Beruflich Dokumente
Kultur Dokumente
Cold Agglutination
Hochgeladen von
Sajjad AhmadOriginalbeschreibung:
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Cold Agglutination
Hochgeladen von
Sajjad AhmadCopyright:
Verfügbare Formate
COLD AGGLUTINATION:
Background
Cold agglutinin disease is a form of autoimmune hemolytic anemia caused by cold-reacting
autoantibodies. Autoantibodies that bind to the erythrocyte membrane leading to premature
erythrocyte destruction (hemolysis) characterize autoimmune hemolytic anemia. Autoimmune
hemolytic anemia is classified as primary or secondary and is subclassified according to
autoantibody type.
In primary autoimmune hemolytic anemia, no underlying systemic disease explains the presence
of autoantibodies, whereas secondary autoimmune hemolytic anemia results from a systemic
disease. The autoantibody may be immunoglobulin G (IgG), immunoglobulin M (IgM), or,
rarely, immunoglobulin A (IgA); it may be warm reacting or cold reacting. (See the image
below.)
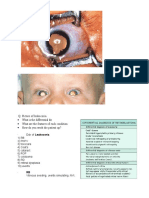
Peripheral blood smear showing several clumps of RBCs with the largest in the center. These are typical
of aggregates seen in persons with cold agglutinin disease.
Autoimmune hemolytic anemia syndromes associated with cold-reacting autoantibodies include
cold agglutinin disease and, to a much lesser extent, paroxysmal cold hemoglobinuria. (Most
paroxysmal cold hemoglobinuria cases are not caused by a cold agglutinin.)
IgM antibodies generally cause cold agglutinin disease. Donath-Landsteiner hemolytic anemia
(previously referred to as paroxysmal cold hemoglobinuria) is caused by IgG antibodies.
Primary and secondary disease
Primary cold agglutinin disease is usually associated with monoclonal cold-reacting
autoantibodies. Primary cold agglutinin disease is chronic and occurs after the fifth decade of
life, with a peak incidence in the seventh and eighth decades. (See Epidemiology.)
Secondary cold agglutinin disease may be associated with either monoclonal or polyclonal cold-
reacting autoantibodies. It predominantly is caused by infection and lymphoproliferative
disorders. Monoclonal secondary disease is usually chronic, occurring in adults. Polyclonal
secondary cold agglutinin disease, which occurs in children and young adults, is usually
transient.
Requirements for induction of active hemolytic anemia
Several factors play a role in determining the ability of a cold agglutinin to induce an active
hemolytic anemia.
[1, 2]
These include the following:
Ability to initiate
Extent of antibody-induced complement activation
Concentration of the antibody
Range of temperatures, including the highest temperature at which the antibody interacts with
the RBC (its thermal amplitude)
Qualitative binding of IgM to the RBC
Modification of the antibody's ability to fix complement components onto the RBCs
History
A common complaint among patients with cold agglutinin disease is painful fingers and toes
with purplish discoloration associated with cold exposure. In chronic cold agglutinin disease, the
patient is more symptomatic during the colder months.
Cold agglutininmediated acrocyanosis differs from Raynaud phenomenon. In Raynaud
phenomena, caused by vasospasm, a triphasic color change occurs, from white to blue to red,
based on vasculature response. No evidence of such a response exists in cold agglutinin
disease.
[24]
Other symptoms of cold agglutinin disease include the following:
Respiratory symptoms - May be present in patients with M pneumoniae infection
Hemoglobinuria (the passage of dark urine that contains hemoglobin) - A rare symptom
that results from hemolysis, this may be reported following prolonged exposure to cold;
hemoglobinuria is more commonly seen in paroxysmal cold hemoglobinuria
Chronic fatigue - Due to anemia
Anemia in patients with cold agglutinin disease may be mild, moderate, or severe. Along with
fatigue, symptoms of anemia include pallor, dyspnea, and poor feeding.
Other symptoms of cold agglutinin disease, such as a history of weight loss and adenopathy, can
be related to the underlying disease state associated with the production of cold agglutinins.
The severity of the clinical manifestations of the cold agglutinins themselves varies from an
inconsequential laboratory finding, in cases of the benign variety, to serious manifestations, such
as acute hemolytic crises and Raynaud-type phenomena, in cases of the more malignant variety.
