Beruflich Dokumente
Kultur Dokumente
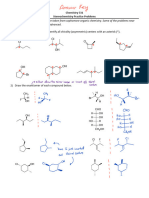
Isomerism
Hochgeladen von
Shivam GuptaCopyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Isomerism
Hochgeladen von
Shivam GuptaCopyright:
Verfügbare Formate
Isomerism
Introduction
The existence of two or more compounds with same molecular formula but different properties (physical, chemical or both) is
known as isomerism; and the compounds themselves are called isomers. The term was given by Berzelius. The difference in
properties of two isomers is due to the difference in the arrangement of atoms within their molecules. Isomerism may be of two
types:
Structural isomerism
When the isomers differ only in the arrangement of atoms or groups within the molecule, without any reference to space, these are
known as structural isomers
and the phenomenon as
structural isomerism.
Thus the structural isomers have the same molecular formula, but possess different structural formulae. Structural isomerism may
again be of several types.
(i) Chain, nuclear or skeleton isomerism
This type of isomerism is
due to the difference in the nature of the carbon chain (i.e. straight or branched)
which forms the nucleus of the molecule, e.g.,
(ii) Position isomerism
It is due to the difference in the position of the substituent atom or group or an unsaturated linkage in the same carbon chain.
Examples are
(iii) Functional isomerism
This type of isomerism is due to difference in the nature of functional group present in the isomers, e.g.,
(iv) Metamerism
It is due to the difference in nature of alkyl groups attached to the same functional group. This type of isomerism is shown by
compounds of the same homologous series. For example,
(v) Tautomerism
Tautomerism may be defined as the phenomenon in which a single compound exists in two readily interconvertible structures that
differ markedly in the relative position of at least one atomic nucleus, generally hydrogen. The two different structures are known
as tautomers of each other.
Sometimes the term tautomerism is also called as desmotropism (Greek desmos-bond; tropos-turn), since the interconversion of
the two forms involves a change of bonds or dynamic isomerism as the two forms are in dynamic equilibrium with each other.
Other names for tautomerism are kryptomerism, allelotropism or merotropy; however, tautomerism is the most widely accepted
term.
There are several types of tautomerism of which keto-enol tautomerism is the most important. In this type, one form (tautomer)
exists as a ketone while the other exists as an enol. The two simplest examples are of acetone and phenol.
However, the most widely studied example of keto-enol tautomerism is that of acetoacetic ester (ethyl acetoacetate).
Isomerism
The two forms are readily interconvertible by acid or base catalysts, and under ordinary conditions surface of the glass is sufficient
to catalyse the interconversion. The exact composition of the equilibrium depends upon the nature of the compound, solvent,
temperature, etc. The conversion of a keto form into enol from is known as enolisation. The two forms of acetoacetic ester have
been isolated under suitable conditions.
Keto-enol tautomerism in acetoacetic ester is proved by the fact that under ordinary conditions the compound gives the propert ies
of the ketonic group as well as that of the enolic group.
Note that in all the examples of keto-enol tautomerism the two isomeric forms are interconvertible by the migration of a proton
from one atom (carbon) to the other with the simultaneous shifting of bonds.
Remember that keto-enol tautomerism is possible only in those aldehydes and ketones which have at least one a -hydrogen
atom which can convert the ketonic group to the enolic group. Examine the following compounds.
Alkyl cyanides (RCN) and alkyl isocyanides (RNC) are also examples of tautomerism.
Similarly, nitro compounds also show tautomerism.
Distinction of tautomerism from resonance :
The tautomeric forms are quite chemically distinct entities and can be separated (in suitable cases e.g. acetoacetic ester) and
characterised. On the other hand, resonating forms differ only in the distribution of electrons and can never be separated fr om one
another since neither of them has any real existence. The important differences between resonance and tautomerism can be
summarised as below.
1. Tautomerism involves a change in the position of atom (generally hydrogen), while resonance involves a change in the pos ition
of the unshared or
only.
2. Tautomers are definite compounds and may be separated and isolated. Resonating structures are only imaginary and cant be
isolated.
3. The two tautomeric forms have different structures (i.e. functional groups). The various resonating structures have the same
functional group.
4. Tautomers are in dynamic equilibrium with each other, resonating structures are not in dynamic equilibrium.
5. Tautomerism has no effect on bond length, while resonance affects the bond length (single bond is shortened while the double
bond becomes longer).
