Beruflich Dokumente
Kultur Dokumente
EKC 245 - Mathematical Methods For Chemical Engineering
Hochgeladen von
Breaker SelvenOriginaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
EKC 245 - Mathematical Methods For Chemical Engineering
Hochgeladen von
Breaker SelvenCopyright:
Verfügbare Formate
UNIVERSITI SAINS MALAYSIA
Second Semester Examination
2010/2011 Academic Session
April/May 2011
EKC 245 Mathematical Methods For Chemical Engineering
[Kaedah Matematik Kejuruteraan Kimia]
Duration : 3 hours
[Masa : 3 jam]
Please ensure that this examination paper contains SIX printed pages and THREE
printed page of Appendix before you begin the examination.
[Sila pastikan bahawa kertas peperiksaan ini mengandungi ENAM muka surat yang
bercetak dan TIGA
muka surat Lampiran sebelum anda memulakan peperiksaan
ini.]
Instruction: Answer ALL
questions.
[Arahan: Jawab SEMUA
soalan.]
In the event of any discrepancies, the English version shall be used.
[Sekiranya terdapat sebarang percanggahan pada soalan peperiksaan, versi Bahasa
Inggeris hendaklah digunapakai].
2/-
- 2 - [EKC 245]
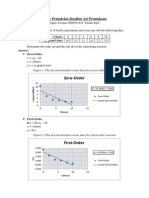
1. The concentration of biochemical oxygen demand (BOD), m, in a wastewater
treatment tank decreases according to
Kepekatan keperluan oksigen biokimia (BOD), m, di dalam tangki rawatan air sisa
berkurang berdasarkan
m =75e
-1.5t
+20e
-0.075t
Determined the time, t required for the BOD concentration to be reduced to 15 using
Newton-Raphson method with initial guess of t =6 and a stopping criterion of 0.5%.
Verify your result.
Tentukan masa, t yang diperlukan bagi mengurangkan kepekatan BOD kepada 15
dengan menggunakan kaedah Newton-Raphson, anggaran awal t = 6 dan kriteria
berhenti 0.5%. Buktikan keputusan anda.
[25 marks/markah]
2. Figure Q.2. shows three reactors linked by pipes. As indicated, the rate of transfer of
chemicals through each pipe is equal to a flowrate (Q, with units of cubic meters per
second) multiplied by the concentration of the reactor from which the flow originates
(c, with units of milligrams per cubic meter). If the system is at a steady state, the
transfer into each reactor will balance the transfer out. The flowrates are given as Q
13
=20, Q
12
=45, Q
21
=15, Q
23
=30 and Q
33
=60. Develop mass-balance equations for
the reactors and indentify their concentrations using
Rajah S.2. menunjukkan tiga reaktor dihubungkan dengan paip. Seperti tertera, kadar
pemindahan bahan kimia melalui setiap paip bersamaan dengan kadar alir (Q,
dengan unit meter padu per saat) didarab dengan kepekatan reaktor daripada aliran
asal tersebut (c, dengan unit milligram per meter padu). Jika sistem tersebut dalam
keadaan mantap, pemindahan masuk ke setiap reaktor seimbang dengan pemindahan
keluar. Kadar aliran diberi sebagai Q
13
= 20, Q
12
= 45, Q
21
= 15, Q
23
= 30 dan Q
33
= 60. Terbitkan persamaan keseimbangan jisim untuk semua reaktor dan tentukan
kepekatan masing-masing menggunakan
[a] Gauss elimination method
Kaedah penyisihan Gauss
[b] Gauss-Seidel method
Kaedah Gauss-Siedel
[25 marks/markah]
3/-
- 3 - [EKC 245]
Figure Q.2.
Rajah S.2.
3. [a] A temperature function across a board is
Fungsi suhu merentasi satu papan ialah
f(x,y) =5x
2
- xy + y
2
+3y
[i] Find the maximum temperature based on analytical solution.
