Cats: 24 mg/cat orally q12h.
b Diphenhydramine 12 mg/kg i.m.
q12h (dogs and cats).
4. Corticosteroids: Dexamethasone
0.51.0 mg/kg i.v.
5. Monitor for development of fluid
overload.
6. Monitor for development of hypotension
or oliguria.
7. Administer H
2
blockers, colloids,
dopamine and/or aminophylline as
needed.
8. Samples of both donor and recipient
blood should be evaluated for
haemolysis, and saved for microbial
culture and infectious disease testing
if required.
9. Broad-spectrum antibiotics may be
administered if bacterial contamination
is suspected.
Delayed haemolytic reactions may
be recognised by an unexpected
decline in the PCV or by jaundice
developing at some time 221 days
post-transfusion. These often do not
require any specific therapy.
Finally
It is important to note on the patients file
that it has received a blood product
transfusion, and to make the owner aware
that this information should be passed
along to other veterinary surgeons that may
be caring for their pet in the future.
Das könnte Ihnen auch gefallen
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Von EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Bewertung: 4.5 von 5 Sternen4.5/5 (119)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaVon EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaBewertung: 4.5 von 5 Sternen4.5/5 (265)
- The Little Book of Hygge: Danish Secrets to Happy LivingVon EverandThe Little Book of Hygge: Danish Secrets to Happy LivingBewertung: 3.5 von 5 Sternen3.5/5 (399)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryVon EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryBewertung: 3.5 von 5 Sternen3.5/5 (231)
- Grit: The Power of Passion and PerseveranceVon EverandGrit: The Power of Passion and PerseveranceBewertung: 4 von 5 Sternen4/5 (587)
- Never Split the Difference: Negotiating As If Your Life Depended On ItVon EverandNever Split the Difference: Negotiating As If Your Life Depended On ItBewertung: 4.5 von 5 Sternen4.5/5 (838)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeVon EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeBewertung: 4 von 5 Sternen4/5 (5794)
- Team of Rivals: The Political Genius of Abraham LincolnVon EverandTeam of Rivals: The Political Genius of Abraham LincolnBewertung: 4.5 von 5 Sternen4.5/5 (234)
- Shoe Dog: A Memoir by the Creator of NikeVon EverandShoe Dog: A Memoir by the Creator of NikeBewertung: 4.5 von 5 Sternen4.5/5 (537)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyVon EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyBewertung: 3.5 von 5 Sternen3.5/5 (2219)
- The Emperor of All Maladies: A Biography of CancerVon EverandThe Emperor of All Maladies: A Biography of CancerBewertung: 4.5 von 5 Sternen4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreVon EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreBewertung: 4 von 5 Sternen4/5 (1090)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersVon EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersBewertung: 4.5 von 5 Sternen4.5/5 (344)
- Her Body and Other Parties: StoriesVon EverandHer Body and Other Parties: StoriesBewertung: 4 von 5 Sternen4/5 (821)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceVon EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceBewertung: 4 von 5 Sternen4/5 (894)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureVon EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureBewertung: 4.5 von 5 Sternen4.5/5 (474)
- The Unwinding: An Inner History of the New AmericaVon EverandThe Unwinding: An Inner History of the New AmericaBewertung: 4 von 5 Sternen4/5 (45)
- The Yellow House: A Memoir (2019 National Book Award Winner)Von EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Bewertung: 4 von 5 Sternen4/5 (98)