Isomerism
6. Tautomerism does not lower the energy of the molecule and hence does not play any role in stabilising the molecule, while
resonance decreases the energy and hence increases the stability of the molecule.
8. Tautomerism can occur in planar as well as non-planar molecules, while resonance occurs only in planar molecules.
Distinction of tautomerism from isomerism. In fact there is no sharp line of distinction between isomers and tautomers since
some substances which are isomers under normal conditions can be converted into tautomeric forms under more drastic conditions.
For example, propyl and iso-propyl bromides are isomeric compounds under normal conditions but form an equilibrium mixture on
heating at 250C in a sealed tube.
And hence dynamic isomerism is a better term for this phenomenon than tautomerism.
Distinction of tautomerism from molecular rearrangement. Although there is no sharp difference between tautomerism and
molecular rearrangement, yet the two can be distinguished by the fact that the former is a rapid and reversible phenomenon
whereas the latter is neither reversible nor rapid.
Stereo isomerism
When isomers have the same structural formula but differ in relative arrangement of atoms or groups in space within the molecule,
these are known as stereoisomers and the phenomenon as stereoisomerism. The spatial arrangement of atoms or groups is also
referred to as configuration of the molecule and thus we can say that the stereoisomers have the same structural formula but
different configuration. Stereoisomerism is of two types.
(i) Geometrical isomerism
The isomers which possess the same structural formula but differ in the spatial arrangement of the groups around the double
bond are known as geometrical isomers and the phenomenon is known as geometrical isomerism. This isomerism is shown by
alkenes or their derivatives. When similar groups lie on the same side, it is the cis-isomer; while when the similar groups lie on
opposite sides, the isomer is trans. For example,
Remember that geometrical isomerism is possible only when each of the doubly bonded carbon atom has two different groups (see
examples above). Thus compounds of the following type will not show geometrical isomerism.
Distinction between cis -and trans- isomers. (a) Generally, the cis-isomer (e.g. maleic acid) cyclises on heating to form the
corresponding anhydride while the trans-isomer does not form its anhydride at all.
(b) The cis-isomer of a symmetrical alkene (alkenes in which both the carbon atoms have similar groups) has a definite dipole
moment, while the trans-isomer has zero dipole moment. For example, 1, 2-dichloroethylene and butene-2.
In trans-isomer of the symmetrical alkenes, the effect produced in one half of the molecule is cancelled by that in the other half of
the molecule.
In case of unsymmetrical alkenes, the cis-isomer has higher dipole moment than the corresponding trans-isomer. For example,
The E and Z Nomenclature of Geometrical Isomers. As discussed earlier, the geometrical isomerism is possible in structures of
the following three types.
In the first two types, the geometrical isomers are labelled as cis and trans on the basis of the fact that the common groups are on
the same or opposite sides of the double bond. But in type 3 where all the four substituents are different, cis-trans type of
isomerism cannot be applied. Moreover, the cis-trans system (also syn-anti system in oximes) is often ambiguous because the
cofigurational descriptions have not been defined according to any general and clear set of rules. So an unambiguous system of
configurational assignments for all types of structures showing geometrical isomerism was developed in 1968. This system is
known as E-Z system of nomenclature and is based upon the sequence rules of Cahn, Ingold and Prelog originally developed for
naming optical isomers on the R-S system. The following procedure is followed in specifying the configuration of such
compounds.
i) Assign the priority order to the two groups attached to each of the doubly bonded carbon
atoms in accordance with the sequence rules. Sequence rules are for determining the priority
order to atoms or groups attached to doubly bonded carbon atoms.
(a) Higher priority is assigned to atoms (directly attached to the carbon atom) of higher
atomic number.
(b) If isotopes of the same element are attached, the isotope with higher mass number will
have a higher priority. If the priority cannot be decided by this rule, it is then determined by
comparing the next atom in the group and so on.
(c) A doubly or triply bonded atom is considered equivalent to two or three such atoms. Thus
a carbonyl group is considered as if carbon has two single bonds with oxygen, i.e.,
By the application of these rules some common substituents have been given the following
priority sequence:-
(ii) Select the atom/group with higher priority on each doubly bonded carbon. If the
atoms/groups of higher priority (denoted by 1) on each carbon are on the same side of the
double bond, the isomer is assigned the configuration Z (from the German word, zusammen
meaning together). On the other hand, if the atoms/groups of higher priority on each carbon
are on the opposite sides of the double bond, the isomer is assigned the configuration E (from
the German word entgegen meaning against).