Carikan suhu maksima berdasarkan penyelesaian analitikal.
[6 marks/markah]
[ii] Perform two iteration of steepest ascent method on the function starting
at point (1,1).
Lakukan dua lelaran kaedah naik tercuram pada fungsi tersebut
bermula dari titik (1,1).
[8 marks/markah]
[iii] List out the advantages and disadvantages of steepest ascent/descend
method.
Senaraikan kebaikan dan keburukan kaedah naik/turun tercuram.
[4 marks/markah]
[b] Reactant A is being converted into product X in a system. The rate of reaction
for each species is given as follows:
Bahan tindak balas A ditukar kepada produk X dalam suatu sistem. Kadar
tindak balas bagi setiap spesis adalah diberi seperti di bawah:
X A
A
C k C k
dt
dC
2
2
1
+ =
X
A X
KC
C k
dt
dC
+
=
1
2
1
4/-
3 1
2
100 mg/s
Q
13
c
1
Q
12
c
1
Q
21
c
2
Q
23
c
2
Q
33
c
3
300 mg/s
- 4 - [EKC 245]
where C
i
=concentration of species i; t =time in s and rate constants k
1
=
0.514 (mol/ dm
3
)
-1
s
-1
; k
2
=0.238 s
-1
; K =0.752 (mol/ dm
3
)
-1
.
Find the
concentration of A, and X after 1 s. The initial feed to the reactor is pure A
with concentration 2 mol/dm
3
.
di mana C
i
= kepekatan spesis i; t = masa dalam saat dan pemalar kadar k
1
= 0.514 (mol/ dm
3
)
-1
s
-1
; k
2
= 0.238 s
-1
; K = 0.752 (mol/ dm
3
)
-1
.
Carikan
kepekatan A dan X selepas 1 saat. Suapan awal ke reaktor ialah A tulen
dengan kepekatan 2 mol/dm
3
[7 marks/markah]
.
4. [a] [i] A heated plate is subjected to two boundary temperatures held at constant
value (in degree Celsius) at certain positions and two boundaries being
insulated as shown in Figure Q.4.[a]. Use Liebmanns method to solve
for the temperature at the nodes in the grid (calculate the temperature at 6
points) of the heated plate to compute the steady state distribution of
temperature. Employ overrelaxation with a value of 1.5 for the
weighting factor and iterate 1 time. Start from the point i =1, j =0 with
the assumption that all the unknown points are zeros.
Sebuah plat panas dengan dua suhu sempadan yang dikekal (darjah
Celsius) pada suhu tetap di titik-titik tertentu dan dua sempadan ditebat
adalah ditunjukkan dalam Rajah S.4.[a]. Gunakan kaedah Liebmann
untuk menyelesaikan suhu di titik-titik grid (kirakan suhu bagi 6 titik)
pada plat panas untuk mencari taburan suhu pada keadaan mantap.
Gunakan santaian dengan nilai 1.5 bagi faktor pemberat dan lelar 1 kali.
Mula dari titik i =1, j = 0 dengan anggapan bahawa semua titik anu
adalah sifar.
Figure Q.4.[a].
Rajah S.4.[a].
[10 marks/markah]
5/-
Insulated
Tertebat
Insulated
Tertebat
100
75
50
75 50 30
- 5 - [EKC 245]
[ii] Given the temperature (in degree Celsius) distribution of a heated plate
at steady state is given as shown in Figure Q.4.[b]. Calculate the fluxes
for the two nodes in the grid of the plate.
Taburan suhu (dalam darjah Celsius) bagi sebuah plat panas pada
keadaan mantap adalah seperti dalam Rajah S.4.[b]. Hitungkan fluks
bagi dua titik pada plat panas tersebut.
Figure Q.4.[b].
Rajah S.4.[b].
Assume that the plate is 20 cm x 30 cm and is made out of aluminum.
Anggapkan plat tersebut adalah 20 sm x 30 sm dan diperbuat daripada
aluminium.
