Beruflich Dokumente
Kultur Dokumente
Organic 1 Summary Notes
Hochgeladen von
cgao30Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Organic 1 Summary Notes
Hochgeladen von
cgao30Copyright:
Verfügbare Formate
S
a
v
i
t
a
P
a
I
I
.
c
o
m
Organic Chemistry Summary Notes
Characteristics of Organic Compounds
1. Covalent bonds
2. Linear (higher m.p. and b.p. because of bigger surface area), branched
(lower m.p. and b.p. because of smaller surface area), ring
3. Saturated (single bonds only) / Unsaturated (double/triple bonds)
4. Functional group
Meth (1C), Eth (2C), Prop (3C), But (4C), Pent (5C), etc.
5. Homologous series
Related compounds sharing the same functional group; addition of
methylene (-CH
2
) each time
6. lsomerism
Structural
i. -ol ether
ii. -al -one Different connected structures
iii. -oic ester
Stereo-isomerism: geometrical
i. C=C
ii. Ring
7. a) Structural formula
b) Expanded formula
c) Condensed formula
d) Line structure
8. Ane
C
n
H
2n+2
, o bond
--Ene
CnH
2n
, o + bonds
--yne
C
n
H
2n-2
, o + + bonds
--Aromatic
Benzene/benzene derivatives
Delocalized electrons add to stability, thus unreactive
Nomenclature
1. Find longest chain (functional group)
2. ldentify substituents
3. Number the parent chain
4. List substituents in alphabetical order and give their locant, (lowest
possible number)
5. Name the parent chain usually only one suffix (max of 2 suffix)
(Refer to sheet "Naming Organic Compounds for further nomenclature)
S
a
v
i
t
a
P
a
I
I
.
c
o
m
Alkanes
o bonded
Tetrahedral (Bond angle: 109.5 degrees)
Stable due to strong C-C, C-H bonds
Non-polar (does not dissolve in water, only non-polar solvents)
Low mp/bp because of weak lMFAs (only LDF)
Mp/bp goes up as molecular mass increases LDF increases
Combustion
o Complete, Excess O
2
CO
2
+ H
2
O + Heat
o lncomplete, Limited O
2
C + CO + H
2
O (used as fuels)
Substitution Reactions
UV
o CH
4
+ Br
2
CH
3
Br + HBr
Alkene
o + bonds
Trigonal Planar (Bond angle: 120 degrees)
Non-polar
LDF (3 bonds Unstable Addition Reactions)
Halogenation
o Test for unsaturated bonds (Alkenes)
o CH
2
= CH
2
+ Br
2
CH
2
Br CH
2
Br
Red/Org. Colourless
Hydrogenation: C=C + H
2
C-C (makes margarine, heterogenous
catalysis); Ni/200 C
Hydration: C=C + HOH C-C-OH (Markovnikov's Rule: Hydrogen goes
to the carbon already attached to the most hydrogens), dilute sulphuric
acid required
Hydrogen Halide: C=C + HX H-C-C-X (Markovnikov's Rule)
Oxidizing Agent (Test for Unsaturated bonds diol)
o KMnO
4
/H
+
Purple Colourless
o K
2
Cr
2
O
7
/H
+
Orange green
o C=C + [O] + H-OH OHCC OH
Polymerisation (Ms. Pall: Can't make out the section! Sowwy )
Cis/Trans ln alkenes, the orientation of substituents matter, since they
are fixed in position.
Br Br Br
C = C C = C
Br
Trans Cis
More Stable, higher mp/bp Less Stable,lower mp/bp
(due to steric hinderance), more polar
S
a
v
i
t
a
P
a
I
I
.
c
o
m
Organic Reaction Mechanisms
Definition: Reaction Mechanisms
During any reaction, bonds are broken and made. As bonds are attractive forces
between positive and negative bits of particles, making and breaking bonds
means moving electrons around. A mechanism is a description of a successful
collision (an effective collision) between the reactants and the electron
rearrangement that happens as reactants are changed into products.
1) HomoIytic Substitution
(A free radical photochemical chain reaction)
A common example is the reaction between methane and chlorine in the
presence of UV light.
