Beruflich Dokumente
Kultur Dokumente
In Situ ATR-FTIR Studies On MgCl2-Diisobutyl Phthalate Interactions Diester Support Info La203972k - Si - 001
Hochgeladen von
Eero IiskolaOriginaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
In Situ ATR-FTIR Studies On MgCl2-Diisobutyl Phthalate Interactions Diester Support Info La203972k - Si - 001
Hochgeladen von
Eero IiskolaCopyright:
Verfügbare Formate
1
SUPPORTING INFORMATION
Synthesis of 4-fluoro-diisobutyl phthalate
4-Fluorophthalic anhydride (2 g, 12 mmol) was added to dry isobutanol (25 cm
3
) in a round-
bottomed flask equipped with a Ar-inlet and reflux condenser fitted with a CaCl
2
-drying tube.
Concentrated sulphuric acid (96.7 %, 30 mg, 15 l, 0.015 mol dm
-3
) was carefully added with stirring.
Upon bubbling Ar through the mixture, it was stirred for 18 h at 105 C after which it was concentrated
to ca. 10 cm
3
and transferred to a separating funnel using 40 cm
3
dichloromethane. The organic layer
was washed with 50 cm
3
cold water, cold 0.6 M NaHCO
3
solution and cold saturated NaCl solution.
After drying the organic layer with Na
2
SO
4
, the solvent was evaporated to afford 4-fluoro-diisobutyl
phthalate as colourless oil (3.185g, 10.8 mmol, 89.6%).
Scheme S1. Full esterification of 4-fluorophthalic anhydride with isobutanol.
1
HNMR spectra of 4-fluoro-diisobutyl phthalate
2
Figure S1.
1
HNMR spectra of 4-fluoro-diisobutyl phthalate [
H
(CDCl
3
) 7.80 (1-H, m,
Ar-H), 7.38 (1-H, m, Ar-H), 7.21 (1-H, m, Ar-H), 4.08 (4-H, t, -O-CH
2
-), 2.02 (2-H, m, -
CH(CH
3
)
2
), 1.01 (12-H, d, -CH(CH
3
)
2
)].
Comparison of DIBP and FDIBP infrared spectra
Comparison of uncoordinated DIBP and FDIBP IR spectra is given in Figure S2. The peaks marked
(C-C) represents alkyl group stretching bands of the donors. The peaks denoted as (C=O)
representing the respective carbonyl stretching bands of uncoordinated DIBP and FDIBP, are separated
by only 3 cm
-1
(see Table S1), an indication that the fluorine atom of FDIBP has negligible influence on
the stretching frequency of the C=O bonds when compared to DIBP. As expected, the two aromatic ring
3
mode peaks, marked (aromatic) (at c.a. 1600 and 1580 cm
-1
for both compounds), are symmetric for
DIBP but asymmetric for FDIBP. The (C-F) band of FDIBP is clearly visible at 1203 cm
-1
.
Figure S2. Comparative IR spectra of diisobutyl phthalate (top) and 4-fluoro-diisobutyl
phthalate (bottom)
In Figure S3, the ATR FTIR spectra of the spin coated MgCl
2
/ethanol, MgCl
2
/ethanol/DIBP and
MgCl
2
/ethanol/FDIBP films having donor to MgCl
2
ratios 0, 0.1 and 0.1 respectively, are compared.
The carbonyl stretching mode and aromatic ring modes of uncoordinated DIBP and FDIBP is also
shown (in dotted lines) for a comparison. The shift in peak maxima of carbonyl bands indicates the
coordination of DIBP and FDIBP. The peak around 3400 cm
-1
corresponds to O-H stretching frequency
of residual ethanol in the MgCl
2
film. The peak around 2950 cm
-1
corresponds to the C-C stretching
frequency of alkyl groups in ethanol and donors.
4
Figure S3. Comparative IR spectra of spin-coated MgCl
2
/Ethanol (top),
MgCl
2
/Ethanol/DIBP (middle) and MgCl
2
/Ethanol/FDIBP (bottom) films, along with
carbonyl stretching and aromatic ring modes of uncoordianted DIBP and FDIBP (dotted
lines). Donor/MgCl
2
molar ratios are 0 (top), 0.1 (middle) and 0.1 (bottom) respectively.
