Beruflich Dokumente
Kultur Dokumente
Biochemsitry 3304 Midterm 3 - Key
Hochgeladen von
abelopez120 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
426 Ansichten12 SeitenMidterm bark uh
Copyright
© © All Rights Reserved
Verfügbare Formate
PDF, TXT oder online auf Scribd lesen
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenMidterm bark uh
Copyright:
© All Rights Reserved
Verfügbare Formate
Als PDF, TXT herunterladen oder online auf Scribd lesen
0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
426 Ansichten12 SeitenBiochemsitry 3304 Midterm 3 - Key
Hochgeladen von
abelopez12Midterm bark uh
Copyright:
© All Rights Reserved
Verfügbare Formate
Als PDF, TXT herunterladen oder online auf Scribd lesen
Sie sind auf Seite 1von 12
Biochemsitry 3304 Midterm 3
1. Which of the following general properties are properties of enzymes?
a. Very high acceleration of reaction rates.
b. Physiological reaction conditions.
c. Highly specific reactions for substrate selectivity and stereospecificity.
d. All of the above are properties of enzymes.
2. Enzymes act on specific substrates using complementarity. What would be the most significant kind of
complementarity would be utilized by the enzyme Succinate Dehydrogenase to differentiate succinate from the
structurally similar malonate?
a. Electronic complementarity
b. Geometric complementarity
c. Charge complementarity
d. Stereochemical complementarity
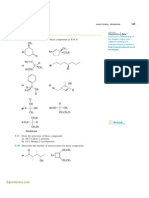
3. TEV Protease is a highly specific protease and cleaves the sequence ENLYFNG at the NG bond. What would you
predict to be the effect of replacing the L-Phenylalanine residue with a D-Phenylalanine?
a. No effect.
b. Cleavage would be more efficient.
c. Cleavage would be a little less efficient.
d. Cleavage would likely not occur or be drastically reduced.
4. The best idea of complementarity suggested in Question 3 would be which of the following?
a. Electronic complementarity
b. Geometric complementarity
c. Charge complementarity
d. Stereochemical complementarity
5. Vitamins are examples of what kind of enzymatic species?
a. Coenzyme molecule.
b. Cofactor molecule.
c. Structural molecule.
d. Organic molecule.
6. Metal ions such as Cu++, Fe++ and Zn++ are examples of what kind of enzymatic species?
a. Coenzyme molecule.
b. Cofactor molecule.
c. Structural molecule.
d. Organic molecule.
7. The following diagram is for both standard and catalyzed reactions converting Substrate S into Product P.
Which double arrow denotes the activation energy for the uncatalyzed reaction?
a. a
b. b
c. c
d. d
8. Which double arrow denotes the difference in activation energy between the catalyzed and uncatalyzed reaction?
a. a
b. b
c. c
d. d
9. Which double arrow denotes the G for the conversion of S to P?
a. a
b. b
c. c
d. d
10. The diagram in Question suggests what about how a catalyst can accelerate a reaction?
a. The activation energy for a catalyzed reaction is less than that for the uncatalyzed reaction.
b. The catalyzed reaction transition state is stabilized compared to the uncatalyzed reaction.
c. The free energy change of the reaction is unchanged.
d. (a) is a consequence of (b), which is the major mechanism for accelerating a reaction with a catalyst.
11. For the diagram in Question 7, the rate of the catalyzed reaction is 148.4 times that of the uncatalyzed reaction.
What is the stabilization energy of the catalyzed reaction at standard temperature?
a. 5.4 kJ/mol
b. 11.3 kJ/mol
c. 12.4 kJ/mol
d. 15.4 kJ/mol
The following first step of an enzymatic reaction is referred to for Questions 12 and 13.
12. What is the type of mechanism used in this example?
a. Covalent catalysis.
b. Metal catalysis.
c. General Acid-Base catalysis.
d. Cannot determine.
13. Would you predict that DNA could be cleaved by this enzyme?
a. Yes
b. No
c. More slowly
d. Cannot determine with this information.