[k=0.49 cal/s.cm.
o
C]
Hint: Fouriers law of heat conduction:
Petunjuk: Hukum Fourier bagi konduksi haba:
i
T
k q
i
= ' where q
i
=heat flux in the direction of the i dimension
[cal/cm
2
.s]
i
T
k q
i
= ' di mana q
i
= fluks haba pada arah dimensi i [cal/sm
2
[5 marks/markah]
.s]
6/-
50.0
52.34
43.0
56.11
0 0
33.89
33.3
- 6 - [EKC 245]
[b] A 4 cm long thin rod is insulated at all point except at its ends. At time t =0,
the temperature of the whole rod is 30
o
C and the boundary conditions are
fixed for all times at T(0) =120
o
C and T(10) =45
o
C. Given x = 1 cm, t =1
s and k =0.944 cm
2
/s. Calculate the temperature distribution at t =1s using
the simple implicit method. The Laplacian difference equations for the system
is given as
Satu rod halus yang panjangnya 4 sm ditebat pada semua titik kecuali pada
kedua-dua hujungnya. Pada masa t = 0, suhu seluruh rod tersebut ialah 30
o
C
dan keadaan sempadan dikekal pada T(0) = 120
o
C dan T(10) = 45
o
C. Diberi
x = 1 sm, t = 1 s dan k = 0.944 sm
2
/s. Kirakan taburan suhu pada t = 1s
menggunakan kaedah implisit mudah. Persamaan Laplace bagi sistem
tersebut diberi sebagai
l
i
l
i
l
i
l
i
T T T T = + +
+
+
+ +
1
1
1 1
1
) 2 1 ( where = k t/(x)
2
[10 marks/markah]
- oooOooo -
- 7 - [EKC 245]
Appendix
Matrix of Polynomial Regression
(
(
(
i i
i i
i o
i i i
i i i
i i
y x
y x
y
a
a
a
x x x
x x x
x x n
2
2
1
4 3 2
3 2
2
Matrix of Multiple Linear Regression
(
(
(
i i
i i
i
i i i i
i i i i
i i
y x
y x
y
a
a
a
x x x x
x x x x
x x n
2
1
2
1
0
2
2 2 1 2
2 1
2
1 1
2 1
Differentiation Formulas
Forward finite-divided-difference
First derivative
h
x f x f
x f
i i
i
) ( ) (
) (
1 '
=
+
O(h)
h
x f x f x f
x f
i i i
i
2
) ( 3 ) ( 4 ) (
) (
1 2 '
+
=
+ +
O(h
2
)
Second derivative
2
1 2 "
) ( ) ( 2 ) (
) (
h
x f x f x f
x f
i i i
i
+
=
+ +
O(h)
2
1 2 3 "
) ( 2 ) ( 5 ) ( 4 ) (
) (
h
x f x f x f x f
x f
i i i i
i
+ +
=
+ + +
O(h
2
)
Backward finite-divided-difference
First derivative
h
x f x f
x f
i
i
) ( ) (
) (
1 1 '
= O(h)
h
x f x f x f
x f
i i i
i
2
) ( ) ( 4 ) ( 3
) (
2 1 '
+
= O(h
2
)
Second derivative
2
2 1 "
) ( ) ( 2 ) (
) (
h
x f x f x f
x f
i i i
i
+
= O(h)
2
3 2 1 "
) ( ) ( 4 ) ( 5 ) ( 2
) (
h
x f x f x f x f
x f
i i i i
i
+
= O(h
2
)
2/-
- 8 - [EKC 245]
Centred finite-divided-difference
First derivative
h
x f x f
x f
i i
i
2
) ( ) (
) (
1 1 ' +
= O(h
2
)
h
x f x f x f x f
x f
i i i i
i
12
) ( ) ( 8 ) ( 8 ) (
) (
2 1 1 2 ' + +
+ +
= O(h
4
)
Second derivative
2
1 1 "
) ( ) ( 2 ) (
) (
h
x f x f x f
x f
i i i
i
+
+
= O(h
2
)
2
2 1 1 2 "
12
) ( ) ( 16 ) ( 30 ) ( 16 ) (
) (
h
x f x f x f x f x f
x f
i i i i i
i
+ +
+ +
= O(h
4
)
Derivatives of unequally spaced data
) )( (
2
) (
) )( (
2
) (
) )( (
2
) ( ) ( '
1 1 1
1
1
1 1
1 1
1 1 1
1
1
i i i i
i i
i
i i i i
x i
i
i i i i
i i
i
x x x x
x x x
x f
x x x x
x x x
x f
x x x x
x x x
x f x f
+
+
=
+ +
+
+
+
+
+