Patient Education
It is essential to educate patients with chronic cold agglutinin disease about the importance of
keeping all body parts warm at all times and avoiding cooling of body parts. Appropriate
clothing is necessary in cold environments, and avoidance of cold foods and working in cold
storage areas is also important.
Patients must comprehend the importance of their daily folic acid intake, which supplies a
needed hematinic. Folic acid could easily become a rate-limiting hematinic in a patient with a
chronic hemolytic process.
Teach patients to watch for signs of anemia, such as dyspnea, palpitations, and pallor, and to
observe for signs of hemolysis, such as jaundice and dark urine.
REfrencesPrevious
13. Gane E, Hyland R, Ding X, Pang P, McHutchison J, Symonds W, Stedman C. ELECTRON: 100%
Suppression of Viral Load through 4 Weeks' Post-treatment for Sofosbuvir + Ledipasvir (GS-5885) +
Ribavirin for 12 Weeks in Treatment-nave and -experienced Hepatitis C Virus GT 1 Patients. 20th
Conference on Retroviruses and Opportunistic Infections, March 3-6, 2013; abstract 41LB
14. Lawitz E, Ghalib R, Rodriguez-Torres M, Younossi Z, Corregidor A, Jacobson I, Callewaert K, Symonds W,
Picchio G, Lindsay K. Suppression of Viral Load through 4 Weeks Post-Treatment Results of a Once-daily
Regimen of Simeprevir + Sofosbuvir with or without Ribavirin in Hepatitis C Virus GT 1 Null Responders.
20th Conference on Retroviruses and Opportunistic Infections, March 3-6, 2013; abstract 155LB
15. Osinusi A, Bon D, Herrmann E, Teferi G, Talwani R, Masur H, Symonds W, McHutchison J, Fauci A,
Kottilil S, and NIAID SPARE Study Team. High Efficacy of Sofosbuvir with Weight-based Ribavirin for 24
Weeks in Difficult-to-Treat Patients. 20th Conference on Retroviruses and Opportunistic Infections, March 3-
6, 2013; abstract 157
16. King M, Xie W, Larsen L, Cohen D, Podsadecki T, Bernstein B. Risk of Virologic Relapse in Hepatitis C
Virus GT1-infected Subjects after 8, 12, and 24 Weeks of ABT-450/r+ABT-267+ABT-33+Ribavirin:
Identifying Optimal Treatment Duration. 20th Conference on Retroviruses and Opportunistic Infections,
March 3-6, 2013; abstract 39
17. Lawitz E, Cohen D, Poordad F, Kowdley K, Everson G, Freilich B, Jensen D, Heckaman M, Pilot-Matias T,
Bernstein B. 12 Weeks of ABT-450/Ritonavir, Non-nucleoside Inhibitor and Ribavirin Achieved SVR24 in
>90% of Treatment-nave Hepatitis C Virus GT1 Patients and 47% of Prior Non-responders. 20th Conference
on Retroviruses and Opportunistic Infections, March 3-6, 2013; abstract 38
18. Heuft M, van den Berk G, Houba S, Smissaert T, Blok W, Regez R, Dijksman L, Brinkman K. Protective
Effect of HBV-active c-ART against primary HBV-Infection. 20th Conference on Retroviruses and