14. The following mechanism is for Pyruvate Decarboxylase.
What type of catalysis mechanism would best describe this reaction pathway as shown?
a. Covalent catalysis.
b. Metal catalysis.
c. General Acid-Base catalysis.
d. Cannot determine.
15. The following is a step in the mechanism of Lysozyme.
What other amino acid would you expect to be able to substitute for Glu35 based on pH and ability to stabilize the
transition state oxonium ion?
a. Histidine
b. Serine
c. Valine
d. Arginine
16. Acetylcholine esterase is a serine esterase critical for hydrolyzing the neurotransmitter acetylcholine. Nerve gases
such as Sarin and DIPF inhibit this enzyme as a primary mode of their toxicity. What is the most correct mechanism for
this inhibition?
a. These agents bind to the enzyme and mimic the substrate, acetylcholine.
b. These agents bind to the enzyme and mimic the products, acetic acid and choline.
c. These agents form a stable covalent bond with the active site serine and mimic the transition state.
d. These agents release HF from their reaction with the active site serine.
17. What three amino acids comprise the catalytic triad for serine proteases?
a. Lysine-Histidine-Serine
b. Aspartic or Glutamic Acid-Histidine-Serine
c. Tyrosine-Histidine-Serine
d. Arginine-Histidine-Serine
18. Many proteases are produced as longer precursors. Why would this be the case?
a. Maintains a blocked or disordered active site until activated by proteolysis or cleavage of the inactive precursor.
b. Prevention of activation of the protease when synthesized, thus protecting the synthesizing organ.
c. Regulated activation of the protease when and where it is required.
d. All of the above.
19. A First Order reaction is linear in respect to which variables?
a. [A] and t
b. 1/[A] and t
c. ln[A] and t
d. k and t
20. A Second Order reaction is linear with respect to which variables?
a. [A] and t
b. 1/[A] and t
c. ln[A] and t
d. k and t
21. The following reaction data was obtained for an experiment. Is the data most consistent with a First or Second Order
reaction? HINT: The graphing diagrams below are to assist you in plotting these data.
T [A]
0 10.0
4 3.0
8 1.8
16 1.0
a. First order b. Second order c. Zero order d. Cannot determine
22. From the graphing in Question 21, what is the value for the k constant for the reaction?
a. 0.02
b. 0.05
c. 0.1
d. 0.5
23. What are the simplifying assumptions made for Michaelis-Menten enzyme kinetics?
a. Substrate concentration greatly exceeds enzyme concentration.
b. The enzyme-substrate complex concentration remains in steady state.
c. Equilibrium between the enzyme, substrate, and enzyme-substrate complex.
d. (b) and (c) are the fundamental simplifying assumptions with (b) deriving from performing the experiment under
conditions of (a).
24. Which of the following equations is the Michaelis-Menten Equation for a competitive inhibitor?
a. v
o
= Vmax [S] / Km + [S]
b. v
o
= Vmax [S] / Km + [S]
c. v
o
= Vmax [S] / Km + [S]
d. v
o
= Vmax [S] / Km + [S]
25. A reaction was analyzed with only 2 points. Assuming linearity, estimate the Vmax and Km from these
measurements. Hint: Slope = rise/run
v
o
(M/s)
[S] (M)
10 2
40 10
a. Vmax = 80 M/s, Km = 10 M
b. Vmax = 120 M/s , Km = 20 M
c. Vmax = 160 M/s , Km = 30 M
d. Vmax = 200 M/s, Km = 40 M
26. What is the type of inhibition for the following graph?
a. Competitive
b. Uncompetitive
c. Mixed
d. Pure noncompetitive
27. What type of inhibition for the following graph?
a. Competitive
b. Uncompetitive
c. Mixed
d. Pure noncompetitive
28. What type of inhibition for the following graph?
a. Competitive
b. Uncompetitive
c. Mixed
d. Pure noncompetitive
Analysis of an inhibitor on an enzyme reaction rate produced the following graph:
29. What type of inhibition is this?
a. Competitive
b. Uncompetitive
c. Mixed
d. Pure noncompetitive
30. The value at the X-intercept (1) has what value?
a. -1/Km
b. 1/Km
c. /Km
d. /Km, but with =
31. The value at the Y-intercept (2) has what value?
a. 1/Vmax
b. /Vmax
c. /Vmax
d. None of the above.