{E = hf, for a quantum of UV light, f = 1 x 10
15
s
-1
; E = 6.6 x 10
-19
J. For 1 mole of
quantum E = 6.6 x 10
-19
J (N
A
/mol) = 400 kJ/mol}
To break a Cl Cl bond (Bond dissociation energy = 242 kJ/mol)
To break a C C bond (bond dissociation energy = 348 kJ/mol)
Evidence for Mechanism
Reaction takes place in sunlight, not darkness
Small amounts of C
2
H
6
detected
lf Pb(CH
3
)
4
added (this dissociates CH
3
), reaction takes place in the
dark as well.
Stage 1 lnitiation: The Production of Free Radicals
A chlorine molecule absorbs UV radiation, breaking the bond in the molecule
homolytically producing two free radicals.
Because the reaction is started by light it is a photochemical reaction.
Cl Cl 2Cl (Very low Ea of 25 kJ/mol)
Stage 2 Propagation
The reaction between a free radical and a molecule to make another free radical.
There are many possible steps:
(1) Cl + CH
4
CH
3
+ HCl (2) Cl + CH
4
CH
3
Cl + H
Reaction #1 is more likely, since the making of H Cl is highly exothermic (431
kJ/mol) compared to the making of a C Cl bond (350 kJ/mol) Because each
step makes another reactive free radical, the reaction is a chain reaction.
Step 3 Termination
The reaction between two free radicals makes an unreactive molecule. Three
possible reactions:
(1) 2Cl Cl
2
(2) 2CH
3
C
2
H
6
(3) Cl + CH
3
CH
3
Cl
S
a
v
i
t
a
P
a
I
I
.
c
o
m
Because any two free radicals can meet, this type of reaction always produces a
mixture of products.
NOTE:
1) Reaction between toluene, Benzene CH3 + X2 Same Mechanism
2) Rate of reaction F2 > Cl2 > Br2 > l2 (Slow and reversible) due to H-X bond
releases more energy in H F than H l.
2) EIectrophiIic Addition
Addition to Alkenes is referred to as: Heterolytic Electrophilic Addition
The bonding between two doubly bonded carbon atom is stronger than the single
bond in alkanes.
C C C = C
(348 kJ/mol, longer bond) (612 kJ/mol, shorter bond)
This makes the alkenes more thermally stable than alkanes. Since the strength
of the double bond is not twice that of a single bond, thus the double bond is a
different kind of bond.
bond
p-p overlap
Perpendicular to bond axis = bond
Evidence for Mechanism
1. a) Br
2
+ inert solvent Br C C Br
b) Br
2
+ polar solvent Br C C OH
2. Br
2
+ M
+
Cl
-
Br C C Cl
Mechanism: H
C=CH
+ X
Rate law: Rate k|alkene||X2| (bimolecular)
C=C C = C
Br
+
H
+
Br
X
lnduced dipole Permanent dipole
-electrons becoming o electrons as they bond to a new atom to the carbon
skeleton.
Two regions of negative charge
are a reactive site for electrophilic
attack. The -electron clouds
make alkenes to be more reactive
than alkanes.
(The -electron cloud may even
induce dipole in nearby particles)
S
a
v
i
t
a
P
a
I
I
.
c
o
m
Slow
First Step
To attack -electrons of the alkene on an electrophile Rep
n
(What does that
stand for?) between -electron of the double bond and the X-X bond, inducing a
dipole in the X
X
. Further approach, allows X X to undergo heterolytic
fission, and the formation of a weak complex between the two ends of the
Br Br
molecule and the -electrons of the C=C bond.
C=C
Br
+
C C + Br
Br
Br
The carbocation rapidly reacts with the nucleophilic Br
-
from the backside to the
bond that was made in the heterolysis.