Now let us consider the example of an alkene in which one of the doubly bonded carbon
atoms has Br and I and the other has F and Cl. Now since I has a higher atomic number than
Br, it is assigned higher priority (1); similarly Cl is of higher priority than F on the second
olefinic carbon atom. Thus the E and Z configuration of the two isomers of 1-bromc-2-
chloro-2-fluoro-1-iodoethene are assigned as below.
Thus the cis- and trans-isomers of 2-butene become Z-and E-2-butenes respectively.
Similarly, following structures are assigned to the configuration mentioned below them.
Aromatic aldoximes and aromatic ketoximes also show geometrical isomerism. In aldoximes,
when H and OH groups are on the same side, the isomer is known as syn (analogous to cis)
and when these groups are on the opposite sides, the isomer is known as
anti
(analogous to
trans).
In ketoximes the prefixes syn and anti indicate which group of ketoxime is syn (on the same
side) or anti (on the opposite sides) to the OH group. For example,
However, remember that all aromatic ketoximes do not show geometrical isomerism e.g.,
(C
6
H
5
)
2
C = NOH, (benzophenone oxime) having two similar aryl groups does not show
geometrical isomerism.
Interconversion of Cis- Trans isomers
The cis and trans isomers of alkenes do not interconvert under ordinary conditions because of
, bond strength of 68 kcal/mole. This amount of energy is available only at high temprature or
with ultraviolet light, so these two isomers exist as stable compounds at room temprature.
The geometrical isomers can be interconverted if energy of more than 68 kcal/mole (the
energy), is applied by heat or uv light; then the
is broken and allows free rotation to occur about the carbon carbon
Interconversion of double bond diastereomers can also be brought Via epoxidation
deoxygenation sequence. The nucleophile attack by phosphours regents example, triphenyl
phosphine at the oxirane carbon leads to inversion of configuration and yields a charge
separated intermediate (a betaine). This undergoes elimination Via a four center cyclic
transition state which requires a 180 rotation around the C C bond to establish the
appropriate geometry. Therefore, if these are cis in the oxirane they become trans in the
alkene.
Conversion of cis into trans or vice versa by heat or uv medium or by free radical initiator is
known as stereomutation. In the presence of free radical double bond first gets converted into
single bond then free rotation around this single bond results in inversion of configuration.
Finally, regeneration of double bond occurs.
Geometrical isomerism also occurs in some saturated cyclic diols, di halide and di carboxylic
acids.
(ii) Optical isomerism
This type of isomerism arises from different arrangements of atoms or groups in three
dimensional space resulting in two isomers which are mirror image of each other. Optical
isomers contain an asymmetric (chiral) carbon atom ( a carbon atom attached to four
different atoms or groups) in their molecules.
For example, lactic acid having four different groups on the central carbon atom is
optically active;
while succinic acid having two similar atoms on the central carbon atom is
optically inactive.
Optical isomers have similar chemical and physical properties and differ only in their
behaviour towards plane polarised light
. The isomer which rotates the plane polarised light to left is known as
laevo (l)
while that which rotates the plane polarised light to the right is known as
dextro (d).
For example,
Note that thedandl
forms of a compound are
nonsuperimposible mirror image of each other
and such pairs are known as
enantiomorphs
or
enantiomers. A compound can exist in enantiomeric forms if it has an asymmetric carbon
atom and is devoid of the elements of symmetry, viz. (i)
a plane of symmetry, (ii) a centre of symmetry and (iii) an alternating axis of symmetry. If a
molecule possesses any of the above elements of symmetry, it is symmetrical; on the other
hand, if it does not possess either of these elements of symmetry, it is asymmetric and hence
is optically active and can exist ind
andlforms.
The number of optical isomers in a molecule containing
n
number of
different asymmetric carbon atoms
is given by the relation 2
n
. Furtermore, there will be 2
n1
pairs of enantiomer and the same number of racemic modifications.
Racemic modification is an equimolecular mixture of d and l forms of the
same compound. The process of converting d or l form of an optically active compound
into dl
(racemic)
form is known as racemisation.
