Beruflich Dokumente
Kultur Dokumente
Experiment Rate of Reaction - Size
Hochgeladen von
Syakir Izzat0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
133 Ansichten2 SeitenCopyright
© © All Rights Reserved
Verfügbare Formate
PDF, TXT oder online auf Scribd lesen
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
© All Rights Reserved
Verfügbare Formate
Als PDF, TXT herunterladen oder online auf Scribd lesen
0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
133 Ansichten2 SeitenExperiment Rate of Reaction - Size
Hochgeladen von
Syakir IzzatCopyright:
© All Rights Reserved
Verfügbare Formate
Als PDF, TXT herunterladen oder online auf Scribd lesen
Sie sind auf Seite 1von 2
Sir HONG Chemistry Group 4541/3
Sir HONG Chemistry Group 1
Effect of size of reactant on the rate of reaction
Aim : To investigate the effect of the size of reactant on the rate of reaction.
Problem Statement : Is the rate of reaction between marble chips and dilute hydrochloric acid
affected by the size of the marble chips?
Hypothesis :
Variables
a) Manipulated :
b) Responding :
c) Fixed :
Apparatus : 250 cm
3
conical flask, 50 cm
3
measuring cylinder, delivery tube with rubber
stopper, burette, retort stand with clamps, weighing balance, stopwatch and
basin.
Materials : 0.2 moldm
-3
hydrochloric acid, water, marble chips and marble powder.
Procedures :
1. A burette is filled with water until full. It is then inverted over water in a basin and clamped
vertically.
2. The water level in the burette is adjusted and the initial reading of the burette is recorded.
3. The apparatus as shown in Diagram is set up.
4. 20 cm
3
of 0.2 moldm
-3
hydrochloric acid is measured and poured into the conical flask.
5. 2.0 g of marble chips are added into the conical flask. The flask is closed immediately with
stopper. At the same time, a stopwatch is started.
6. The conical flask is swirled gently. Carbon dioxide gas liberated is collected in the burette by
downward displacement of water.
7. The volume of gas collected in the burette is recorded at 0.5 minute intervals until no more gas is
liberated.
8. Steps 1 to 7 are repeated using 2.0 g of the marble powder. All the other conditions remain
unchanged.
Marble chips
Hydrochloric acid
Water
Carbon dioxide gas
Sir HONG Chemistry Group 4541/3
Sir HONG Chemistry Group 2
Observation :
i) Marble chips
Time / s 0 30 60 90 120 150 180 210 240 270 300 330 360
Burette reading / cm
3
Volume of gas / cm
3
ii) Marble powder
Time / s 0 30 60 90 120 150 180 210 240 270 300 330 360
Burette reading / cm
3
Volume of gas / cm
3
Discussion :
1. What is the total volume of gas liberated by 20 cm
3
of 0.2 moldm
-3
hydrochloric acid?
2. Write a chemical equation for the reaction between marble chips and dilute hydrochloric acid.
3. Write an ionic equation for the reaction between marble chips and dilute hydrochloric acid.
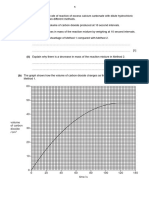
4. Draw a graph of total volume of carbon dioxide gas collected against time for both the marble
chips and powder.
5. Based on the graph, what is the relationship between size of the marble chips and rate of the
reaction?