Fast
Br
C C C C
Br Br Br
NOTE
1. When a substance such as HX adds onto an alkene, the H- always adds onto
the C-atom with the most H's already attached to it. Markovnikov's Rule,
due to the delocalisation of the charge on C+ (C+3 > C+2 > C+1)
2. Hydration of alkenes C=C +
OSO
2
OH C C OH
3. Test for alkenes (1) Br2, (2) Oxidizing agent like OH C C OH
Das könnte Ihnen auch gefallen
- Commonwealth Statutory Declaration Form (May 2011) PDFDokument2 SeitenCommonwealth Statutory Declaration Form (May 2011) PDFcgao30Noch keine Bewertungen
- Commonwealth Statutory Declaration Form (May 2011) PDFDokument2 SeitenCommonwealth Statutory Declaration Form (May 2011) PDFcgao30Noch keine Bewertungen
- Pythagoras WorksheetDokument2 SeitenPythagoras Worksheetcgao30Noch keine Bewertungen
- CD30 Literature Review PlanDokument1 SeiteCD30 Literature Review Plancgao30Noch keine Bewertungen
- Student Elective Rotation GuideDokument3 SeitenStudent Elective Rotation Guidecgao30Noch keine Bewertungen
- CPPREP4002 - Annotated Unit GuideDokument8 SeitenCPPREP4002 - Annotated Unit Guidecgao30Noch keine Bewertungen
- Theme IV Yr 5d Content Guide - MatrixDokument9 SeitenTheme IV Yr 5d Content Guide - Matrixcgao30Noch keine Bewertungen
- CD marker panel guide for flow cytometry cell identificationDokument2 SeitenCD marker panel guide for flow cytometry cell identificationcgao30Noch keine Bewertungen
- Benign Lesions of The Vulva & Vagina (Table)Dokument3 SeitenBenign Lesions of The Vulva & Vagina (Table)cgao30Noch keine Bewertungen
- Lit Reviews For RX Students v7Dokument20 SeitenLit Reviews For RX Students v7Joshua McdonaldNoch keine Bewertungen
- CD30 Literature Review PlanDokument1 SeiteCD30 Literature Review Plancgao30Noch keine Bewertungen
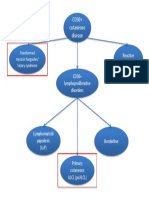
- CD30+ cutaneous lymphoproliferative disordersDokument1 SeiteCD30+ cutaneous lymphoproliferative disorderscgao30Noch keine Bewertungen
- CD30 Literature Review PlanDokument1 SeiteCD30 Literature Review Plancgao30Noch keine Bewertungen
- CD30 Draft CollationDokument8 SeitenCD30 Draft Collationcgao30Noch keine Bewertungen
- Paediatrics: Acyanotic Heart DiseaseDokument5 SeitenPaediatrics: Acyanotic Heart Diseasecgao30Noch keine Bewertungen
- Preterm LabourDokument3 SeitenPreterm Labourcgao30Noch keine Bewertungen
- Pathology Lecture 7 - LiverDokument11 SeitenPathology Lecture 7 - Livercgao30Noch keine Bewertungen
- Intro To Motor Systems: (Why Else Would You Go To The Gym, Right?)Dokument13 SeitenIntro To Motor Systems: (Why Else Would You Go To The Gym, Right?)cgao30Noch keine Bewertungen
- Fibroids: 1. Red DegenerationDokument2 SeitenFibroids: 1. Red Degenerationcgao30Noch keine Bewertungen
- EndometriosisDokument1 SeiteEndometriosiscgao30Noch keine Bewertungen
- Cell Respiration Notes (3&8)Dokument9 SeitenCell Respiration Notes (3&8)cgao30Noch keine Bewertungen
- Matrix TopicsDokument3 SeitenMatrix Topicscgao30Noch keine Bewertungen
- Skin Terms: DescriptorsDokument2 SeitenSkin Terms: Descriptorscgao30Noch keine Bewertungen
- Pelvic Organ ProlapseDokument5 SeitenPelvic Organ Prolapsecgao30Noch keine Bewertungen
- O&G GlossaryDokument3 SeitenO&G Glossarycgao30Noch keine Bewertungen
- Pathology Lecture 7 - LiverDokument11 SeitenPathology Lecture 7 - Livercgao30Noch keine Bewertungen
- Inflammatory Conditions (Disease Mechanisms)Dokument3 SeitenInflammatory Conditions (Disease Mechanisms)cgao30Noch keine Bewertungen