Since the rotation of
d
is cancelled by equal but opposite rotation of
l
, racemic mixture
(r)
is always optically inactive. This type of optical inactivity is known
as optical inactivity due to external compensation
. Now sincedl mixture(rform) can be separated intodandlform
(resolution)
, optical activity can be restored in therform.
The number of optical isomers in a compound containing
n
number of similar asymmetric carbon atoms is always less than 2
n
.
The classical and most important example is
tartaric acid,
CH(OH)COOH.CH(OH)COOH which can exist in the following isomeric forms.
(i)
dTartaric acid.
It rotates the plane polarised light to the right. The rotation due to the upper half is
strengthened by rotation due to the lower half. It has no plane of symmetry.
(ii)
l Tartaric acid
. It rotates the plane polarised light to left. Here again rotation due to upper half is
strengthened by rotation due to lower half. It also has no plane of symmetry. The
d and
l
tartaric acids are mirrorimage of each other
(enantiomers).
(iii)
r Tartaric acid.
It is equimolecular mixture of the
d andl
forms and hence optically inactive (eg.r
lactic acid) due to external compensation.
(iv)
m
Tartaric acid. It possesses a plane of symmetry
(denoted by dotted line) and hence superimposes on its mirror image (i.e., they are identical)
and hence it is optically inactive. The optical inactivity is said to be due to
internal compensation
as the rotation due to the upper half of the molecule is balanced by the equal but opposite
rotation due to the lower half. The
meso
isomer cannot be resolved into active (d andl ) isomers (difference from racemic tartaric
acid).
Isomerism
Remember that stereoisomers which are not mirror image (enantiomers) are known as diastereomers or diastereoisomers. Thus
m tartaric acid constitutes the diastereomer of d as well as of l tartaric acid.
Prediction of number of optical isomers
(i) When the molecule is unsymmetrical
Number of d and l isomers (a) = 2
n
(active)
Number of meso forms (m) = 0
Where n is the number of chiral carbon atom (s).
Common example is CH
3
.CHBr.COOH 2
1
= 2
(ii) When the molecule is symmetrical and has even number of chiral carbon atoms
Number of d and l isomers (a) = 2
(n1)
Number of meso forms (m) = 2
(n/2 1)
Common example is tartaric acid, HOOC. CHOH. CHOH.COOH
(iii) When the molecule is symmetrical and has an odd number of chiral carbon atoms.
Number of d and l forms (a) = 2
(n1)
2
(n/2 )
Number of meso forms (m) = 2
(n/2 )
Optical Isomerism in compounds containing no chiral carbon atom
As described earlier that the basic requirement for a compound to be optically active is its nonsuperimposibility of its mirror
image. Although the largest number of known optically active compounds are optically active due to the presence of chiral car bon
atom, some compounds are also known which do not possess any chiral carbon atom, but on the whole their molecules are chiral
(such molecules were earlier called dissymmetric); hence they are optically active. Various types of compounds belonging to t his
group are allenes, alkylidene cycloalkanes, spiro compounds (spiranes) and properly substitut ed biphenyls.
Allenes. Allenes are the organic compounds of the following general formulae.
Allenes exhibit optical isomerism provided the two groups attached to each terminal carbon atom are different, i.e.
Alkylidenes cycloalkanes and spiro compounds. When one or both of the double bonds in allenes are replaced by one and two
rings, the resulting systems are respectively known as alkylidene cycloalkanes and spiranes.
Biphenyls. Suitably substituted diphenyl compounds are also devoid of individual chiral carbon atom, but the molecules are chiral
due to restricted rotation around the single bond between the two benzene nuclei and hence they must exist as two non
superimposible mirror images of each other. Such type of stereoisomerism which is due to restricted rotation about single bond, is
known as atropisomerism and the stereoisomers are known as atropisomers. Examples,
Racemic Mixture or Racemic Modification
As described earlier, a racemic modification is an equimolecular mixture of a pair of enantiomers, i.e., (+) and () forms and is
denoted by Racemic mixture is generally obtained in the following two ways.
(i) By mixing equal amounts of the two enantiomers.