Integration Formulas
Trapezoidal Rule
2
) ( ) (
) (
b f a f
b a I
+
=
n
x f x f x f
a b I
n
i
n i o
2
) ( ) ( 2 ) (
) (
1
1
=
+ +
=
Simpsons 1/3 Rule
6
) ( ) ( 4 ) (
) (
2 1
x f x f x f
a b I
o
+ +
n
x f x f x f x f
a b I
n
i
n
j
n j i o
3
) ( ) ( 2 ) ( 4 ) (
) (
1
5 , 3 , 1
2
6 , 4 , 2
=
+ + +
- 9 - [EKC 245]
Useful Formula
ODE solver
Eulers method
y
i+1
=y
i
+y
i
h
4
th
y
order Runge-Kutta
i+1
=y
i
6
1
+ (k
1
+2k
2
+2k
3
+k
4
where k
)h
1
k
=f (x, y)
2
= f (x
i
2
1
+ h, y
i
2
1
+ k
1
k
h)
3
= f (x
i
2
1
+ h, y
i
2
1
+ k
2
k
h)
4
=f (x
i
+ h, y
i
+k
3
h)
Finite difference methods
Laplacian difference equations
T
i+1,j
+T
i-1,j
+T
i,j+1
+T
i,j-1
- 4 T
i,j
=0
Explicit method:
t
T T
t
T
l
i
l
i
+1
;
2
1 1
2
2
2
x
T T T
x
T
l
i
l
i
l
i
+
=
+
Simple implicit method:
t
T T
t
T
l
i
l
i
+1
;
2
1
1
1 1
1
2
2
) (
2
x
T T T
x
T
l
i
l
i
l
i
+ +
+
The Crank Nicolson Method
t
T T
t
T
l
i
l
i
+1
;
(
+
+
+ +
+ +
2
1
1
1 1
1
2
1 1
2
2
) (
2
) (
2
2
1
x
T T T
x
T T T
x
T
l
i
l
i
l
i
l
i
l
i
l
i
Overrelaxation
old
j i
new
j i
new
j i
T T T
, , ,
) 1 ( + =
Das könnte Ihnen auch gefallen
- Process Dynamics and ControlDokument9 SeitenProcess Dynamics and Controlbhaskar537750% (2)
- Module 4: Worked Out Problems:) / Sinh / Sinh (. Sin 1) 1 (2), (Dokument10 SeitenModule 4: Worked Out Problems:) / Sinh / Sinh (. Sin 1) 1 (2), (Karthiik88Noch keine Bewertungen
- Reaction KineticsDokument37 SeitenReaction KineticsNurshuhada NordinNoch keine Bewertungen
- Chemical KineticsDokument7 SeitenChemical Kineticsthinkiit100% (1)
- Thermal (TE-411,412,413,414,511)Dokument25 SeitenThermal (TE-411,412,413,414,511)nved01Noch keine Bewertungen
- Kinetics ReviewDokument5 SeitenKinetics ReviewbrittanypriyaNoch keine Bewertungen
- MeasurementDokument7 SeitenMeasurementEdgar Alexander Montero VeraNoch keine Bewertungen
- Multiple Choice Questions On Mechanics and HeatDokument21 SeitenMultiple Choice Questions On Mechanics and HeatGodwinNoch keine Bewertungen
- HW2 SolutionsDokument8 SeitenHW2 SolutionschNoch keine Bewertungen
- Jan15 QnPapersDokument11 SeitenJan15 QnPapersVigneshwaran KannanNoch keine Bewertungen
- Water Quality ModelingDokument7 SeitenWater Quality ModelingAgnes FerinnaNoch keine Bewertungen
- Homework 5 - 2020 - 01 - v3 - YH (v3) - ALV (v2)Dokument5 SeitenHomework 5 - 2020 - 01 - v3 - YH (v3) - ALV (v2)CARLOS DIDIER GÓMEZ ARCOSNoch keine Bewertungen
- 11 02 2012 Xii Abcd Part Test IIIssssssssssssssssssssssDokument13 Seiten11 02 2012 Xii Abcd Part Test IIIssssssssssssssssssssssvishal1100850% (1)
- G K S S: Che381 Process Dynamics & Control Jan-Apr 2014 End-Semester Exam 3 Hours 100 PointsDokument4 SeitenG K S S: Che381 Process Dynamics & Control Jan-Apr 2014 End-Semester Exam 3 Hours 100 PointsSushmitaNoch keine Bewertungen
- Principles of Matlab (Fall 07) Workout #1Dokument11 SeitenPrinciples of Matlab (Fall 07) Workout #1Karim GaberNoch keine Bewertungen