The peak positions of all relevant IR bands in Figure S3 are given in Table S1.
Donor
max
(C=O) (cm
-1
)
max
(Aromatic ring modes) (cm
-1
)
Uncoordinated DIBP 1725 1599 & 1580
Uncoordinated FDIBP 1728 1608 & 1589
Coordinated DIBP 1705 1596 & 1581
Coordinated FDIBP 1708 1606 & 1590
Table S1. Comparative ATR-FTIR peak vibration frequencies for uncoordinated and
coordinated 4-fluoro-diisobutyl phthalate (FDIBP) and diisobutyl phthalate (DIBP).
Quantification of ethanol in the MgCl
2
/ethanol and MgCl
2
/ethanol/donor films
We recorded XPS spectrum of MgCl
2
/ethanol film (on silicon wafer) at room temperature. The
elemental analysis (C, O, Mg, Cl and Si) was carried out. It is known that in the XPS spectra of the
5
samples contain chlorine, C1s signal is largely overlapped with energy loss features of Cl 2s signal. As a
reference, we measured XPS spectrum of anhydrous MgCl
2
. The C 1s signal of anhydrous MgCl
2
film
was used for the calibration of Cl 2s energy loss features. Based on this information, it is possible to
resolve the C 1s region of MgCl
2
/ethanol film as Cl 2s energy loss and C 1s signal from ethanol. C 1s
signal of ethanol contains two peaks (based on XPS database), C 1s signal from CH
3
- carbon (284.3 eV)
and C 1s signal from -CH
2
-OH carbon (285.8 eV). The contribution of ethanol to C 1s signal is
estimated and used to calibrate (O-H) band in the ATR-FTIR spectra of MgCl
2
/ethanol film measured
at 30 C (after exposing to Ar gas flow for 30 minutes). The estimation of ethanol in the donor
incorporated films were carried out based on the ATR-FTIR spectra of MgCl
2
/ethanol film measured at
30 C. C 1s regions of anhydrous MgCl
2
, MgCl
2
/ethanol film at 30 C, and 150 C are shown in figure
S4.
Figure S4. Comparative XPS spectra (C1s region) of anhydrous MgCl
2
, MgCl
2
/ethanol
6
films before and after annealing.
Peak fitting procedure
Peak fitting simulations were carried out using casa XPS software (http://www.casaxps.com/).
Infrared spectra were transferred to casa format. The preliminary constraints used for peak fitting was
based on values reported in literature. ATR-FTIR experiments showed that uncoordinated (pure
condensed phase) DIBP band appears at 1725 cm
-1
(component a). The optimum positions of other two
components were optimized to obtain an acceptable fit for entire set of spectra. The constraints used for
peak fitting is shown in Table S2. The components b and c were assigned to DIBP coordinated on
MgCl
2
(104) and DIBP coordinated on MgCl
2
(110) respectively. The peak fitting demanded in an
increase of the FWHM of the DIBP on MgCl
2
(110) when increasing the temperature. This might
indicate that these adsorption sites are more heterogeneous than the DIBP on MgCl
2
(104) sites.
Symbol Wavenumber (cm
-1
) FWHM
a
(uncoordinated DIBP)
1726 - 1724 20 - 30
b
[DIBP on MgCl
2
(104)]
1706 - 1704 30 - 35
c
[DIBP on MgCl
2
(110)]
(b-20)* 35 - 50
*The difference between the peak maxima of b and c is
fixed at 20 cm
-1
, which is the value reported in literature for
the of DIBP on MgCl
2
(104) and DIBP on MgCl
2
(110)
7
bands.
Table S2. Constraints used for peak fitting simulations.
As an example, the carbonyl stretching and aromatic ring mode bands of DIBP in the
DIBP/MgCl
2
film (with 15 mol % DIBP loading) at various stages of in-situ experiment is shown in
Figure S5. The changes in the intensity of components are clearly visible in the figure. The component
d represents the (O-H) scissoring band of moisture. Component e and f represents two aromatic
ring stretch bands of DIBP.