- On Fire: The (Burning) Case for a Green New DealVon EverandOn Fire: The (Burning) Case for a Green New DealBewertung: 4 von 5 Sternen4/5 (73)
- Chapter 16 - BiologicsDokument54 SeitenChapter 16 - BiologicsRobert Dominic Gonzales100% (2)
- One Step Hepatitis B Virus Test KitDokument34 SeitenOne Step Hepatitis B Virus Test KitGail IbanezNoch keine Bewertungen
- Blood TransfusionDokument37 SeitenBlood TransfusionSumathi Akshaya100% (1)
- Companion July 2014 Low ResDokument36 SeitenCompanion July 2014 Low ReslybrakissNoch keine Bewertungen
- How To February 2014 Anesteziezi o PasareDokument6 SeitenHow To February 2014 Anesteziezi o PasarelybrakissNoch keine Bewertungen
- Advanced portable immune-analyzer for speed and reliabilityDokument2 SeitenAdvanced portable immune-analyzer for speed and reliabilityezfia67% (3)
- XPress Studio HD 5.1 InstallIDokument31 SeitenXPress Studio HD 5.1 InstallIlybrakissNoch keine Bewertungen
- Xpress Pro 4.5 UGSuppDokument248 SeitenXpress Pro 4.5 UGSupplybrakissNoch keine Bewertungen
- Pexip Infinity Management API V32.aDokument53 SeitenPexip Infinity Management API V32.aozz12345Noch keine Bewertungen
- MotivationalDokument43 SeitenMotivationallybrakissNoch keine Bewertungen
- Infinity Connect Webapp Userguide V32.aDokument35 SeitenInfinity Connect Webapp Userguide V32.alybrakissNoch keine Bewertungen
- Document ActDokument15 SeitenDocument ActlybrakissNoch keine Bewertungen
- Pexip Infinity Scheduling Meetings Quickguide V32.aDokument4 SeitenPexip Infinity Scheduling Meetings Quickguide V32.alybrakissNoch keine Bewertungen
- Infinity Connect Desktop Userguide V1.12.aDokument25 SeitenInfinity Connect Desktop Userguide V1.12.alybrakissNoch keine Bewertungen
- Infinity Connect Mobile Userguide V1.10.aDokument17 SeitenInfinity Connect Mobile Userguide V1.10.alybrakissNoch keine Bewertungen
- LE 069 LE 078 InstructionsDokument4 SeitenLE 069 LE 078 InstructionslybrakissNoch keine Bewertungen
- MesopotamDokument35 SeitenMesopotamlybrakissNoch keine Bewertungen
- AramanicDokument50 SeitenAramaniclybrakissNoch keine Bewertungen
- Collected Works of PoeDokument208 SeitenCollected Works of PoeTrần Tú BíchNoch keine Bewertungen
- About AbrahamDokument25 SeitenAbout AbrahamlybrakissNoch keine Bewertungen
- The Call of CthulhuDokument14 SeitenThe Call of CthulhuLOKADNoch keine Bewertungen
- C Enu Faci PT DragosteDokument95 SeitenC Enu Faci PT DragostelybrakissNoch keine Bewertungen
- 1017 Intelligence Quotient IQ Test Interview Questions Answers GuideDokument7 Seiten1017 Intelligence Quotient IQ Test Interview Questions Answers GuideRaniel MamaspasNoch keine Bewertungen
- A Little PrincessDokument196 SeitenA Little PrincessShona ButtNoch keine Bewertungen
- 1017 Intelligence Quotient IQ Test Interview Questions Answers GuideDokument7 Seiten1017 Intelligence Quotient IQ Test Interview Questions Answers GuideRaniel MamaspasNoch keine Bewertungen
- 1017 Intelligence Quotient IQ Test Interview Questions Answers GuideDokument7 Seiten1017 Intelligence Quotient IQ Test Interview Questions Answers GuideRaniel MamaspasNoch keine Bewertungen
- How-To-june-2014 CompanionHow To Approach The Canine AthleteInDokument6 SeitenHow-To-june-2014 CompanionHow To Approach The Canine AthleteInlybrakissNoch keine Bewertungen
- Stiri WWWDokument5 SeitenStiri WWWlybrakissNoch keine Bewertungen
- How-To-April-2014 Companion How To Manage Urethral in Dogs Urethral ObstructionDokument6 SeitenHow-To-April-2014 Companion How To Manage Urethral in Dogs Urethral ObstructionlybrakissNoch keine Bewertungen
- How-To-september-2014 2014how To Manage Feline Ureteric ObstructionDokument5 SeitenHow-To-september-2014 2014how To Manage Feline Ureteric ObstructionlybrakissNoch keine Bewertungen