Conclusion :
Das könnte Ihnen auch gefallen
- Lab Report Preparation of Standard SolutionDokument9 SeitenLab Report Preparation of Standard SolutionCRSFZ88% (8)
- O Level Biology Practice For Structured Questions Movement Of SubstancesVon EverandO Level Biology Practice For Structured Questions Movement Of SubstancesNoch keine Bewertungen
- Chemistry IA Example 2Dokument12 SeitenChemistry IA Example 2Vanessa Tumanggor100% (1)
- Experiment 2 Calibration of Volumetric Glassware PDFDokument22 SeitenExperiment 2 Calibration of Volumetric Glassware PDFAfiqah Rahah100% (1)
- RTS Chemistry SPM Question Bank Chapter 10Dokument8 SeitenRTS Chemistry SPM Question Bank Chapter 10Scorched ZenNoch keine Bewertungen
- Module Form 5 .Rate of ReactionDokument8 SeitenModule Form 5 .Rate of ReactionChew Gee Lan100% (1)
- Common Laboratory EquipmentDokument4 SeitenCommon Laboratory EquipmentMG Untalan BauzonNoch keine Bewertungen
- Magnesium Reacts With Dilute Hydrochloric Acid in A Conical Flask Which Is Connected To An Inverted Measuring Cylinder in A Trough of WaterDokument4 SeitenMagnesium Reacts With Dilute Hydrochloric Acid in A Conical Flask Which Is Connected To An Inverted Measuring Cylinder in A Trough of Waterวสุ เกตุสิริNoch keine Bewertungen
- Laboratory Manual For Engineering Chemistry Practical: First / Second Semester B.EDokument50 SeitenLaboratory Manual For Engineering Chemistry Practical: First / Second Semester B.EDaizy GillNoch keine Bewertungen
- Handbook Titration 6.0-MB PDF-EnglishDokument190 SeitenHandbook Titration 6.0-MB PDF-EnglishRahul100% (1)
- As and A Level Chemistry Core Practical 2 Solid Acid Solution (Student, Teacher, Technician Worksheets)Dokument5 SeitenAs and A Level Chemistry Core Practical 2 Solid Acid Solution (Student, Teacher, Technician Worksheets)TedNoch keine Bewertungen
- Experiment 3 CHM476Dokument10 SeitenExperiment 3 CHM476Hazwan Hamim100% (1)
- Rate of Reaction Bwat SendiriDokument4 SeitenRate of Reaction Bwat SendiriNor Ashikin IsmailNoch keine Bewertungen
- Standardization of Hydrochloric AcidDokument7 SeitenStandardization of Hydrochloric AcidDenise Chow86% (21)
- Chapter 10 No 9Dokument8 SeitenChapter 10 No 9Ain FzaNoch keine Bewertungen
- Chapter 10: Rate of ReactionDokument4 SeitenChapter 10: Rate of ReactionAin FzaNoch keine Bewertungen
- Effect of Surface Area On The Rate of ReactionDokument9 SeitenEffect of Surface Area On The Rate of Reactionhas65Noch keine Bewertungen
- 1.3 Rate of Reaction (1.2c)Dokument75 Seiten1.3 Rate of Reaction (1.2c)Sha Tasha Natasha0% (1)
- Chemistry Extended Essay Final DraftDokument7 SeitenChemistry Extended Essay Final DraftLynn SleimanNoch keine Bewertungen
- Effect of Surface Area On The Rate of ReactionDokument10 SeitenEffect of Surface Area On The Rate of ReactionsuffyandaimNoch keine Bewertungen
- Surface Area: Experiment 1.1: To Investigate The Effect of The of A Reactant On The Rate of ReactionDokument75 SeitenSurface Area: Experiment 1.1: To Investigate The Effect of The of A Reactant On The Rate of ReactionRakesh NairNoch keine Bewertungen
- Chemistry Report (Size of Reactant)Dokument3 SeitenChemistry Report (Size of Reactant)Ain NajwaNoch keine Bewertungen
- Rate of Reaction Lab (Marble Chips)Dokument1 SeiteRate of Reaction Lab (Marble Chips)Naldo 64Noch keine Bewertungen
- Experiment 1.1Dokument2 SeitenExperiment 1.1marlina4Noch keine Bewertungen
- Experiment 3 - KineticsDokument7 SeitenExperiment 3 - Kineticsdiyana a.fNoch keine Bewertungen
- Chemistry SPM Potential Questions-Form5chap1 2Dokument15 SeitenChemistry SPM Potential Questions-Form5chap1 2EloiseCalaisNoch keine Bewertungen
- Gabrielle Robinson - 601 Labs 2021Dokument13 SeitenGabrielle Robinson - 601 Labs 2021Gabrielle RobinsonNoch keine Bewertungen
