Beruflich Dokumente
Kultur Dokumente
Tut3 Q1
Hochgeladen von
DiablofireZACopyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Tut3 Q1
Hochgeladen von
DiablofireZACopyright:
Verfügbare Formate
T
2
169.44 degC =
Thermophysical Properties:
Used Steam Tables in Cengel & Boles:
Point 1: h
0.9MPa
2773.9
kJ
kg
:= h
1.0MPa
2778.1
kJ
kg
:=
h
1
p
1
.9 MPa
1 MPa .9 MPa
h
1.0MPa
h
0.9MPa
( )
h
0.9MPa
+ := h
1
2.774 10
3
kJ
kg
=
Point 2: h
f165degC
697.34
kJ
kg
:= h
f170degC
719.21
kJ
kg
:=
h
2
T
2
165 degC
170 degC 165 degC
h
f170degC
h
f165degC
( )
h
f165degC
+ :=
h
2
716.761
kJ
kg
=
Point 3: T
3
10 degC := h
3
42.01
kJ
kg
:=
Solution:
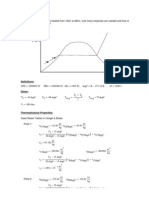
Tutorial 3 - Question 1
Dry saturated steam at 8 bar(g) is used in a plant and the condensate runs to a waste at a
temperature 6oC below steam temperature. How much of the enthalpy of saturated steam is lost, and
what is the percentage of the total enthalpy of saturated steam which is being wasted?
s [kJ/kg.K]
T [oC]
1
2
3
Definitions:
MW 1000000 W MPa 1000000 Pa kPa 1000 Pa degC K 273.16 K kJ 1000 J
Given:
p
1
0.8 .101325 + ( ) MPa := p
1
0.901MPa =
T
0.9MPa
175.38 degC := T
1.0MPa
179.91 degC :=
T
1
p
1
.9 MPa
1 MPa .9 MPa
T
1.0MPa
T
0.9MPa
( )
T
0.9MPa
+ := T
1
175.44 degC =
T
2
T
1
6 degC :=
Solution:
h
cond
h
2
h
3
:= h
cond
674.751
kJ
kg
=
%waste
h
cond
h
1
:= %waste 24.324 % =
Das könnte Ihnen auch gefallen
- Fokus Deutsch - Episode 01Dokument11 SeitenFokus Deutsch - Episode 01DiablofireZA33% (3)
- Tutorial 3 - Question 8Dokument2 SeitenTutorial 3 - Question 8DiablofireZANoch keine Bewertungen
- Fokus Deutsch - Series 104Dokument4 SeitenFokus Deutsch - Series 104DiablofireZA100% (2)
- Renewable Energies 2Dokument29 SeitenRenewable Energies 2DiablofireZANoch keine Bewertungen
- Tutorial 3 - Question 7Dokument1 SeiteTutorial 3 - Question 7DiablofireZANoch keine Bewertungen
- Tutorial 3 - Question 6Dokument1 SeiteTutorial 3 - Question 6DiablofireZANoch keine Bewertungen
- Tutorial 3 - Question 5Dokument1 SeiteTutorial 3 - Question 5DiablofireZANoch keine Bewertungen
- Jacketed Pan: Tutorial 3 - Question 4Dokument1 SeiteJacketed Pan: Tutorial 3 - Question 4DiablofireZANoch keine Bewertungen
- Tutorial 3 - Question 3Dokument1 SeiteTutorial 3 - Question 3DiablofireZANoch keine Bewertungen
- Tut 3 - Question 2Dokument2 SeitenTut 3 - Question 2DiablofireZANoch keine Bewertungen
- Energiestelsels / Energy Systems M434: Neem Kennis: Geen Memo Sal Vir Hierdie Tutoriaal Beskikbaar Gestel Word Nie!!Dokument1 SeiteEnergiestelsels / Energy Systems M434: Neem Kennis: Geen Memo Sal Vir Hierdie Tutoriaal Beskikbaar Gestel Word Nie!!DiablofireZANoch keine Bewertungen
- Tut 3Dokument5 SeitenTut 3DiablofireZANoch keine Bewertungen
- Energiestelsels / Energy Systems M434: Neem Kennis: Geen Memo Sal Vir Hierdie Tutoriaal Beskikbaar Gestel Word Nie!!Dokument1 SeiteEnergiestelsels / Energy Systems M434: Neem Kennis: Geen Memo Sal Vir Hierdie Tutoriaal Beskikbaar Gestel Word Nie!!DiablofireZANoch keine Bewertungen
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceVon EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceBewertung: 4 von 5 Sternen4/5 (895)
- Never Split the Difference: Negotiating As If Your Life Depended On ItVon EverandNever Split the Difference: Negotiating As If Your Life Depended On ItBewertung: 4.5 von 5 Sternen4.5/5 (838)
- The Yellow House: A Memoir (2019 National Book Award Winner)Von EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Bewertung: 4 von 5 Sternen4/5 (98)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeVon EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeBewertung: 4 von 5 Sternen4/5 (5794)
- Shoe Dog: A Memoir by the Creator of NikeVon EverandShoe Dog: A Memoir by the Creator of NikeBewertung: 4.5 von 5 Sternen4.5/5 (537)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaVon EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaBewertung: 4.5 von 5 Sternen4.5/5 (266)
- The Little Book of Hygge: Danish Secrets to Happy LivingVon EverandThe Little Book of Hygge: Danish Secrets to Happy LivingBewertung: 3.5 von 5 Sternen3.5/5 (400)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureVon EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureBewertung: 4.5 von 5 Sternen4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryVon EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryBewertung: 3.5 von 5 Sternen3.5/5 (231)
- Grit: The Power of Passion and PerseveranceVon EverandGrit: The Power of Passion and PerseveranceBewertung: 4 von 5 Sternen4/5 (588)
- The Emperor of All Maladies: A Biography of CancerVon EverandThe Emperor of All Maladies: A Biography of CancerBewertung: 4.5 von 5 Sternen4.5/5 (271)
- The Unwinding: An Inner History of the New AmericaVon EverandThe Unwinding: An Inner History of the New AmericaBewertung: 4 von 5 Sternen4/5 (45)
- On Fire: The (Burning) Case for a Green New DealVon EverandOn Fire: The (Burning) Case for a Green New DealBewertung: 4 von 5 Sternen4/5 (74)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersVon EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersBewertung: 4.5 von 5 Sternen4.5/5 (345)
- Team of Rivals: The Political Genius of Abraham LincolnVon EverandTeam of Rivals: The Political Genius of Abraham LincolnBewertung: 4.5 von 5 Sternen4.5/5 (234)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreVon EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreBewertung: 4 von 5 Sternen4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyVon EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyBewertung: 3.5 von 5 Sternen3.5/5 (2259)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Von EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Bewertung: 4.5 von 5 Sternen4.5/5 (121)
- Her Body and Other Parties: StoriesVon EverandHer Body and Other Parties: StoriesBewertung: 4 von 5 Sternen4/5 (821)