Opportunistic Infections, March 3-6, 2013; abstract 33
19. Boyd A, Miailhes P, Maylin S, Gozlan J, Lascoux-Combe C, Delaugerre C, Girard P-M, Lacombe K.
Intensification with Pegylated Interferon during Treatment with Tenofovir in HIV/Hepatitis B Virus Co-
infected Patients. 20th Conference on Retroviruses and Opportunistic Infections, March 3-6, 2013; abstract
668
Das könnte Ihnen auch gefallen
- Uworld - PEDIATRICSDokument50 SeitenUworld - PEDIATRICSNikxy100% (1)
- WHO Vaccine Manual PDFDokument112 SeitenWHO Vaccine Manual PDFRagel CorpsNoch keine Bewertungen
- 253 Complexes For Biomedis Trinity - DescriptionDokument74 Seiten253 Complexes For Biomedis Trinity - Descriptionjanhendrik4444100% (1)
- Photo QuizDokument21 SeitenPhoto Quizabas_maytham1021100% (3)
- Acute GlomerulonephritisDokument4 SeitenAcute GlomerulonephritisJulliza Joy PandiNoch keine Bewertungen
- President CEO Healthcare Administrator in Nashville TN Resume Kerry GillihanDokument4 SeitenPresident CEO Healthcare Administrator in Nashville TN Resume Kerry GillihanKerryGillihanNoch keine Bewertungen
- Autoimmune Hemolytic AnemiaDokument7 SeitenAutoimmune Hemolytic AnemiaHoopmen SilaenNoch keine Bewertungen
- ABC Quality and Patient Saftey Workshop FinalDokument68 SeitenABC Quality and Patient Saftey Workshop Finalyousrazeidan1979Noch keine Bewertungen
- Acute Glomerulonephritis: Group 8 PresentationDokument25 SeitenAcute Glomerulonephritis: Group 8 PresentationcollinsmagNoch keine Bewertungen
- DHA Exam and Review MaterialsDokument8 SeitenDHA Exam and Review MaterialsRrichard Prieto Mmallari100% (4)
- DHA Exam and Review MaterialsDokument8 SeitenDHA Exam and Review MaterialsRrichard Prieto Mmallari100% (4)
- DHA Exam and Review MaterialsDokument8 SeitenDHA Exam and Review MaterialsRrichard Prieto Mmallari100% (4)
- Glomerulonephritis: Lecturer Prof. Yu.R. KovalevDokument39 SeitenGlomerulonephritis: Lecturer Prof. Yu.R. Kovalevalfaz lakhani100% (1)
- Apoptosis and CancerDokument20 SeitenApoptosis and CancerSajjad Ahmad0% (1)
- Master Techniques in Orthopaedic SurgeryDokument1.031 SeitenMaster Techniques in Orthopaedic Surgerysasa0687100% (6)
- Uric AcidDokument10 SeitenUric AcidSajjad AhmadNoch keine Bewertungen
- Legal Medicine: Legal Medicine (2011) Antonio Rebosa, LL.B, M.DDokument6 SeitenLegal Medicine: Legal Medicine (2011) Antonio Rebosa, LL.B, M.DarciblueNoch keine Bewertungen
- Acute Glomerulonephritis: Background, Pathophysiology, EtiologyDokument5 SeitenAcute Glomerulonephritis: Background, Pathophysiology, Etiology'Riku' Pratiwie TunaNoch keine Bewertungen
- Acute GlomerulonephritisDokument12 SeitenAcute Glomerulonephritiskuchaibaru90Noch keine Bewertungen
- Ha Ii by AbdifatahDokument36 SeitenHa Ii by AbdifatahAbdifatah AhmedNoch keine Bewertungen
- Cold Agglutinin DiseaseDokument8 SeitenCold Agglutinin Diseasehtunnm@gmail.comNoch keine Bewertungen
- Fulltext - Hematology v3 Id1118Dokument3 SeitenFulltext - Hematology v3 Id1118Thành Nguyễn VănNoch keine Bewertungen
- Cold Agglutinin DiseaseDokument4 SeitenCold Agglutinin Diseasenavneet21usNoch keine Bewertungen
- AIHA FinalDokument85 SeitenAIHA FinalAbhineet SalveNoch keine Bewertungen
- 41anurag EtalDokument2 Seiten41anurag EtaleditorijmrhsNoch keine Bewertungen
- Immune Hemolytic Anemia1Dokument6 SeitenImmune Hemolytic Anemia1lubna aloshibiNoch keine Bewertungen
- Immune Hemolytic Anemia1Dokument6 SeitenImmune Hemolytic Anemia1lubna aloshibiNoch keine Bewertungen