- Example Planning Experiment Form 5Dokument14 SeitenExample Planning Experiment Form 5Victoria PetrusNoch keine Bewertungen
- Chemistry Internal AssessmentDokument11 SeitenChemistry Internal AssessmentTinesh GovindarajooNoch keine Bewertungen
- Effect of Catalyst On The Rate of Reaction Teacher's GuideDokument8 SeitenEffect of Catalyst On The Rate of Reaction Teacher's GuideFia RafiahNoch keine Bewertungen
- Unit 1 Manual 2019Dokument18 SeitenUnit 1 Manual 2019JozelleNoch keine Bewertungen
- As Practical Calculations Worksheets - RedDokument14 SeitenAs Practical Calculations Worksheets - RedKeniel YaoNoch keine Bewertungen
- VARIATION OF MICROSTRUCTURE WITH CARBONATION in Lime and Cement Blended PastesDokument32 SeitenVARIATION OF MICROSTRUCTURE WITH CARBONATION in Lime and Cement Blended PastesSaurav BhattacharjeeNoch keine Bewertungen
- Experiment 3Dokument8 SeitenExperiment 3Luxemberg NgNoch keine Bewertungen
- JURNAL8 Ayu 011Dokument7 SeitenJURNAL8 Ayu 011Ayu SuwarniNoch keine Bewertungen
- Myp 4 Criteria B and C Summative AssessmentDokument7 SeitenMyp 4 Criteria B and C Summative AssessmentBRIGHTON ONYANGONoch keine Bewertungen
- Exp 2Dokument9 SeitenExp 2Naduni RanasingheNoch keine Bewertungen
- Chem LabsDokument13 SeitenChem LabsArya SinghNoch keine Bewertungen
- 7.1 Measuring The Rate of ReactionDokument33 Seiten7.1 Measuring The Rate of ReactionCikFasyareena MaoNoch keine Bewertungen
- Enthalpy of Decomposition (Final)Dokument8 SeitenEnthalpy of Decomposition (Final)Mirfayz RuzimurodovNoch keine Bewertungen
- Rate of Reaction : Amount of Product Reactant TimeDokument4 SeitenRate of Reaction : Amount of Product Reactant TimeSubesh ShanmugamNoch keine Bewertungen
- Rate of Reaction Part 1Dokument3 SeitenRate of Reaction Part 1Subesh ShanmugamNoch keine Bewertungen
- Chemistry P3 0003Dokument9 SeitenChemistry P3 0003Karoki Francis KagombeNoch keine Bewertungen
- (M1.2) REVIEW & COMPLETE - Practical 4.1 The Kinetics of The Reaction Between CaCO3 and HCLDokument3 Seiten(M1.2) REVIEW & COMPLETE - Practical 4.1 The Kinetics of The Reaction Between CaCO3 and HCLSalma ShakiraNoch keine Bewertungen
- 64edf0f4e41caDokument6 Seiten64edf0f4e41caDanzell JonathanNoch keine Bewertungen
- The Effects of Dissolved Sodium Chloride (Nacl) On Well Injectivity During Co2 Storage Into Saline AquifersDokument20 SeitenThe Effects of Dissolved Sodium Chloride (Nacl) On Well Injectivity During Co2 Storage Into Saline AquifersMuhammad FatchurroziNoch keine Bewertungen
- Wood Ash 5Dokument11 SeitenWood Ash 5r;rNoch keine Bewertungen
- Determination of The Valency of MagnesiumDokument7 SeitenDetermination of The Valency of MagnesiumJiaxinOoiNoch keine Bewertungen
- Rates of ReactionDokument77 SeitenRates of ReactionFatema KhatunNoch keine Bewertungen
- Lab ReportDokument4 SeitenLab ReportTISHANPRAKASH A/L SIVARAJAH MoeNoch keine Bewertungen
- Lab Report 2 Water of HydrationDokument4 SeitenLab Report 2 Water of HydrationsayaanaNoch keine Bewertungen
- My TestDokument33 SeitenMy TestqusaielnoorNoch keine Bewertungen
- Mariah Campbell Chemistry Assignment 4-2-1Dokument3 SeitenMariah Campbell Chemistry Assignment 4-2-1Mariah CampbellNoch keine Bewertungen
- G10 Labs Sep 2023Dokument21 SeitenG10 Labs Sep 2023dejonae651Noch keine Bewertungen
- Design Lab 2Dokument3 SeitenDesign Lab 2Protiti MondalNoch keine Bewertungen
- ChemistryLPB Answers 230212 150001Dokument45 SeitenChemistryLPB Answers 230212 150001T. RodolfoNoch keine Bewertungen
- Science Form 2 Set 5: Sample Task ReportDokument3 SeitenScience Form 2 Set 5: Sample Task ReportcnidNoch keine Bewertungen
- Quality Control Baking Soda Lab ReportDokument22 SeitenQuality Control Baking Soda Lab ReportKatrina Le100% (6)
- Activity 6.4 PG 152 F4 (Experiment Test Book)Dokument3 SeitenActivity 6.4 PG 152 F4 (Experiment Test Book)leftwingNoch keine Bewertungen