(ii) By synthesis. The synthesis of a chiral compound from achiral compound in the absence of optically active agent or circularly
polarised light always produces a racemic modification. For example, the formation of lactonitrile from acetaldehyde always
results in a racemic modification in the following manner:-
Resolution
Separation of dlmixture of a compound into d and l isomers is known as resolution. This can be done by several methods, viz.
mechanical, biochemical and chemical method. Chemical method involves the formation of diastereomers and is found to be the
best method for resolution.
Walden inversion (Optical inversion). The conversion of dform of an optically active compound into lform of the same or of
different compound or viceversa is known as Walden inversion or optical inversion (P. Walden in 1895). For example, d
malic acid when treated with PCl5 gives l chlorosuccinic acid, i.e., inversion in configuration has taken place. The l
chlorosuccinic acid may also be converted back to malic acid with or without change in configuration which actually depends upon
the nature of the reagent.
Thus among other factors, nature of the reagent plays an important role in Walden inversion. It has been observed that mild or
weak reagent like Ag2O do not cause Walden inversion while strong reagents like KOH and PCl5 cause Walden inversion.
Remember that Walden inversion follows SN2 mechanism which involves the inversion of configuration while SN1 mechanism
involves racemisation.
Das könnte Ihnen auch gefallen
- Gamsat Chemistry Sample Questions PDFDokument6 SeitenGamsat Chemistry Sample Questions PDFBandita DattaNoch keine Bewertungen
- Tricks of Isomerism in Coordination Compounds Chemistry For NEET & JEE 2019Dokument5 SeitenTricks of Isomerism in Coordination Compounds Chemistry For NEET & JEE 2019misostudy0% (1)
- Isomerism WorksheetDokument42 SeitenIsomerism WorksheetChinmay100% (2)
- PMR Spectroscopy: Solved Problems Volume : IIVon EverandPMR Spectroscopy: Solved Problems Volume : IIBewertung: 5 von 5 Sternen5/5 (3)
- 531 - Stereochem Practice KeyDokument4 Seiten531 - Stereochem Practice KeyAli MuhtashamNoch keine Bewertungen
- 11 AllDokument28 Seiten11 AllEdson EmidioNoch keine Bewertungen
- Solved Example: Chemistry For Neet & AiimsDokument24 SeitenSolved Example: Chemistry For Neet & AiimsAnup KNoch keine Bewertungen
- GOC Practice Assignment CHCDokument26 SeitenGOC Practice Assignment CHCjanviNoch keine Bewertungen
- Isomerism Page # 3Dokument52 SeitenIsomerism Page # 3RameshKumarNoch keine Bewertungen
- Structural IsomerismDokument9 SeitenStructural IsomerismJue MayaNoch keine Bewertungen
- 04 IsomerismDokument19 Seiten04 IsomerismSoham RaneNoch keine Bewertungen
- JEE (Main+Advanced) 2023: Chem. Worksheet-5 IupacDokument2 SeitenJEE (Main+Advanced) 2023: Chem. Worksheet-5 IupacSoham X ANoch keine Bewertungen
- Carbonyl Compound WorksheetDokument25 SeitenCarbonyl Compound WorksheetOmendra SinghNoch keine Bewertungen
- Isomerism Sheet - by NJ Sir PDFDokument42 SeitenIsomerism Sheet - by NJ Sir PDFVikas Rana100% (2)
- Part - I: Objective Questions: Section A: Geometrical IsomerismDokument10 SeitenPart - I: Objective Questions: Section A: Geometrical IsomerismTejas pawarNoch keine Bewertungen
- Iit Jam Chemistry Core2014Dokument8 SeitenIit Jam Chemistry Core2014Mahendra GanuboyinaNoch keine Bewertungen