- 2.13.08 Cold Agglutinin RogersDokument27 Seiten2.13.08 Cold Agglutinin RogersJessica StewartNoch keine Bewertungen
- 2.13.08 Cold Agglutinin RogersDokument27 Seiten2.13.08 Cold Agglutinin RogersYeni Chie Aneuk TuleutNoch keine Bewertungen
- Reporte de Caso EmbaraoDokument3 SeitenReporte de Caso EmbaraoPaola TabaresNoch keine Bewertungen
- 1086IC Ic 40 63Dokument4 Seiten1086IC Ic 40 63Radias ZasraNoch keine Bewertungen
- Cold Agglutinin DiseaseDokument49 SeitenCold Agglutinin DiseaseMilind KothiyalNoch keine Bewertungen
- Immune Hemolytic Anemia Modul 4Dokument10 SeitenImmune Hemolytic Anemia Modul 4Dinda SaviraNoch keine Bewertungen
- Cold Antibody Autoimmune Hemolytic Anemias Jan2008Dokument15 SeitenCold Antibody Autoimmune Hemolytic Anemias Jan2008simos_spamNoch keine Bewertungen
- Diseases of The Hematopoietic SystemDokument35 SeitenDiseases of The Hematopoietic Systemfentahunbekele282Noch keine Bewertungen
- Cryo Globulin Emi ADokument34 SeitenCryo Globulin Emi AWanjohi MosesNoch keine Bewertungen
- DengueDokument3 SeitenDenguemommydaddyNoch keine Bewertungen
- Glomerulonephritis: Nameesha Natasha Naidu 20130105Dokument26 SeitenGlomerulonephritis: Nameesha Natasha Naidu 20130105AliMalikNoch keine Bewertungen
- Reactivearthritis: Steven K. SchmittDokument13 SeitenReactivearthritis: Steven K. SchmittrachelNoch keine Bewertungen
- ASPGNDokument17 SeitenASPGNAdLi DLi DLiNoch keine Bewertungen
- Post Streptococus GNDokument14 SeitenPost Streptococus GNRahma Cita HalidaNoch keine Bewertungen
- Evan Syndrome A Case ReportDokument3 SeitenEvan Syndrome A Case ReportEditor IJTSRDNoch keine Bewertungen
- Anti-Glomerular Basement Membrane Disease An Update On Subgroups, Pathogenesis and TherapiesDokument7 SeitenAnti-Glomerular Basement Membrane Disease An Update On Subgroups, Pathogenesis and TherapiesLucas SilveiraNoch keine Bewertungen
- Cryo Globulin Emi ADokument6 SeitenCryo Globulin Emi AMeow CattoNoch keine Bewertungen
- Immune Hemolytic AnemiaDokument25 SeitenImmune Hemolytic AnemiaMuhammad DaviqNoch keine Bewertungen
- Journal ReadingDokument15 SeitenJournal ReadingAndreia StephanieNoch keine Bewertungen
- Treatment of Acquired HemophiliaDokument7 SeitenTreatment of Acquired HemophiliaLuana MNoch keine Bewertungen
- Autoimmune Hemolytic Anemia: Bradley C. Gehrs and Richard C. FriedbergDokument14 SeitenAutoimmune Hemolytic Anemia: Bradley C. Gehrs and Richard C. FriedbergNadiya UlfaNoch keine Bewertungen
- Agn PDFDokument6 SeitenAgn PDFMohamed ZiadaNoch keine Bewertungen
- Anemia of InflammationDokument47 SeitenAnemia of InflammationGebruNoch keine Bewertungen
- Hypogammaglobulinemia and ArthritisDokument36 SeitenHypogammaglobulinemia and ArthritisNilesh NolkhaNoch keine Bewertungen
- Cvs146 Slide Demam Rematik Dan Penyakit Jantung RematikDokument40 SeitenCvs146 Slide Demam Rematik Dan Penyakit Jantung RematikMarogi Al AnsorianiNoch keine Bewertungen
- Pathology SGD 4: Diseases-Of-The-Immune-System: Group-4Dokument23 SeitenPathology SGD 4: Diseases-Of-The-Immune-System: Group-4Kalpana JenaNoch keine Bewertungen
- Cryoglobulinemia ReviewDokument7 SeitenCryoglobulinemia ReviewJosé Manuel Valencia GallardoNoch keine Bewertungen
- Acute GlomerulonephritisDokument28 SeitenAcute GlomerulonephritisPaul SinsNoch keine Bewertungen
- Autoimmune Hemolytic Anemia Diagnosis and Differential DiagnosisDokument10 SeitenAutoimmune Hemolytic Anemia Diagnosis and Differential DiagnosisMARCO MONTES REYESNoch keine Bewertungen