32. In these data, the lowest line has no inhibitor with a Y-intercept of 0.05 s/nM. The next higher line has 100 nM of an
inhibitor and a Y-intercept of 0.25 s/nM. What is the for this inhibitor?
a. 1
b. 2
c. 5
d.10
33. From this , what is the Ki for this inhibitor given the inhibitor concentration in Question 32?
a. 2.5 nM
b. 25 nM
c. 100 nM
d. 250 nM
34. Analysis of a novel enzyme apparently using multiple substrates (S
A
and S
B
) produces the Reaction Rate (v
o
) versus
Substrate A reaction profile observed in Curve 1 at a constant level of Substrate B. Adding more Substrate B shifts the
observed rate to Curve 2. What could explain this observation?
a. Substrate B inhibits the reaction of the enzyme with Substrate A.
b. Substrate B activates the reaction of the enzyme with Substrate A.
c. The enzyme appears to be cooperative and Substrate B appears to increase the cooperativity.
d. Both (b) and (c) are possible.
35. Degradation of nutrients and cell components to be used for energy or to rebuild other cellular components and
structures is an example of what type of metabolism?
a. Catabolism
b. Anabolism
c. Reduction/Oxidation
d. Aerobic
36. How is metabolism controlled?
a. Genetic regulation of the same metabolic pathways in different organs and tissues like isozymes.
b. Different pathways for catabolic and anabolic metabolism.
c. Allosteric signaling and covalent modifications of enzymes involved in metabolic processes.
d. All of these are significant mechanisms to control metabolism.
37. What type of organic molecules assist many enzymatic reactions involved in metabolism?
a. Proteins
b. Carbohydrates
c. Nucleic acids
d. Vitamins
38. What is the equilibrium constant for phosphoenolpyruvate (PEP) hydrolyzing to pyruvate and phosphate at human
physiological temperature? Hint: Remember the energy of hydrolysis of PEP is about twice that of ATP.
a. 2.9 x 10E10
b. 7.3 x 10E10
c. 2.8 x 10E11
d. 7.2 x 10E11
39. What electrochemical potential would you observe for a highly spontaneous redox reaction?
a. Positive
b. Negative
c. Near zero
d. Zero
40. Undersea bacteria can use alternative sources of energy including sulfurous materials like H
2
S. Using the standard
half-cell potentials for these reactions, calculate the electrochemical potential and direction for this reaction?
Pyruvate- + 2H+ + 2 e- = lactate- Eo = -0.185V
S + 2H+ + 2 e- = H
2
S Eo = -0.230V
a. -0.045V, lactate- + S = H2S + pyruvate-
b. 0.045V, lactate- + S = H2S + pyruvate-
c. 0.045V, pyruvate- + H2S = lactate- + S
d. -0.045V, pyruvate- + H2S = lactate- + S
41. Calculate the G for this reaction using the Faraday Constant = 96,485 J/V mol.
a. -4.3 kJ/mol
b. -8.7 kJ/mol
c. -10.5 kJ/mol
d. -18.5 kJ/mol
42. What carbohydrate is the following structure?
a. -D-Glucose
b. -D-Mannose
c. -D-Mannose
d. -D-Glucose
43. Which of the following linear structures would correlate with the above cyclic structure?
a. b. c. d.
44. A highly purified sample of polysaccharide was mixed with Tollens Reagent. This reagent provides Ag+ ions
complexed with ammonia to form a diamminesilver(I)+ cation. When mixed with the polysaccharide, the container
became rapidly coated with a mirror-like silver surface. What is the conclusion from this test?
a. The polysaccharide formed a silver complex and precipitated.
b. The polysaccharide is a reducing sugar.
c. The polysaccharide is not a reducing sugar.
d. Cannot determine from this information.