- PRACTICE SHEET - 03 (Chemistry) : CH - CH - CH CN CN CNDokument3 SeitenPRACTICE SHEET - 03 (Chemistry) : CH - CH - CH CN CN CNABD 17Noch keine Bewertungen
- Dipole Moments in Organic CHEMISTRYDokument18 SeitenDipole Moments in Organic CHEMISTRYBalraj Dhillon100% (2)
- Isomerism: One or More Than One Answer Type Questions: 1. Which One of The Following Pairs of Isomers Are EnentiomersDokument11 SeitenIsomerism: One or More Than One Answer Type Questions: 1. Which One of The Following Pairs of Isomers Are EnentiomerskamalNoch keine Bewertungen
- Ape Assignment 3Dokument7 SeitenApe Assignment 3Atharva KulkarniNoch keine Bewertungen
- CH CH CHCH CH H CH CH CH CH CH CH H CH: Byvineet Khatri SirDokument13 SeitenCH CH CHCH CH H CH CH CH CH CH CH H CH: Byvineet Khatri Sirsarvesh goyalNoch keine Bewertungen
- 13 DPP 03g Goc Excel Acid+BaseDokument5 Seiten13 DPP 03g Goc Excel Acid+BasekljNoch keine Bewertungen
- Carbonyl CompoundsDokument10 SeitenCarbonyl CompoundsMahendra ChouhanNoch keine Bewertungen
- Reaction Mechanism PDFDokument14 SeitenReaction Mechanism PDFSreeragNoch keine Bewertungen
- Alkene DPPDokument20 SeitenAlkene DPPKalyan ReddtNoch keine Bewertungen
- BOC Complete 1 To 5 DPPDokument5 SeitenBOC Complete 1 To 5 DPPBhawna SharmaNoch keine Bewertungen
- Aldol Reaction - ChemistryDokument7 SeitenAldol Reaction - ChemistryGamer HelperNoch keine Bewertungen
- GRiGNARD REAGENT!!Dokument22 SeitenGRiGNARD REAGENT!!GazalNoch keine Bewertungen
- Assignment: Organic ChemistryDokument6 SeitenAssignment: Organic ChemistryWalid EbaiedNoch keine Bewertungen
- Navneet Jethwani Geometrical Optics: Organic ChemistryDokument40 SeitenNavneet Jethwani Geometrical Optics: Organic ChemistrySubhrota Pradhan100% (1)
- Cls Jeead-18-19 Xii Che Target-7 Set-2 Chapter-12Dokument47 SeitenCls Jeead-18-19 Xii Che Target-7 Set-2 Chapter-12DxNoch keine Bewertungen
- Isomerism: IndexDokument38 SeitenIsomerism: IndexDEV UPPALNoch keine Bewertungen
- DPP Module-1 01JA To 06JA OrganicDokument21 SeitenDPP Module-1 01JA To 06JA OrganicAkkaldevi Saivinayak CRNoch keine Bewertungen
- 0optical Isomerism - QuizDokument3 Seiten0optical Isomerism - QuizSanjay Mani Tripathi50% (2)
- IIT JEE Main Advanced Physical Chemistry 12th Solid State PDFDokument29 SeitenIIT JEE Main Advanced Physical Chemistry 12th Solid State PDFKalpana Saravana KumarNoch keine Bewertungen
- Quantitative and QualitativeDokument15 SeitenQuantitative and QualitativesquadralsupremeNoch keine Bewertungen
- DPP 1 Optical Isomerism VKP Sir-3706Dokument3 SeitenDPP 1 Optical Isomerism VKP Sir-3706Sanjay Mani TripathiNoch keine Bewertungen
- 6.DAY-8 CHE - Organic Chemistry Electron Migration Effects & Reagents - 25-05-2020 PDFDokument7 Seiten6.DAY-8 CHE - Organic Chemistry Electron Migration Effects & Reagents - 25-05-2020 PDFRamakrishna ReddyNoch keine Bewertungen
- Reduction, Oxidation - Hydrolysis Exercise PDFDokument24 SeitenReduction, Oxidation - Hydrolysis Exercise PDFGOURISH AGRAWAL100% (3)
- Isomerism DPPDokument4 SeitenIsomerism DPPRAGHUL MNoch keine Bewertungen
- Carbocation RearrangementDokument4 SeitenCarbocation RearrangementManas J. AggarwalNoch keine Bewertungen
- Atomic Structure IITDokument16 SeitenAtomic Structure IITAdiChemAdi69% (13)
- Substitution - EliminationDokument36 SeitenSubstitution - EliminationSachin SinghalNoch keine Bewertungen