Figure S5. Comparative in-situ ATR-IR spectra after peak fitting (DIBP to Mg mol ratio
of 0.15).
Molar extinction coefficient calibration
From the peak fitting simulations on DIBP spectra, it is clear that the DIBP on MgCl
2
(104)
8
sites are transferred to MgCl
2
(110) sites. The transfer is almost complete at lower loading. However
the total peak area remained constant. Because of this observation, it is assumed that that molar
extinction coefficient of DIBP adsorbed on MgCl
2
is independent of its chemical environment. In order
to confirm the above assumption, we used XPS as an independent calibration method. FDIBP/MgCl
2
films were prepared from the solution of MgCl
2
and FDIBP in ethanol (FDIBP to Mg mol ratio of
0.10). Following the same in-situ ATR-FTIR experiment procedure used for DIBP, the spectra of
FDIBP/MgCl
2
films were collected. We propagate the same peak fitting procedure to FDIBP spectra,
by making required change in the absolute wavenumbers (+3 cm
-1
). The donor (here FDIBP)
conversion from MgCl
2
(104) sites to MgCl
2
(110) sites was observed (Figure S6). In this case also, the
integrated peak intensity remained constant. Before and after annealing, XPS measurements of similar
films were carried out. It is found that, before and after annealing, the concentration of FDIBP in those
films was same (Figure S7).
9
Figure S6. Comparative in-situ ATR-IR spectra after peak fitting (FDIBP to Mg mol
ratio of 0.10). Changes in the intensity of different components with respect to
temperature are shown as inset.
Figure S7. Comparative XPS spectra (F1s region) of MgCl
2
/FDIBP films before and
after annealing (FDIBP to Mg mol ratio of 0.10).
Electron diffraction patterns of MgCl
2
and DIBP/MgCl
2
films
The diffraction patterns of MgCl
2
and MgCl
2
/DIBP films (annealed and non-annealed) were
compared (Figure S8). The most intense signal in diffraction pattern of all these films corresponds to a
d spacing of 5.9 , which is diagnostic for the stacking of MgCl
2
structural layers (003 planes for the
modification, 001 planes for the and modifications). The intensity of this signal in the diffraction
pattern is related to stacking order of MgCl
2
layers. From the diffraction pattern, it is clear that the
stacking was more ordered in MgCl
2
films compared to the MgCl
2
/DIBP films. In the diffraction
pattern of non-annealed MgCl
2,
two more signals were also observed at a d spacing of 4.4 and 4.8
(Figure S7a). The signal at 4.8 and 4.4 is correspond to MgO (overtones of signals at 2.2 and 2.4 ),
which was probably formed due to the action of electron beam on residual ethanol and MgCl
2
.
10
Figure S8. Electron diffraction patterns of MgCl
2
and MgCl
2
/DIBP films: (a) MgCl
2
film
at 30 C. (b) MgCl
2
film after annealing at 150 C for 1h. (c) MgCl
2
/DIBP film at 30 C.
(d) MgCl
2
/DIBP film after annealing at 150 C for 1h. In all figures, the intense signal (at
d spacing of 5.9 ) represents the basal plane of MgCl
2
.