45. The following structure is for hyaluronic acid glycosaminoglycan. The repeating structure is a disaccharide unit.
What monosaccharides comprise this disaccharide unit?
a. glucose and N-acetyl-glucosamine
b. galactose and N-acetyl-glucosamine
c. glucuronic acid and N-acetyl-glucoseamine
d. N-acetyl-galactoseamine and glucuronic acid
46. What linkage is between these sugar residues (not the linkage between the disaccharide units at the ends, but
between the sugars in the disaccharide unit itself)?
a. -(1-2)
b. -(1-3)
c. -(1-3)
d. -(1-4)
47. Why would the highly branched storage polysaccharide glycogen be important for energy storage rather than having
all of the carbohydrates as single or smaller monosaccharides?
a. Monosaccharides at high concentration would produce very significant osmotic pressure within cells.
b. Monosaccharides can produce energy rapidly.
c. Monosaccharides are easily transported.
d. Monosaccharides are the primary structure necessary for metabolic processing.
48. Which of the following is important properties of peptidoglycans involved in bacterial cell wall synthesis?
a. Peptidoglycans are a crosslinked meshwork of carbohydrates and short peptides.
b. Gram positive bacteria have thick peptidoglycan cell walls.
c. Peptidoglycans often have isopeptide bonds and amino acids with alternative chirality.
d. (a) and (c) are both important for peptidoglycans in bacteria.
49. The following peptide sequence from CD34, a human hematopoetic progenitor cell antigen, is known to be heavily
glycosylated. Where would you predict glycosylation to occur?
MSLDNNGTAT PELPTQGTFS NVSTNVSYQE
Section 1 Section 2 Section 3
a. Section 1 and Section 2
b. Section 2 and Section 3
c. Section 1 and Section 3
d. Section 1, 2 and 3.
50. Which of the following enzyme(s) are important for defense against bacteria with peptidoglycan cell walls?
a. RNAse
b. Lysozyme
c. Trypsin
d. Acetylcholine Esterase
Das könnte Ihnen auch gefallen
- BIOCHEMISTRY, CELL AND MOLECULAR BIOLOGY: Passbooks Study GuideVon EverandBIOCHEMISTRY, CELL AND MOLECULAR BIOLOGY: Passbooks Study GuideNoch keine Bewertungen
- Biol 130 Notes 2012Dokument121 SeitenBiol 130 Notes 2012Nick O'HaraNoch keine Bewertungen
- AS Biology BYB1 Core Principles 10.4 Enzymes TestDokument5 SeitenAS Biology BYB1 Core Principles 10.4 Enzymes TestormattNoch keine Bewertungen
- Quiz 1: Introduction To Molecular BiologyDokument4 SeitenQuiz 1: Introduction To Molecular BiologyLuis MartinezNoch keine Bewertungen
- The Vitamins: Chemistry, Physiology, Pathology, MethodsVon EverandThe Vitamins: Chemistry, Physiology, Pathology, MethodsW. H. SebrellBewertung: 4 von 5 Sternen4/5 (2)
- Basic Concepts in PharmacologyDokument25 SeitenBasic Concepts in PharmacologyRizka Amaliah NasutionNoch keine Bewertungen
- Separation and Characterization Techniques For Proteins and AminoDokument34 SeitenSeparation and Characterization Techniques For Proteins and AminoAnna Donato100% (2)
- Channels, Carriers, and Pumps: An Introduction to Membrane TransportVon EverandChannels, Carriers, and Pumps: An Introduction to Membrane TransportNoch keine Bewertungen