- Anti-Glomerular Basement Membrane VasculitisDokument8 SeitenAnti-Glomerular Basement Membrane VasculitisDra Daphne Rivero GallegosNoch keine Bewertungen
- Cryoglobulinemia: Dr. Luthfi Ahmad Dr. Suriani Alimudin, SP - PD, K-AIDokument16 SeitenCryoglobulinemia: Dr. Luthfi Ahmad Dr. Suriani Alimudin, SP - PD, K-AILuthfi Ziad AhmadNoch keine Bewertungen
- 10 Primary Glumerulopathies III - GKDokument2 Seiten10 Primary Glumerulopathies III - GKGerarld Immanuel KairupanNoch keine Bewertungen
- Dr. Ali's Uworld Notes For Step 2 CKDokument46 SeitenDr. Ali's Uworld Notes For Step 2 CKuyesNoch keine Bewertungen
- Warm Autoimmune Hemolytic Anemia (AIHA) in AdultsDokument45 SeitenWarm Autoimmune Hemolytic Anemia (AIHA) in AdultsMilind KothiyalNoch keine Bewertungen
- Allergy Immunology (3) (MedicalBooksVN - Com)Dokument9 SeitenAllergy Immunology (3) (MedicalBooksVN - Com)malbertal1Noch keine Bewertungen
- Intravenous ImmunoglobulinDokument5 SeitenIntravenous ImmunoglobulinTeslim RajiNoch keine Bewertungen
- Glomerular DsDokument18 SeitenGlomerular Dsnathan asfahaNoch keine Bewertungen
- A Rare Cause of AA Amyloidosis and End-Stage Kidney Failure: AnswersDokument3 SeitenA Rare Cause of AA Amyloidosis and End-Stage Kidney Failure: AnswersSezen YılmazNoch keine Bewertungen
- A Case Report On Sickle Cell Disease With Hemolyti PDFDokument4 SeitenA Case Report On Sickle Cell Disease With Hemolyti PDFAlhaji SwarrayNoch keine Bewertungen
- A Young Traveller Presenting With Typhoid Fever After Oral Vaccination: A Case ReportDokument9 SeitenA Young Traveller Presenting With Typhoid Fever After Oral Vaccination: A Case ReportMarscha MaryuanaNoch keine Bewertungen
- Nephrithic Sydrome PDFDokument20 SeitenNephrithic Sydrome PDFLeónGarcíaNoch keine Bewertungen
- New Insights in The Pathogenesis of Autoimmune Hemolytic AnemiaDokument7 SeitenNew Insights in The Pathogenesis of Autoimmune Hemolytic AnemiaABHINABA GUPTANoch keine Bewertungen
- Chapter No. 9, TransportDokument1 SeiteChapter No. 9, TransportSajjad AhmadNoch keine Bewertungen
- PbHealthDeptAlliedHospProf SR 2012 20120414Dokument14 SeitenPbHealthDeptAlliedHospProf SR 2012 20120414Sajjad AhmadNoch keine Bewertungen
- SKM FCC Franchise Application FormDokument1 SeiteSKM FCC Franchise Application FormSajjad AhmadNoch keine Bewertungen
- ABL Kinase Domain MutationDokument13 SeitenABL Kinase Domain MutationSajjad AhmadNoch keine Bewertungen
- Sops For Xpert Mtb/Rif Assay I UDokument3 SeitenSops For Xpert Mtb/Rif Assay I USajjad AhmadNoch keine Bewertungen
- Volunteering Program User ManualDokument12 SeitenVolunteering Program User ManualSajjad AhmadNoch keine Bewertungen
- Defense SysemDokument45 SeitenDefense SysemSajjad AhmadNoch keine Bewertungen
- Fusion Gene in CancerDokument26 SeitenFusion Gene in CancerSajjad AhmadNoch keine Bewertungen
- Antigen Presenting CellsDokument27 SeitenAntigen Presenting CellsSajjad AhmadNoch keine Bewertungen
- Adaptive ImmunityDokument14 SeitenAdaptive ImmunitySajjad AhmadNoch keine Bewertungen
- Calcium Significance & RegulationDokument25 SeitenCalcium Significance & RegulationSajjad AhmadNoch keine Bewertungen
- ChromatographyDokument11 SeitenChromatographySajjad Ahmad100% (1)
- ReceptorsDokument46 SeitenReceptorsSajjad Ahmad100% (1)
- Antibodies StructureDokument21 SeitenAntibodies StructureSajjad AhmadNoch keine Bewertungen