- Practica 2Dokument4 SeitenPractica 2angelicaNoch keine Bewertungen
- Ee 5307 HomeworksDokument15 SeitenEe 5307 HomeworksManoj KumarNoch keine Bewertungen
- ME3122E - Tutorial Solution 3Dokument8 SeitenME3122E - Tutorial Solution 3LinShaodun100% (3)
- Solutions To CL 444 Tests Test 1Dokument10 SeitenSolutions To CL 444 Tests Test 1Sumit VermaNoch keine Bewertungen
- Chemical Kinetics1Dokument59 SeitenChemical Kinetics1farooq_bagbanNoch keine Bewertungen
- ODE & LSE AssignmentDokument5 SeitenODE & LSE AssignmentmasterrkNoch keine Bewertungen
- Matlab Exercise 1Dokument2 SeitenMatlab Exercise 1maheshluintelNoch keine Bewertungen
- University of Mauritius: December 2008Dokument4 SeitenUniversity of Mauritius: December 2008Damree EmirNoch keine Bewertungen
- A Step Change of Magnitude 4 Is Introduced Into A System Having The Transfer FunctionDokument8 SeitenA Step Change of Magnitude 4 Is Introduced Into A System Having The Transfer FunctionFarid SarrafNoch keine Bewertungen
- Mekelle University: College of Natural & Computational Sciences Department of MathematicsDokument6 SeitenMekelle University: College of Natural & Computational Sciences Department of Mathematicsdavid seaNoch keine Bewertungen
- We M5Dokument14 SeitenWe M5knyogishNoch keine Bewertungen
- Isro Scientist Me 2017 Paper Fe5f57b7Dokument25 SeitenIsro Scientist Me 2017 Paper Fe5f57b7logesh. rNoch keine Bewertungen
- Sri Lanka Technological Campus: Bachelor of Technology Year - Semester - End-Semester ExaminationDokument10 SeitenSri Lanka Technological Campus: Bachelor of Technology Year - Semester - End-Semester Examinationearl pannilaNoch keine Bewertungen
- 12S ME304 MT2 SolutionsDokument5 Seiten12S ME304 MT2 SolutionsOğulcan AytaçNoch keine Bewertungen
- 2014 3P4 Midterm 1 SolutionsDokument9 Seiten2014 3P4 Midterm 1 SolutionsIsibor CaptainNoch keine Bewertungen
- Numerical Solution of Initial Value ProblemsDokument17 SeitenNumerical Solution of Initial Value ProblemslambdaStudent_eplNoch keine Bewertungen
- Chapter 4-: Determination of Rate Law For Batch ReactorDokument25 SeitenChapter 4-: Determination of Rate Law For Batch ReactorWonda 005Noch keine Bewertungen
- PEME200001 Mathematical Techniques 2Dokument10 SeitenPEME200001 Mathematical Techniques 2Andrew AndersonNoch keine Bewertungen
- Gate Previous Year QuestionsDokument53 SeitenGate Previous Year QuestionsPOOJA VERMANoch keine Bewertungen
- Experiment-3 Heat Transfer in Agitated Vessel: Sarthak Lathiya Hto Lab 18BT01035Dokument10 SeitenExperiment-3 Heat Transfer in Agitated Vessel: Sarthak Lathiya Hto Lab 18BT01035SARTHAK LATHIYANoch keine Bewertungen
- Komputasi Numerik Ujian Akhir Semester: Muhammad Zaki Zahirsyah 1406574522Dokument22 SeitenKomputasi Numerik Ujian Akhir Semester: Muhammad Zaki Zahirsyah 1406574522zakizahirsyahNoch keine Bewertungen