- Selected Topics in the History of Biochemistry. Personal Recollections. Part IIIVon EverandSelected Topics in the History of Biochemistry. Personal Recollections. Part IIIBewertung: 1 von 5 Sternen1/5 (1)
- Stereochemistry QuestionsDokument7 SeitenStereochemistry Questionsalyson_lNoch keine Bewertungen
- Introduction To ElectrophoresisDokument52 SeitenIntroduction To ElectrophoresisMegha AnandNoch keine Bewertungen
- Biochemistry Midterm 1 QuestionsDokument2 SeitenBiochemistry Midterm 1 Questionselfin_treeNoch keine Bewertungen
- COLLEGE CHEMISTRY: Passbooks Study GuideVon EverandCOLLEGE CHEMISTRY: Passbooks Study GuideNoch keine Bewertungen
- Sample Exam 4 Fall 11Dokument14 SeitenSample Exam 4 Fall 11janohxNoch keine Bewertungen
- LXTDokument69 SeitenLXTTendai ChirisaNoch keine Bewertungen
- Sermons to Remember: Volume I: God's Words to the NationsVon EverandSermons to Remember: Volume I: God's Words to the NationsNoch keine Bewertungen
- Protein Purification and AnalysisDokument16 SeitenProtein Purification and AnalysisSujan BoseNoch keine Bewertungen
- Amino Acids MnemonicDokument4 SeitenAmino Acids MnemonicakileshNoch keine Bewertungen
- Plasma Protein Metabolism: Regulation of Synthesis, Distribution, and DegradationVon EverandPlasma Protein Metabolism: Regulation of Synthesis, Distribution, and DegradationMarcus RothschildBewertung: 5 von 5 Sternen5/5 (1)
- Full Blood Count Apr04, DR Eva RaikDokument7 SeitenFull Blood Count Apr04, DR Eva RaikDanielcc LeeNoch keine Bewertungen
- Jan BI1 - 2001Dokument13 SeitenJan BI1 - 2001skarun1Noch keine Bewertungen
- Clinical EnzymologyDokument18 SeitenClinical EnzymologyNur LiyanaNoch keine Bewertungen
- Biology Midterm Study GuideDokument7 SeitenBiology Midterm Study Guideapi-313911292Noch keine Bewertungen
- Final Exam Biology BDokument13 SeitenFinal Exam Biology Bapi-237801056Noch keine Bewertungen
- AYFS DiscussionGuide OnlineDokument71 SeitenAYFS DiscussionGuide OnlineRichard Lancaster-ShanksNoch keine Bewertungen
- FST Undergraduate Handbook 2020-2021Dokument392 SeitenFST Undergraduate Handbook 2020-2021Robin WilliamsNoch keine Bewertungen
- Biochem CompreDokument72 SeitenBiochem CompreStd DlshsiNoch keine Bewertungen
- 6 - Clinical Biochemistry - 1001411Dokument14 Seiten6 - Clinical Biochemistry - 1001411bsmallahNoch keine Bewertungen
- Biochemistry - Final Exam Biology 020.305 December 15, 2011 Instructions For ExaminationDokument11 SeitenBiochemistry - Final Exam Biology 020.305 December 15, 2011 Instructions For ExaminationJenna ReynoldsNoch keine Bewertungen
- PCOL2012 Unit of Study Outline 2016Dokument10 SeitenPCOL2012 Unit of Study Outline 2016ncisisthebestNoch keine Bewertungen
- Cytochrome P450 - Structure Mechanism and Biochemistry 2005Dokument701 SeitenCytochrome P450 - Structure Mechanism and Biochemistry 2005Naxo SottorffNoch keine Bewertungen
- Question CH06+answer PDFDokument8 SeitenQuestion CH06+answer PDFCris-Anne Juangco III100% (1)
- BIOC 307 Old Exam 1Dokument17 SeitenBIOC 307 Old Exam 1Katie RoseNoch keine Bewertungen