- A - 1 (Isomerism, Reaction Mechanism) - Question PaperDokument11 SeitenA - 1 (Isomerism, Reaction Mechanism) - Question PaperSachin DedhiaNoch keine Bewertungen
- 3.AcidBases FinalDokument35 Seiten3.AcidBases FinalSoham RaneNoch keine Bewertungen
- Carboxylic Acid and Amines Worksheet PDFDokument22 SeitenCarboxylic Acid and Amines Worksheet PDFd anjilappaNoch keine Bewertungen
- Alkyl Halides and Aryl Halides - QBDokument23 SeitenAlkyl Halides and Aryl Halides - QBNETHAKANI SUJATHA100% (1)
- Coordination Compounds 19-06-2020Dokument6 SeitenCoordination Compounds 19-06-2020Vanshaj GuptaNoch keine Bewertungen
- Goc & Eas Test-IiDokument7 SeitenGoc & Eas Test-IiAniket GuptaNoch keine Bewertungen
- Exercise # 1: Career Point Pre-Medical (Topic Wise MCQ)Dokument20 SeitenExercise # 1: Career Point Pre-Medical (Topic Wise MCQ)Sanjay Mani TripathiNoch keine Bewertungen
- Electrochemistry IPEDokument18 SeitenElectrochemistry IPEAdiChemAdi100% (3)
- 03ElectronicdisplacementEffects Exercise Send1Dokument33 Seiten03ElectronicdisplacementEffects Exercise Send1Aaryan Keshan100% (1)
- Answers of Organic Chemistry DPP For GOC (Conceptual Improvement of GOC)Dokument5 SeitenAnswers of Organic Chemistry DPP For GOC (Conceptual Improvement of GOC)Krishna SinglaNoch keine Bewertungen
- Solution - Colligative Properties Solutions PDFDokument25 SeitenSolution - Colligative Properties Solutions PDFGOURISH AGRAWALNoch keine Bewertungen
- Alcohol, Phenol and EtherDokument35 SeitenAlcohol, Phenol and EtherSubhrota PradhanNoch keine Bewertungen
- ISI R: Organic ChemistryDokument28 SeitenISI R: Organic Chemistrysarvesh goyalNoch keine Bewertungen
- Aromaticity (2) IIT JAM PDFDokument3 SeitenAromaticity (2) IIT JAM PDFDIKSHA SARASWATNoch keine Bewertungen
- Jee TSC Iupac Nomenclature Etoos Sheet PDFDokument16 SeitenJee TSC Iupac Nomenclature Etoos Sheet PDFShadow100% (1)
- Mole Concept 2Dokument38 SeitenMole Concept 2R S.NagiNoch keine Bewertungen
- R IS IR: Iupac & NomenclatureDokument11 SeitenR IS IR: Iupac & NomenclatureDhruv KuchhalNoch keine Bewertungen
- IsomerismDokument3 SeitenIsomerismhaqfazal2007Noch keine Bewertungen
- Nucleophilic SubstitutionDokument18 SeitenNucleophilic SubstitutionShivam GuptaNoch keine Bewertungen
- Common Names and Geneva SystemDokument26 SeitenCommon Names and Geneva SystemShivam GuptaNoch keine Bewertungen
- Jee MainDokument23 SeitenJee MainKrishnagopal KunduNoch keine Bewertungen
- Fundamental Organic ChemistryDokument42 SeitenFundamental Organic ChemistryShivam GuptaNoch keine Bewertungen
- Anims and UreaDokument25 SeitenAnims and UreaShivam GuptaNoch keine Bewertungen
- Alkyl HalidesDokument20 SeitenAlkyl HalidesShivam Gupta0% (1)
- Al KynesDokument16 SeitenAl KynesShivam GuptaNoch keine Bewertungen
- Group 6-ADokument9 SeitenGroup 6-AShivam GuptaNoch keine Bewertungen
- Alkanes Paraffins: Preparation (I) From Grignard ReagentDokument25 SeitenAlkanes Paraffins: Preparation (I) From Grignard ReagentShivam GuptaNoch keine Bewertungen
- Alkadienes & Its PropertiesDokument24 SeitenAlkadienes & Its PropertiesShivam Gupta67% (3)
- Aldehyde and KetonesDokument45 SeitenAldehyde and KetonesShivam GuptaNoch keine Bewertungen
- Alcohols, Diols, TriolsDokument32 SeitenAlcohols, Diols, TriolsShivam GuptaNoch keine Bewertungen