Das könnte Ihnen auch gefallen
- Shoe Dog: A Memoir by the Creator of NikeVon EverandShoe Dog: A Memoir by the Creator of NikeBewertung: 4.5 von 5 Sternen4.5/5 (537)
- Measurement of Gases Present or Generated During Fires: Standard Guide ForDokument16 SeitenMeasurement of Gases Present or Generated During Fires: Standard Guide ForYogiIndraPrayogaNoch keine Bewertungen
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeVon EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeBewertung: 4 von 5 Sternen4/5 (5794)
- UntitledDokument21 SeitenUntitledPravinNoch keine Bewertungen
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceVon EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceBewertung: 4 von 5 Sternen4/5 (895)
- Course Outline CEV444 (Sep 2017-Jan 2018)Dokument7 SeitenCourse Outline CEV444 (Sep 2017-Jan 2018)Solehah OmarNoch keine Bewertungen
- The Yellow House: A Memoir (2019 National Book Award Winner)Von EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Bewertung: 4 von 5 Sternen4/5 (98)
- Cotton/Wool Printing With Natural Dyes Nano-Particles: January 2014Dokument11 SeitenCotton/Wool Printing With Natural Dyes Nano-Particles: January 2014B161260013 Ц.АНУNoch keine Bewertungen
- Grit: The Power of Passion and PerseveranceVon EverandGrit: The Power of Passion and PerseveranceBewertung: 4 von 5 Sternen4/5 (588)
- Vibrational Studies of Na SO K SO Nahso and Khso Crystals: Azha - Periasamy, S.Muruganand and M.PalaniswamyDokument9 SeitenVibrational Studies of Na SO K SO Nahso and Khso Crystals: Azha - Periasamy, S.Muruganand and M.PalaniswamyMelin YohanaNoch keine Bewertungen
- The Little Book of Hygge: Danish Secrets to Happy LivingVon EverandThe Little Book of Hygge: Danish Secrets to Happy LivingBewertung: 3.5 von 5 Sternen3.5/5 (400)
- G. C. Jones, B. Jackson (Auth.) - Infrared Transmission Spectra of Carbonate Minerals-Springer Netherlands (1993)Dokument241 SeitenG. C. Jones, B. Jackson (Auth.) - Infrared Transmission Spectra of Carbonate Minerals-Springer Netherlands (1993)JorgeNoch keine Bewertungen
- The Emperor of All Maladies: A Biography of CancerVon EverandThe Emperor of All Maladies: A Biography of CancerBewertung: 4.5 von 5 Sternen4.5/5 (271)
- Astm1399 PDFDokument25 SeitenAstm1399 PDFkristoffer_mosshedenNoch keine Bewertungen
- Never Split the Difference: Negotiating As If Your Life Depended On ItVon EverandNever Split the Difference: Negotiating As If Your Life Depended On ItBewertung: 4.5 von 5 Sternen4.5/5 (838)
- Infrared Spectroscopy Materials Science I To 12Dokument522 SeitenInfrared Spectroscopy Materials Science I To 12Luis Roberto Caselin100% (1)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyVon EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyBewertung: 3.5 von 5 Sternen3.5/5 (2259)
- International Journal of Biological MacromoleculesDokument10 SeitenInternational Journal of Biological MacromoleculesAlejandra Gonzalez RuizNoch keine Bewertungen
- On Fire: The (Burning) Case for a Green New DealVon EverandOn Fire: The (Burning) Case for a Green New DealBewertung: 4 von 5 Sternen4/5 (74)
- Oxiranes and Oxirenes: Monocyclic: Ihsan ErdenDokument48 SeitenOxiranes and Oxirenes: Monocyclic: Ihsan ErdencapdesuroNoch keine Bewertungen
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureVon EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureBewertung: 4.5 von 5 Sternen4.5/5 (474)
- Analysis and Characterization of Cefixime by Using IR, HPLC and Gas ChromatographyDokument10 SeitenAnalysis and Characterization of Cefixime by Using IR, HPLC and Gas ChromatographyFallahNoch keine Bewertungen
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryVon EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryBewertung: 3.5 von 5 Sternen3.5/5 (231)
- Vibration-Rotation Spectroscopy of HCL and DCLDokument14 SeitenVibration-Rotation Spectroscopy of HCL and DCLLovely yadavNoch keine Bewertungen