- Dna Notes (3&7) - CompleteDokument11 SeitenDna Notes (3&7) - Completecgao30100% (1)
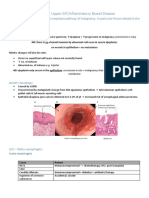
- Pathology Lecture 5 - Upper GITDokument10 SeitenPathology Lecture 5 - Upper GITcgao30Noch keine Bewertungen
- Pathology Lecture 2 - NeoplasiaDokument15 SeitenPathology Lecture 2 - Neoplasiacgao30Noch keine Bewertungen
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeVon EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeBewertung: 4 von 5 Sternen4/5 (5784)
- The Little Book of Hygge: Danish Secrets to Happy LivingVon EverandThe Little Book of Hygge: Danish Secrets to Happy LivingBewertung: 3.5 von 5 Sternen3.5/5 (399)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceVon EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceBewertung: 4 von 5 Sternen4/5 (890)
- Shoe Dog: A Memoir by the Creator of NikeVon EverandShoe Dog: A Memoir by the Creator of NikeBewertung: 4.5 von 5 Sternen4.5/5 (537)
- Grit: The Power of Passion and PerseveranceVon EverandGrit: The Power of Passion and PerseveranceBewertung: 4 von 5 Sternen4/5 (587)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureVon EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureBewertung: 4.5 von 5 Sternen4.5/5 (474)
- The Yellow House: A Memoir (2019 National Book Award Winner)Von EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Bewertung: 4 von 5 Sternen4/5 (98)
- Team of Rivals: The Political Genius of Abraham LincolnVon EverandTeam of Rivals: The Political Genius of Abraham LincolnBewertung: 4.5 von 5 Sternen4.5/5 (234)
- Never Split the Difference: Negotiating As If Your Life Depended On ItVon EverandNever Split the Difference: Negotiating As If Your Life Depended On ItBewertung: 4.5 von 5 Sternen4.5/5 (838)
- The Emperor of All Maladies: A Biography of CancerVon EverandThe Emperor of All Maladies: A Biography of CancerBewertung: 4.5 von 5 Sternen4.5/5 (271)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryVon EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryBewertung: 3.5 von 5 Sternen3.5/5 (231)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaVon EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaBewertung: 4.5 von 5 Sternen4.5/5 (265)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersVon EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersBewertung: 4.5 von 5 Sternen4.5/5 (344)
- On Fire: The (Burning) Case for a Green New DealVon EverandOn Fire: The (Burning) Case for a Green New DealBewertung: 4 von 5 Sternen4/5 (72)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyVon EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyBewertung: 3.5 von 5 Sternen3.5/5 (2219)
- The Unwinding: An Inner History of the New AmericaVon EverandThe Unwinding: An Inner History of the New AmericaBewertung: 4 von 5 Sternen4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreVon EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreBewertung: 4 von 5 Sternen4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Von EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Bewertung: 4.5 von 5 Sternen4.5/5 (119)
- Her Body and Other Parties: StoriesVon EverandHer Body and Other Parties: StoriesBewertung: 4 von 5 Sternen4/5 (821)
- Part Number 26-84: Illustrated Parts BreakdownDokument90 SeitenPart Number 26-84: Illustrated Parts BreakdownJesus OliverosNoch keine Bewertungen
- Astm B 265 - 03Dokument8 SeitenAstm B 265 - 03kaminaljuyuNoch keine Bewertungen