- SE 2008 ElectricalDokument46 SeitenSE 2008 ElectricalMuhammad MujtabaNoch keine Bewertungen
- Ejercicios DSCDokument39 SeitenEjercicios DSCFreyley LeyvaNoch keine Bewertungen
- M6803 Assignment 2012Dokument1 SeiteM6803 Assignment 2012Irving Paul GirsangNoch keine Bewertungen
- hw2 SolDokument11 Seitenhw2 SolSaied Aly SalamahNoch keine Bewertungen
- Systems and Control PDFDokument9 SeitenSystems and Control PDFHamid Farhan0% (1)
- Q. 1 - Q. 25 Carry One Mark EachDokument12 SeitenQ. 1 - Q. 25 Carry One Mark EachPritum SutharNoch keine Bewertungen
- Tut. - No.1 - ME2121 (July 2011)Dokument6 SeitenTut. - No.1 - ME2121 (July 2011)Divij SoodNoch keine Bewertungen
- Simulation Study of The CSTR Reactor For Control PurposesDokument4 SeitenSimulation Study of The CSTR Reactor For Control PurposesEstefannya Carvajal CruzNoch keine Bewertungen
- Process Modelling, Simulation and Control For Chemical Engineering. Solved Problems. Chapter 5: Simulation Ex-AmplesDokument12 SeitenProcess Modelling, Simulation and Control For Chemical Engineering. Solved Problems. Chapter 5: Simulation Ex-AmplesJohn100% (2)
- Environmental Systems and Facility PlanningDokument33 SeitenEnvironmental Systems and Facility PlanningpeagricultureNoch keine Bewertungen
- One-Dimensional, Steady-State Conduction With Thermal Energy GenerationDokument35 SeitenOne-Dimensional, Steady-State Conduction With Thermal Energy GenerationIvan PonceNoch keine Bewertungen
- 7 Dynamics Tutorial AnsDokument20 Seiten7 Dynamics Tutorial AnsselmanNoch keine Bewertungen
- Gujarat Technological University: InstructionsDokument2 SeitenGujarat Technological University: InstructionsBhakti MahbubaniNoch keine Bewertungen
- 2008 ExamaaaaaaaaaaaaaaaDokument7 Seiten2008 ExamaaaaaaaaaaaaaaabbteenagerNoch keine Bewertungen
- Concentration Changes in A CSTR (Continuous Stirred Tank Reactor)Dokument10 SeitenConcentration Changes in A CSTR (Continuous Stirred Tank Reactor)pekanselandarNoch keine Bewertungen
- Numerical Methods Final Requirements ShshsiwzbDokument10 SeitenNumerical Methods Final Requirements ShshsiwzbIñigo Carlos AquinoNoch keine Bewertungen
- Problem Set AdrianDokument60 SeitenProblem Set AdrianTomas Otero IIINoch keine Bewertungen
- CHEE319 Quiz1 2015 SoluDokument6 SeitenCHEE319 Quiz1 2015 SolusunliasNoch keine Bewertungen
- Complex Variables and Statistical Methods - June - 2015Dokument8 SeitenComplex Variables and Statistical Methods - June - 2015Mohan RaoNoch keine Bewertungen
- Solutions To Exercises: Chapter 10: 10.1 Use The Chemical Potential of An Ideal Gas in (10.1.9) and Obtain The BarometricDokument6 SeitenSolutions To Exercises: Chapter 10: 10.1 Use The Chemical Potential of An Ideal Gas in (10.1.9) and Obtain The BarometricSalomé TorresNoch keine Bewertungen