- Ib Chemistry Answers ToolDokument19 SeitenIb Chemistry Answers ToolGeorges FarahNoch keine Bewertungen
- Solubility of Stibnite Ore in HCL Solutions: Sabri Colak, and Sinan YapiciDokument8 SeitenSolubility of Stibnite Ore in HCL Solutions: Sabri Colak, and Sinan YapiciAbdullah UYSALNoch keine Bewertungen
- Chemistry - Higher Level: Pre-Leaving Certificate Examination, 2020 Triailscrúdú Na Hardteistiméireachta, 2020Dokument12 SeitenChemistry - Higher Level: Pre-Leaving Certificate Examination, 2020 Triailscrúdú Na Hardteistiméireachta, 2020Diaa SaberNoch keine Bewertungen
- Chemistry Form 5 Chapter 1 - Rate of ReactionDokument63 SeitenChemistry Form 5 Chapter 1 - Rate of ReactionSiti Nursyafiqah100% (7)
- Tiffany Gayapersad 4S Group 2Dokument2 SeitenTiffany Gayapersad 4S Group 2Tiffany GayapersadNoch keine Bewertungen
- Labarotary Report 1Dokument7 SeitenLabarotary Report 1Nor FazilahNoch keine Bewertungen
- Practical 5: Determination of SugarsDokument11 SeitenPractical 5: Determination of SugarsHazuwanaNoch keine Bewertungen
- Determine Total Acid Content: Physical and Chemical Analysis ReportDokument10 SeitenDetermine Total Acid Content: Physical and Chemical Analysis ReportNguyễn ThuNoch keine Bewertungen
- ChemistryDokument10 SeitenChemistrySabbir AhmmedNoch keine Bewertungen
- 1-4 Concentrate On Keeping Up Your StandardsDokument21 Seiten1-4 Concentrate On Keeping Up Your Standardsmaryamshahzad489Noch keine Bewertungen
- LaboratoryapparatussespptDokument39 SeitenLaboratoryapparatussespptMapeh EditingNoch keine Bewertungen
- Demonstration of Glassware Used in Microbiology LaboratoryDokument14 SeitenDemonstration of Glassware Used in Microbiology LaboratorySujoy Tontubay100% (1)
- 5070 s17 QP 41Dokument12 Seiten5070 s17 QP 41Waleed Bin AhmadNoch keine Bewertungen
- Iso 8655 1 2002Dokument9 SeitenIso 8655 1 2002Ahmad KhreisatNoch keine Bewertungen
- Chemistry Lab Report - Titration With Antacid TabletDokument8 SeitenChemistry Lab Report - Titration With Antacid TabletSubesh Shanmugam100% (1)
- Chem Project AntacidDokument20 SeitenChem Project Antacidapi-371218886% (7)
- Titration Excellence: Downloaded From Manuals Search EngineDokument34 SeitenTitration Excellence: Downloaded From Manuals Search EnginejonahNoch keine Bewertungen
- Titration and Colourimetry CourseworkDokument4 SeitenTitration and Colourimetry CourseworkantoniaNoch keine Bewertungen
- Fresenius 2008T Dialysis System - Maintenance ProceduresDokument67 SeitenFresenius 2008T Dialysis System - Maintenance ProceduresJair Contessoti JuniorNoch keine Bewertungen
- Chemistry Practical 2022 - XIIDokument21 SeitenChemistry Practical 2022 - XIIAayanurNoch keine Bewertungen
- Chemistry Project Part 2Dokument58 SeitenChemistry Project Part 2Shubham BaghelNoch keine Bewertungen
- Chemistry Project: On Acid Content of Fruit JuicesDokument21 SeitenChemistry Project: On Acid Content of Fruit JuicesSagar KumarNoch keine Bewertungen
- 22 Georgescu C 10 19Dokument6 Seiten22 Georgescu C 10 19Cristian-Catalin GavatNoch keine Bewertungen
- Unit 4 - Review On Basic Principles Applied in Analytical ChemistryDokument12 SeitenUnit 4 - Review On Basic Principles Applied in Analytical ChemistryJayson PolinarNoch keine Bewertungen
- Laboratory Apparatus and Their FunctionsDokument7 SeitenLaboratory Apparatus and Their FunctionsLinzieeNoch keine Bewertungen
- T860 Auto Titrator HanonDokument1 SeiteT860 Auto Titrator Hanonstr_ilaNoch keine Bewertungen
- Experiment 1 - Numbers and MeasurementDokument8 SeitenExperiment 1 - Numbers and MeasurementPatricia Ann JoseNoch keine Bewertungen
- Guidelines For Proper Reading of The Retort Condensed Liquid Meniscus in A J TubeDokument7 SeitenGuidelines For Proper Reading of The Retort Condensed Liquid Meniscus in A J TubeWajid NizamiNoch keine Bewertungen