- CEIC2000 Thermodynamics - Lecture 2 The First Law Forms of EnergyDokument49 SeitenCEIC2000 Thermodynamics - Lecture 2 The First Law Forms of EnergyJoshua JohnNoch keine Bewertungen
- DC30-010 - ODYSSEY Operator Manual - Rev PDokument92 SeitenDC30-010 - ODYSSEY Operator Manual - Rev PYolanda Peña100% (1)
- Volumes by Cylindrical ShellsDokument7 SeitenVolumes by Cylindrical Shellseomer1968Noch keine Bewertungen
- Engineering Calculation Sheet Consulting EngineersDokument17 SeitenEngineering Calculation Sheet Consulting EngineersParthiban ArivazhaganNoch keine Bewertungen
- Malla Curricular Ingenieria Civil UNTRMDokument1 SeiteMalla Curricular Ingenieria Civil UNTRMhugo maldonado mendoza50% (2)
- ETABS 2016 Concrete Frame Design: ETABS 2016 16.2.1 License # 14VSTSP6QKXWAC6Dokument2 SeitenETABS 2016 Concrete Frame Design: ETABS 2016 16.2.1 License # 14VSTSP6QKXWAC6Luis Miguel GaviñoNoch keine Bewertungen
- SBT Mechanics TH 1Dokument535 SeitenSBT Mechanics TH 1Shreyas Singh100% (2)
- Moc3011 PDFDokument7 SeitenMoc3011 PDFAlvaro Mompi RuizNoch keine Bewertungen
- BS en 10297 2Dokument36 SeitenBS en 10297 2Sharma ShailenNoch keine Bewertungen
- Learning Objectives-2: Uniform MotionDokument7 SeitenLearning Objectives-2: Uniform MotionBryanHarold BrooNoch keine Bewertungen
- MCR 3U5 CPT Part 2Dokument4 SeitenMCR 3U5 CPT Part 2Ronit RoyanNoch keine Bewertungen
- 4.7.5 Walls: CK Yk CKDokument2 Seiten4.7.5 Walls: CK Yk CKBertin BakariNoch keine Bewertungen
- Numerical Study of Buckling of Thin PlatesDokument7 SeitenNumerical Study of Buckling of Thin PlatesGogyNoch keine Bewertungen
- Storage and Flow of Powder: Mass Flow Funnel FlowDokument9 SeitenStorage and Flow of Powder: Mass Flow Funnel FlowDuc HuynhNoch keine Bewertungen
- Topic 5 - Criticality of Homogeneous ReactorsDokument53 SeitenTopic 5 - Criticality of Homogeneous ReactorsSit LucasNoch keine Bewertungen
- Romax 4000 PDFDokument2 SeitenRomax 4000 PDFALEKSANDARNoch keine Bewertungen
- Phy WsDokument4 SeitenPhy WsPranav VNoch keine Bewertungen
- Bulk Driven MosfetDokument3 SeitenBulk Driven MosfetAyandev BarmanNoch keine Bewertungen
- Grade F: Tabulation Allowable Within TheDokument1 SeiteGrade F: Tabulation Allowable Within ThelechepinitoNoch keine Bewertungen
- Cascho Modelo DDokument16 SeitenCascho Modelo Dfrankz89Noch keine Bewertungen
- Egyptian FractionsDokument80 SeitenEgyptian Fractionsmyasweet22Noch keine Bewertungen
- MV Drop TestDokument5 SeitenMV Drop Testrajinipre-1Noch keine Bewertungen
- Unconfined Compression Test: Experiment No. 3Dokument6 SeitenUnconfined Compression Test: Experiment No. 3Patricia TubangNoch keine Bewertungen
- Instabilities and Nonequilibrium Structures VII & VIIIDokument377 SeitenInstabilities and Nonequilibrium Structures VII & VIIIcoerenciaceNoch keine Bewertungen
- Wireless Pick and Place RobotDokument26 SeitenWireless Pick and Place RobotAshok GudivadaNoch keine Bewertungen
- Introduction to PCA and FA: Dimension Reduction ToolsDokument29 SeitenIntroduction to PCA and FA: Dimension Reduction ToolsPushpaRasnayakeNoch keine Bewertungen
- Grade 12 English Test Review: Key Terms, Grammar, Reading ComprehensionDokument5 SeitenGrade 12 English Test Review: Key Terms, Grammar, Reading ComprehensionLinh HuongNoch keine Bewertungen
- The Consumption of Compressed Air: Applications and ComponentsDokument24 SeitenThe Consumption of Compressed Air: Applications and Componentsibong tiriritNoch keine Bewertungen