- Analytical Modeling of Solute Transport in Groundwater: Using Models to Understand the Effect of Natural Processes on Contaminant Fate and TransportVon EverandAnalytical Modeling of Solute Transport in Groundwater: Using Models to Understand the Effect of Natural Processes on Contaminant Fate and TransportNoch keine Bewertungen
- Logical progression of twelve double binary tables of physical-mathematical elements correlated with scientific-philosophical as well as metaphysical key concepts evidencing the dually four-dimensional basic structure of the universeVon EverandLogical progression of twelve double binary tables of physical-mathematical elements correlated with scientific-philosophical as well as metaphysical key concepts evidencing the dually four-dimensional basic structure of the universeNoch keine Bewertungen
- Uo6 SedimentationDokument8 SeitenUo6 SedimentationBreaker SelvenNoch keine Bewertungen
- A New Biomimetic Sensor Based On Molecularly Imprinted Polymers For Highly Sensitive and Selective Determination of Hexazinone HerbicideDokument8 SeitenA New Biomimetic Sensor Based On Molecularly Imprinted Polymers For Highly Sensitive and Selective Determination of Hexazinone HerbicideBreaker SelvenNoch keine Bewertungen
- Triangle RightDokument1 SeiteTriangle RightBreaker SelvenNoch keine Bewertungen
- ASA2 IOI Pamol Kluang RSPO Public Summarffhhy Report June 2012Dokument37 SeitenASA2 IOI Pamol Kluang RSPO Public Summarffhhy Report June 2012Breaker SelvenNoch keine Bewertungen
- P Safety Ekc367 - 4 - PDFDokument4 SeitenP Safety Ekc367 - 4 - PDFBreaker SelvenNoch keine Bewertungen
- Optimization of A Chem-E-CarDokument18 SeitenOptimization of A Chem-E-CarBreaker SelvenNoch keine Bewertungen
- AbsenceDokument1 SeiteAbsenceBreaker SelvenNoch keine Bewertungen
- IdiomsDokument20 SeitenIdiomsdebatri_mitraNoch keine Bewertungen
- Mapua University: School of EECEDokument8 SeitenMapua University: School of EECEBenj MendozaNoch keine Bewertungen
- Sualaptop365.Edu - VN - Aspire P3-171 Quanta EE3Dokument32 SeitenSualaptop365.Edu - VN - Aspire P3-171 Quanta EE3Saglam Elektronik Ümit AtlarNoch keine Bewertungen
- Asm MDDokument4 SeitenAsm MDroyal31607Noch keine Bewertungen
- Elastic Analysis & Engineering Design Formulae For Bonded JointsDokument14 SeitenElastic Analysis & Engineering Design Formulae For Bonded Jointsscoobymehrotra27Noch keine Bewertungen
- Pilot Lights and Illuminated Pushbuttons Panorama en PDFDokument4 SeitenPilot Lights and Illuminated Pushbuttons Panorama en PDFAndiAttaNoch keine Bewertungen
- Short-Circuit Analysis Models For Unbalanced Inverter-Based Distributed Generation Sources and LoadsDokument11 SeitenShort-Circuit Analysis Models For Unbalanced Inverter-Based Distributed Generation Sources and LoadsRamesh NaiduNoch keine Bewertungen