- Team of Rivals: The Political Genius of Abraham LincolnVon EverandTeam of Rivals: The Political Genius of Abraham LincolnBewertung: 4.5 von 5 Sternen4.5/5 (234)
- Journal of Inorganic and Nuclear Chemistry, 1973, 35, 11, 3763-3767Dokument5 SeitenJournal of Inorganic and Nuclear Chemistry, 1973, 35, 11, 3763-3767Jose Alfredo LugoNoch keine Bewertungen
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaVon EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaBewertung: 4.5 von 5 Sternen4.5/5 (266)
- Lec - 24 - Vibrational Spectroscopy - Theoratical Bacgrouund - Origin of Molecular Vobration - Principles of SpectrosDokument10 SeitenLec - 24 - Vibrational Spectroscopy - Theoratical Bacgrouund - Origin of Molecular Vobration - Principles of SpectrosZahir Rayhan JhonNoch keine Bewertungen
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersVon EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersBewertung: 4.5 von 5 Sternen4.5/5 (345)
- Zn-OH-O - FTIR - para Julio PDFDokument3 SeitenZn-OH-O - FTIR - para Julio PDFJulio Andrés Casal RamosNoch keine Bewertungen
- DmsoDokument8 SeitenDmsovisa1032100% (8)
- The Unwinding: An Inner History of the New AmericaVon EverandThe Unwinding: An Inner History of the New AmericaBewertung: 4 von 5 Sternen4/5 (45)
- Use of Waste Plastic Materials For Road Construction in Ghana - ScienceDirectDokument13 SeitenUse of Waste Plastic Materials For Road Construction in Ghana - ScienceDirectAbera MulugetaNoch keine Bewertungen
- Lab Three Report AspirinDokument15 SeitenLab Three Report AspirinYan UvanilsonNoch keine Bewertungen
- Conceptuation, Formulation and Evaluation of Sustained Release FL Oating Tablets of Captopril Compression Coated With Gastric Dispersible Hydrochlorothiazide Using 23 Factorial Design PDFDokument12 SeitenConceptuation, Formulation and Evaluation of Sustained Release FL Oating Tablets of Captopril Compression Coated With Gastric Dispersible Hydrochlorothiazide Using 23 Factorial Design PDFDIKANoch keine Bewertungen
- Polymer SpectrosDokument408 SeitenPolymer SpectrosIkbal Fikri100% (3)
- IR SlideshowDokument22 SeitenIR SlideshowHafiz Shoaib SarwarNoch keine Bewertungen
- Characterization of Durio Seeds (Durio Zibethinus Murr) Edible Film Template BaruDokument15 SeitenCharacterization of Durio Seeds (Durio Zibethinus Murr) Edible Film Template BaruSelmianti KondoleleNoch keine Bewertungen
- The Infrared Frequencies of DNA BasesDokument9 SeitenThe Infrared Frequencies of DNA BasesvivesurNoch keine Bewertungen
- BE184P Exercise 1.1a SpectrophotometryDokument9 SeitenBE184P Exercise 1.1a SpectrophotometryDen CelestraNoch keine Bewertungen
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreVon EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreBewertung: 4 von 5 Sternen4/5 (1090)
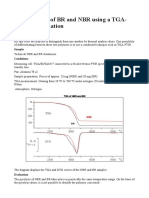
- Identification of BR and NBR Using A TGA-FTIR Combination: PurposeDokument4 SeitenIdentification of BR and NBR Using A TGA-FTIR Combination: Purposelely dwiNoch keine Bewertungen
- GRE Chemistry Test: Practice BookDokument56 SeitenGRE Chemistry Test: Practice BookDave Patrick EscalaNoch keine Bewertungen
- Polypyrrole-Palladium Systems Prepared in PDCL Aqueous SolutionsDokument10 SeitenPolypyrrole-Palladium Systems Prepared in PDCL Aqueous SolutionsemzzNoch keine Bewertungen
- Advanced Drug Delivery Reviews: Leena PeltonenDokument15 SeitenAdvanced Drug Delivery Reviews: Leena PeltonenAathira AjeeshNoch keine Bewertungen
- IRDokument36 SeitenIROctavio GilNoch keine Bewertungen
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Von EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Bewertung: 4.5 von 5 Sternen4.5/5 (121)
- ProofDokument14 SeitenProofSandrita BallesterosNoch keine Bewertungen
- Her Body and Other Parties: StoriesVon EverandHer Body and Other Parties: StoriesBewertung: 4 von 5 Sternen4/5 (821)