- Health Regulation Department FeesDokument8 SeitenHealth Regulation Department FeesDr-Usman KhanNoch keine Bewertungen
- Antibodies StructureDokument21 SeitenAntibodies StructureSajjad AhmadNoch keine Bewertungen
- Real Time PCRDokument25 SeitenReal Time PCRSajjad AhmadNoch keine Bewertungen
- Antibodies StructureDokument21 SeitenAntibodies StructureSajjad AhmadNoch keine Bewertungen
- Case Study Formate MSNDokument2 SeitenCase Study Formate MSNLijoNoch keine Bewertungen
- Perioperative Nursing: Lecturer: Mr. Renato D. Lacanilao, RN, MANDokument27 SeitenPerioperative Nursing: Lecturer: Mr. Renato D. Lacanilao, RN, MANJmarie Brillantes PopiocoNoch keine Bewertungen
- NCP Case PresDokument5 SeitenNCP Case Pressyd19Noch keine Bewertungen
- Solution Manual For Fordneys Medical Insurance 15th Edition Linda SmithDokument24 SeitenSolution Manual For Fordneys Medical Insurance 15th Edition Linda SmithSarahAlexanderrkcq100% (39)
- Kudori Therapy - Updated 9th Dec17Dokument32 SeitenKudori Therapy - Updated 9th Dec17Manickavasagam RengarajuNoch keine Bewertungen
- Primary Lesion: Abdul Rashid Bin Tharek Group 90Dokument27 SeitenPrimary Lesion: Abdul Rashid Bin Tharek Group 90rashidNoch keine Bewertungen
- 5 Year Clinical Follow Up of Prefebricated Precision Attachments A Comparison of Uni and Bilateral Removable Dental ProsthesesDokument7 Seiten5 Year Clinical Follow Up of Prefebricated Precision Attachments A Comparison of Uni and Bilateral Removable Dental Prosthesesalmond_pretzelNoch keine Bewertungen
- BrochureDokument14 SeitenBrochurePrakash KumbharNoch keine Bewertungen
- Acute Cholecystitis: Jochen Schuld Matthias GlanemannDokument3 SeitenAcute Cholecystitis: Jochen Schuld Matthias GlanemannNabilla DamarNoch keine Bewertungen
- ApheresisDokument5 SeitenApheresisGRK BIOMEDNoch keine Bewertungen
- Priyanka Sen Final Practice School Internship ReportDokument35 SeitenPriyanka Sen Final Practice School Internship ReportThakur Aditya PratapNoch keine Bewertungen
- ThoracentesisDokument1 SeiteThoracentesisNiño Cris PatiñoNoch keine Bewertungen
- Unit One Vocabulary: 'prɔvidәntDokument53 SeitenUnit One Vocabulary: 'prɔvidәntKhanh Chi NguyenNoch keine Bewertungen
- Terlipressin Is Superior To Noradrenaline PDFDokument11 SeitenTerlipressin Is Superior To Noradrenaline PDFfcodoc321Noch keine Bewertungen
- S: "Masakit Ang Ulo at Tiyan Niya" As Verbalized byDokument2 SeitenS: "Masakit Ang Ulo at Tiyan Niya" As Verbalized bydenise-iceNoch keine Bewertungen
- CraniomaDokument7 SeitenCraniomaSophia BeecherNoch keine Bewertungen
- Bates Physical Exam Video NotesDokument3 SeitenBates Physical Exam Video Notesdulcedeleche12359Noch keine Bewertungen
- When Are Focused Assessments ConductedDokument26 SeitenWhen Are Focused Assessments ConductedNozomi YukiNoch keine Bewertungen
- Drugs and Pharmaceutical Technology Question BankDokument7 SeitenDrugs and Pharmaceutical Technology Question BankVanitha ENoch keine Bewertungen
- सञ्चयकर्ता स्वास्थ्योपचार योजना सञ्चालन कार्यविधि, २०७७-50e1Dokument13 Seitenसञ्चयकर्ता स्वास्थ्योपचार योजना सञ्चालन कार्यविधि, २०७७-50e1crystalconsultancy22Noch keine Bewertungen
- Literature ReviewDokument3 SeitenLiterature Reviewapi-609233193Noch keine Bewertungen
- LeukocoriaDokument5 SeitenLeukocoriabahaashakirNoch keine Bewertungen
- English GuideDokument85 SeitenEnglish GuideanandkishoreNoch keine Bewertungen
- Failures in ImplantsDokument8 SeitenFailures in ImplantsDr FarhatNoch keine Bewertungen