- Principle of Extraction of MetalsDokument28 SeitenPrinciple of Extraction of MetalsShivam Gupta100% (1)
- Group 1-ADokument14 SeitenGroup 1-AShivam GuptaNoch keine Bewertungen
- Concave Mirrors TeacherDokument5 SeitenConcave Mirrors TeacherShivam GuptaNoch keine Bewertungen
- IIT Maths Sample Paper 2: AlgebraDokument2 SeitenIIT Maths Sample Paper 2: AlgebraShivam GuptaNoch keine Bewertungen
- Curriculum Vitae: Shubhanshu MehrotraDokument3 SeitenCurriculum Vitae: Shubhanshu MehrotraShivam GuptaNoch keine Bewertungen
- CHEM 210 Chapter 5 Wrap-UpDokument27 SeitenCHEM 210 Chapter 5 Wrap-UpTuan NguyenNoch keine Bewertungen
- Organic Isomers Multiple Choice QuestionsDokument3 SeitenOrganic Isomers Multiple Choice QuestionsrajaijahNoch keine Bewertungen
- Stereoisomerism: Review of ConceptsDokument25 SeitenStereoisomerism: Review of Concepts丁文婷Noch keine Bewertungen
- SterioChemistry IitDokument19 SeitenSterioChemistry IitRanjiv GuptaNoch keine Bewertungen
- ws16 Diastereomers Professor Jennifer Poutsma PDFDokument4 Seitenws16 Diastereomers Professor Jennifer Poutsma PDFSankar AdhikariNoch keine Bewertungen
- CHM 241-Practice Exam FinalDokument12 SeitenCHM 241-Practice Exam FinalPreeti SharmaNoch keine Bewertungen
- Hydrocarbons One Shot BouncebackDokument172 SeitenHydrocarbons One Shot BouncebackHarishNoch keine Bewertungen
- 化學奧林匹亞冬令營 有機3 2018Dokument119 Seiten化學奧林匹亞冬令營 有機3 2018楊泰萱Noch keine Bewertungen
- Chiral Chromatography 1998 - Scott & BeesleyDokument552 SeitenChiral Chromatography 1998 - Scott & Beesleytutuncucanan100% (1)
- KnowItAll's ChemWindow ® EditionDokument9 SeitenKnowItAll's ChemWindow ® EditiondnajenNoch keine Bewertungen
- Komlexemaster 7 SemDokument49 SeitenKomlexemaster 7 SemRaheem SimsNoch keine Bewertungen
- 18.09.22 SR - Star Co-Sc (Model-B) Jee Main Ptm-1 QPDokument18 Seiten18.09.22 SR - Star Co-Sc (Model-B) Jee Main Ptm-1 QPDeeip DNoch keine Bewertungen
- Organic Chemistry 4th Edition Smith Test Bank 1Dokument15 SeitenOrganic Chemistry 4th Edition Smith Test Bank 1carolyn100% (47)
- Aromatic CompoundsDokument6 SeitenAromatic CompoundsSANIA FAJAR KHANNoch keine Bewertungen
- Chapter 3Dokument43 SeitenChapter 3Jacquelyn GuintoNoch keine Bewertungen
- Chem 261 Exam 1 S10Dokument4 SeitenChem 261 Exam 1 S10rrf7Noch keine Bewertungen
- Stereo ChemistryDokument57 SeitenStereo ChemistryNehalPatelNoch keine Bewertungen
- Isomerism in Biomolecules PDFDokument2 SeitenIsomerism in Biomolecules PDFfakhribabiker100% (2)
- SP StereochemistryDokument63 SeitenSP StereochemistryMadhumitha KatreddyNoch keine Bewertungen
- Organicchem ProbsetsDokument132 SeitenOrganicchem ProbsetskimyNoch keine Bewertungen
- EXPERIMENT #7: Steam Distillation of Essential Oils: TLC Analysis and Stereoisomerism ObjectivesDokument7 SeitenEXPERIMENT #7: Steam Distillation of Essential Oils: TLC Analysis and Stereoisomerism ObjectivesAji SarosaNoch keine Bewertungen
- Stereo IsomerismDokument24 SeitenStereo IsomerismKrishna ThakurNoch keine Bewertungen
- Isomer Bansal InstituteDokument36 SeitenIsomer Bansal InstituteVanshaj GuptaNoch keine Bewertungen
- Stereochirality R or SDokument52 SeitenStereochirality R or SnifafaniNoch keine Bewertungen
- US8242315Dokument19 SeitenUS8242315梅汉Noch keine Bewertungen
- Stereochemistry CHM456Dokument82 SeitenStereochemistry CHM456notmeNoch keine Bewertungen