- Schematic Diagram of Relay & Tcms Panel T: REV Revised by Checked by Approved byDokument1 SeiteSchematic Diagram of Relay & Tcms Panel T: REV Revised by Checked by Approved byTaufiq HidayatNoch keine Bewertungen
- Dynamic NatDokument7 SeitenDynamic NatArslan SaleemNoch keine Bewertungen
- Aviat Quick Start Guide For Indoor Equipment GroundingDokument20 SeitenAviat Quick Start Guide For Indoor Equipment GroundingKevin_INoch keine Bewertungen
- Security Intelligence Fundamentals: Student NotebookDokument245 SeitenSecurity Intelligence Fundamentals: Student NotebookMohsine AzouliNoch keine Bewertungen
- Ait Unit 3Dokument10 SeitenAit Unit 3Ayushi PatelNoch keine Bewertungen
- 3D TransformationsDokument11 Seiten3D TransformationsMichelle SmithNoch keine Bewertungen
- Origins of Media ExposureDokument24 SeitenOrigins of Media ExposureVeronica StancuNoch keine Bewertungen
- Lecture 1 RoboticsDokument34 SeitenLecture 1 RoboticsArshadNoch keine Bewertungen
- Golden Motor - BLDC MotorsDokument4 SeitenGolden Motor - BLDC MotorsEduardo BarbieriNoch keine Bewertungen
- Amplifiers-Module-01 pg1-5Dokument5 SeitenAmplifiers-Module-01 pg1-5SumdNoch keine Bewertungen
- SAPUI5 TrainingDokument148 SeitenSAPUI5 Trainingadmet615100% (6)
- Homework 3 Answers UpdatedDokument2 SeitenHomework 3 Answers UpdatedMariana OliveiraNoch keine Bewertungen
- EdgeCAM Advanced MillingDokument111 SeitenEdgeCAM Advanced MillingRodrigo Luiz100% (1)
- Wireless Local Area Network (WLAN)Dokument48 SeitenWireless Local Area Network (WLAN)Ali AhmadNoch keine Bewertungen
- Joseph, Sheilo Pearl P. Forensic-4Dokument4 SeitenJoseph, Sheilo Pearl P. Forensic-4Kate BttaNoch keine Bewertungen
- Pre Hackathon Problem Solving Kit v1.1Dokument53 SeitenPre Hackathon Problem Solving Kit v1.1jojiNoch keine Bewertungen
- EN15000 - Service ManualDokument38 SeitenEN15000 - Service ManualLeonid100% (2)
- Marc VolumeD - User Subroutines and Special Routines For Details PDFDokument606 SeitenMarc VolumeD - User Subroutines and Special Routines For Details PDFTao YuanNoch keine Bewertungen
- 9A05704 Advanced Computer ArchitectureDokument4 Seiten9A05704 Advanced Computer ArchitecturesivabharathamurthyNoch keine Bewertungen
- Sale Register Report PMGKAY June 2022 PHHDokument10 SeitenSale Register Report PMGKAY June 2022 PHHsaumyaNoch keine Bewertungen
- FinalTrainingCalendar 2016-17 RPATCDokument34 SeitenFinalTrainingCalendar 2016-17 RPATCsohelalamNoch keine Bewertungen
- Curriculum Vitae: Executive Summary & Covering LetterDokument9 SeitenCurriculum Vitae: Executive Summary & Covering LetterKhalid MahmoodNoch keine Bewertungen
- AirWatch On-Premise Technical Architecture Guide v7 - 3Dokument19 SeitenAirWatch On-Premise Technical Architecture Guide v7 - 3OscarNoch keine Bewertungen
- Control of Switched Reluctance Generator in Wind Energy SystemDokument106 SeitenControl of Switched Reluctance Generator in Wind Energy SystemSung Ryoung LimNoch keine Bewertungen