- How-To-may-2014 CompanionHow To Utilize Radiotherapy For Dogs With Oral TumoursIntroductionDokument6 SeitenHow-To-may-2014 CompanionHow To Utilize Radiotherapy For Dogs With Oral TumoursIntroductionlybrakissNoch keine Bewertungen
- How-To-August-2014 2014how To Deal With A Patient With Thermal BurnsImmediateDokument5 SeitenHow-To-August-2014 2014how To Deal With A Patient With Thermal BurnsImmediatelybrakissNoch keine Bewertungen
- Companion September 2014 Low ResDokument32 SeitenCompanion September 2014 Low ReslybrakissNoch keine Bewertungen
- Companion August 2014 Low ResDokument36 SeitenCompanion August 2014 Low ReslybrakissNoch keine Bewertungen
- Punicalagin ToxicityDokument9 SeitenPunicalagin ToxicitySatish Chandra KushwahaNoch keine Bewertungen
- Forensic DNA Analysis: DNA Is The Genetic MaterialDokument71 SeitenForensic DNA Analysis: DNA Is The Genetic MaterialumashankarbaghelNoch keine Bewertungen
- Procleix Ultrio Plus AssayDokument61 SeitenProcleix Ultrio Plus AssaydatitoxNoch keine Bewertungen
- What Is A Blood TransfusionDokument6 SeitenWhat Is A Blood TransfusionCarlo TolentinoNoch keine Bewertungen
- Catalogo General de Productos Stratec MolecularDokument44 SeitenCatalogo General de Productos Stratec MolecularyudycantNoch keine Bewertungen
- Seminar - Hemorrhage 25-06-2020Dokument75 SeitenSeminar - Hemorrhage 25-06-2020Chandra dimpleNoch keine Bewertungen
- Pemilihan Biomarker Yang Efektif Untuk Penelitian Klinis 14042015Dokument66 SeitenPemilihan Biomarker Yang Efektif Untuk Penelitian Klinis 14042015mahyarani dalimutheNoch keine Bewertungen
- PHLEBOTOMYDokument21 SeitenPHLEBOTOMYmyka brilliant cristobalNoch keine Bewertungen
- Study Guide STH Edition: Clinical LaboratoryDokument30 SeitenStudy Guide STH Edition: Clinical LaboratoryMendoza, Richelle A.Noch keine Bewertungen
- Rapid Igm-Igg Combined Antibody Test For Sars-Cov-2Dokument2 SeitenRapid Igm-Igg Combined Antibody Test For Sars-Cov-2ludiNoch keine Bewertungen
- Calcium: Arsenazo III (Single Reagent)Dokument2 SeitenCalcium: Arsenazo III (Single Reagent)mohmed elsadigNoch keine Bewertungen
- Lec 1A Principles of Clinical ChemDokument6 SeitenLec 1A Principles of Clinical ChemBrian LeungNoch keine Bewertungen
- Adverse Effects of Plasma TherapyDokument80 SeitenAdverse Effects of Plasma TherapyShaiji ShahidNoch keine Bewertungen
- MAGLUMI Vitamin B12 (CLIA) Test KitDokument4 SeitenMAGLUMI Vitamin B12 (CLIA) Test KitMohamed BoulkadidNoch keine Bewertungen
- Nucleic AcidsDokument66 SeitenNucleic Acidspjblk100% (1)
- Lipid Biochemistry: Historical PerspectivesDokument67 SeitenLipid Biochemistry: Historical Perspectivesainnayya devaNoch keine Bewertungen
- Erba XL 640 - MultiXl 20-06-2020Dokument222 SeitenErba XL 640 - MultiXl 20-06-2020niral savaliyaNoch keine Bewertungen
- Introduction To Clinical Chemistry 2Dokument67 SeitenIntroduction To Clinical Chemistry 2quteba alkhwaldhNoch keine Bewertungen
- Igm Arc ChemDokument8 SeitenIgm Arc Chembassam alharaziNoch keine Bewertungen
- A. Automation in Clinical Chemistry NotesDokument13 SeitenA. Automation in Clinical Chemistry NotesBryant joshua leonardo100% (1)
- UNCROSSED BLOOD Transfusion RisksDokument15 SeitenUNCROSSED BLOOD Transfusion RisksHerlina InaNoch keine Bewertungen
- DIALAB Diacal Auto Calibration Serum ProcedureDokument6 SeitenDIALAB Diacal Auto Calibration Serum Procedureg0en2Noch keine Bewertungen
- User Manual For PR SeriesqDokument46 SeitenUser Manual For PR SeriesqTulu TvNoch keine Bewertungen
- Semen SeroDokument74 SeitenSemen SeroAman ButtNoch keine Bewertungen
- ABIM Lab Test Reference RangesDokument22 SeitenABIM Lab Test Reference RangesRealyn Bebero100% (1)
- TPE in Intensive Care - Dr. Teuku Yasir, SpAn-KIC, FIPMDokument17 SeitenTPE in Intensive Care - Dr. Teuku Yasir, SpAn-KIC, FIPMTeuku FauzanoeNoch keine Bewertungen