- Coursesaver (Chad) College Physics OutlinesDokument40 SeitenCoursesaver (Chad) College Physics Outlinescalong558Noch keine Bewertungen
- Backwards ReasoningDokument40 SeitenBackwards Reasoningharshit chaudharyNoch keine Bewertungen
- Haem Lecture 2.2016 PDFDokument12 SeitenHaem Lecture 2.2016 PDFdorsa koraeiNoch keine Bewertungen
- How To Score High in Biochemistry Exams?Dokument2 SeitenHow To Score High in Biochemistry Exams?Prof.PTS100% (5)
- Lecture Notes First Semester Yr 2 BPham BMLS BDSDokument57 SeitenLecture Notes First Semester Yr 2 BPham BMLS BDSKarin AdraiNoch keine Bewertungen
- Coenzymes and CofactorsDokument11 SeitenCoenzymes and CofactorsGovindaraju ShruthiNoch keine Bewertungen
- EnzymesDokument19 SeitenEnzymesوليد. وفي الروح0% (1)
- DAT QuizletDokument78 SeitenDAT QuizletJihee YoonNoch keine Bewertungen
- SCH4C Esters LabDokument8 SeitenSCH4C Esters LabSteve M HallNoch keine Bewertungen
- Research Proposal-SignedDokument3 SeitenResearch Proposal-Signedapi-410826283Noch keine Bewertungen
- Biochem CombinedDokument758 SeitenBiochem CombinedTheBoss 20Noch keine Bewertungen
- Ist Year MbbsDokument3 SeitenIst Year MbbsJaved Gaba0% (1)
- Protein Purification TechniquesDokument102 SeitenProtein Purification TechniquesDawlat SlamaNoch keine Bewertungen
- AutophagyDokument1 SeiteAutophagyCLPHtheoryNoch keine Bewertungen
- A Quick Look at Biochemistry Carbohydrate Metabolism (ELENA1)Dokument15 SeitenA Quick Look at Biochemistry Carbohydrate Metabolism (ELENA1)Elena FloresNoch keine Bewertungen
- Industrial Biochemistry - 609u1Dokument27 SeitenIndustrial Biochemistry - 609u1Palanisamy Selvamani100% (1)
- Chapter 16 The Molecular Basis of InheritanceDokument4 SeitenChapter 16 The Molecular Basis of InheritanceMmmNoch keine Bewertungen
- L 17 Structure and Functions of ProteinsDokument43 SeitenL 17 Structure and Functions of ProteinssNoch keine Bewertungen
- Fe SCN2Dokument4 SeitenFe SCN2Jürgen Nicholas Schwarze100% (1)
- Chapter7 MC QuizDokument19 SeitenChapter7 MC Quizsmishra_97Noch keine Bewertungen
- Sample Exam BiologyDokument4 SeitenSample Exam BiologyHamsaNoch keine Bewertungen
- Group21 Bioinformatics Assignment6 .Primary-structure-Protein-localizationdocxDokument6 SeitenGroup21 Bioinformatics Assignment6 .Primary-structure-Protein-localizationdocxHuỳnh NhưNoch keine Bewertungen
- Jotun Penguard Express CF TDSDokument5 SeitenJotun Penguard Express CF TDSnihad_mNoch keine Bewertungen
- Thermodynamic Analysis of EAF Electrical Energy deDokument17 SeitenThermodynamic Analysis of EAF Electrical Energy deRafaela PradeNoch keine Bewertungen
- Accc/Tw Prague (700) : Data SheetDokument1 SeiteAccc/Tw Prague (700) : Data SheetkmiqdNoch keine Bewertungen
- Remediation of Cadmium and Lead Contamination in Mustard - Maize Cropping SystemDokument5 SeitenRemediation of Cadmium and Lead Contamination in Mustard - Maize Cropping SystemDr Amrit Kumar JhaNoch keine Bewertungen
- Type of Evaporator 1pptDokument12 SeitenType of Evaporator 1pptgrittyptNoch keine Bewertungen
- Nanopartikel AseklofenakDokument9 SeitenNanopartikel AseklofenakReski WahyuNoch keine Bewertungen
- Structure-Property Relationships of Flexible Polyurethane FoamsDokument10 SeitenStructure-Property Relationships of Flexible Polyurethane Foamstoiec hocNoch keine Bewertungen
- Metabolism Exam 2 - GIFT - Spring 2016Dokument9 SeitenMetabolism Exam 2 - GIFT - Spring 2016shafa_nathani100% (2)
- Spectracron 110 FD Alkyd Enamel PDFDokument2 SeitenSpectracron 110 FD Alkyd Enamel PDFSatish Vishnubhotla0% (1)
- R304 0307 Env HHDokument221 SeitenR304 0307 Env HHsyamsundariitmiitmNoch keine Bewertungen
- Enzyme PurificationDokument25 SeitenEnzyme Purificationranjish_007Noch keine Bewertungen
- Sepakat Setia Perunding SDN BHD: Design InformationDokument6 SeitenSepakat Setia Perunding SDN BHD: Design InformationAfiq SyahmiNoch keine Bewertungen
- Bioinorganic ChemistryDokument12 SeitenBioinorganic Chemistrycrisanto valdezNoch keine Bewertungen
- 11-Msds Mobile SHC 624Dokument9 Seiten11-Msds Mobile SHC 624Fatimazahra SahriNoch keine Bewertungen
- Repair Laptop BatteryDokument23 SeitenRepair Laptop Batteryapi-381781593% (15)
- Tugas PPM Deny Saputro Arifin 113170039Dokument9 SeitenTugas PPM Deny Saputro Arifin 113170039Vira IrnandaNoch keine Bewertungen
- Physics For Scientists and Engineers, 6e: Chapter 41 - Quantum MechanicsDokument15 SeitenPhysics For Scientists and Engineers, 6e: Chapter 41 - Quantum MechanicsTom TrầnNoch keine Bewertungen
- Spectrolab m12 enDokument8 SeitenSpectrolab m12 enBHAART PANCHAL100% (1)
- Cover Lab ReportDokument5 SeitenCover Lab ReportadlenaNoch keine Bewertungen
- Potent Antimutagenic Activity of White Tea in Comparison With Green Tea in The Salmonella AssayDokument15 SeitenPotent Antimutagenic Activity of White Tea in Comparison With Green Tea in The Salmonella AssayPhan Anh TrịnhNoch keine Bewertungen
- Revamps 2018 PDFDokument60 SeitenRevamps 2018 PDFkeo landNoch keine Bewertungen
- European Steel and Alloy Grades: HDT560F (1.0959)Dokument2 SeitenEuropean Steel and Alloy Grades: HDT560F (1.0959)farshid KarpasandNoch keine Bewertungen
- Background Information: Methodology: ProcedureDokument6 SeitenBackground Information: Methodology: ProcedureAlexandra Ramos MNoch keine Bewertungen
- Supercritical Fluid Technology For Drug Product Development (2004)Dokument688 SeitenSupercritical Fluid Technology For Drug Product Development (2004)Regiani Almeida Rezende100% (1)
- Sample Problem 1: X Direction X Direction XDokument4 SeitenSample Problem 1: X Direction X Direction XHarold MangaNoch keine Bewertungen
- Sensors Evaluation 2019 Oct 19 (19) - DOC#NDL:NDA:DGA:ON:072002 PDFDokument1 SeiteSensors Evaluation 2019 Oct 19 (19) - DOC#NDL:NDA:DGA:ON:072002 PDFDivyansh KohliNoch keine Bewertungen
- Heat Transfer Lab Experiment Report PDFDokument5 SeitenHeat Transfer Lab Experiment Report PDFNasih AhmadNoch keine Bewertungen
- Shell Gadus S2 OG Clear Oil 20000Dokument2 SeitenShell Gadus S2 OG Clear Oil 20000Xavier DiazNoch keine Bewertungen
- Corrosion HDBK S2Dokument296 SeitenCorrosion HDBK S2Aleksandra AleksicNoch keine Bewertungen
- Gas Natural TransmisionDokument43 SeitenGas Natural Transmisionangel3reyesNoch keine Bewertungen