Beruflich Dokumente
Kultur Dokumente
c1
Hochgeladen von
promitiamOriginaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
c1
Hochgeladen von
promitiamCopyright:
Verfügbare Formate
CHAPTER 2
CHLORINATEDSOLVENT CHEMISTRY: STRUCTURES,
NOMENCLATURE AND PROPERTIES
David M. Cwiertny
1
and Michelle M. Scherer
2
1
University of California at Riverside, Riverside, CA 92521;
2
The University of Iowa, Iowa
City, IA 52242
2.1 INTRODUCTION
This chapter summarizes the principles of chlorinated solvent remediation, provides over-
views of the biotic and abiotic reactions that can transform and detoxify these compounds, and
discusses the remediation challenges posed by the properties and behavior of these compounds
in the subsurface environment.
2.2 STRUCTURE AND NOMENCLATURE
Chlorinated solvents are organic compounds generally constructed of a simple hydrocar-
bon chain (typically one to three carbon atoms in length) to which at least one chlorine atom is
covalently bonded. For the current discussion, chlorinated solvents will be further divided into
three categories based upon common structural characteristics: chlorinated methanes, chlori-
nated ethanes and chlorinated ethenes. Examples from each solvent class are shown in
Figure 2.1. Additional information pertaining to the nomenclature of these chemical species
is provided in Table 2.1.
Chlorinated methanes represent the most structurally simple solvent class and consist of
a single carbon center (known as a methyl carbon) to which as many as four chlorine atoms
are covalently bonded. From the perspective of groundwater contamination, perhaps the
most well known chlorinated methane is carbon tetrachloride (CT). Also known by its
International Union of Pure and Applied Chemistry (IUPAC) name of tetrachloromethane,
CT consists of a fully chlorinated methyl carbon. By IUPAC conventions, the modifier of
tetra serves as an indicator of the number of chlorine atoms bound to the carbon center. For
chlorinated methanes other than CT, hydrogen atoms usually make up the remainder of the
substituents necessary to satisfy the methyl carbons bonding requirements. Named in a
similar fashion by IUPAC, the chlorinated methanes with a lower degree of halogenation are
trichloromethane (commonly referred to as chloroform [CF]), dichloromethane (DCM, more
commonly called methylene chloride [MC]) and chloromethane (CM, also referred to as
methyl chloride).
Chlorinated ethanes consist of two carbon centers joined by a single covalent bond.
Common groundwater pollutants from this class include 1,1,1-trichloroethane (1,1,1-TCA) and
1,2-dichloroethane. In regards to the nomenclature associated with chlorinated ethanes, a
similar convention to that used for chlorinated methanes is employed in which the prefix
attached to chloroethane indicates the total number of chlorine atoms on the solvent
molecule. Common acronyms for this class follow the pattern in which the first letter (or series
of letters) refers to the number of total halogen substituents (e.g., T for trichloro- or Te for
H.F. Stroo and C.H. Ward (eds.), In Situ Remediation of Chlorinated Solvent Plumes,
doi: 10.1007/978-1-4419-1401-9_2, #Springer Science+Business Media, LLC 2010
29
tetrachloro-), the second letter refers to the halogen identity (e.g., C for chlorine) and the last
letter, in all cases A, refers to ethane.
In addition, the numbers preceding the name or abbreviation indicate the location of the
chlorine substituents on the two possible carbon centers. For example, 1,1,2,2-tetrachloroethane
(1,1,2,2-TeCA) possesses two chlorine atoms on each of its carbon centers, whereas the three
chlorine atoms of 1,1,1-TCA are all located on the same carbon. In certain instances, there can
be more than one way in which the same number of chlorine atoms distribute themselves on the
carbon centers, as is the case for 1,1,2-TCA and 1,1,1-TCA. These compounds, which share the
same chemical formula (C
2
H
3
Cl
3
) yet differ in the sequence in which their atoms are connected,
are referred to as structural isomers (Vollhardt and Schore, 1994).
Chlorinated ethenes (sometimes referred to as chlorinated ethylenes) also possess two
carbon centers, but unlike chlorinated ethanes, these carbon atoms are joined by a carbon-
carbon double bond known as a p-bond (pi-bond) system. Another important difference
between chlorinated ethanes and chlorinated ethenes is the maximum number of atoms
bound to the carbon centers in each case. The double-bonded carbon centers in chlorinated
ethenes can accommodate at most two halogen (or hydrogen) substituents, whereas the single-
bonded ethanes can accommodate three halogen (or hydrogen) substituents.
Examples of chlorinated ethenes that are important groundwater pollutants include tetra-
chloroethene, commonly referred to as perchloroethene (PCE), and trichloroethene (TCE).
Another chlorinated ethene of note is the monochlorinated species that is most commonly
referred to as vinyl chloride (VC). The nomenclature associated with the chlorinated ethenes
follows a similar convention to that used with the chlorinated methanes and ethanes
H
Cl
Cl
H
C
Cl
Cl
Cl
Cl
C
Cl
H H
H
C C
Cl
H
H
H
Cl
Cl
C C
Cl H
Cl Cl
C C
Cl
Cl Cl
C C
Cl
Cl
H
H
Cl
Cl
C C
Cl
dichloromethane
(DCM)
carbon tetrachloride
(CT)
1,1,1 - trichloroethane
(1,1,1-TCA)
vinyl chloride
(VC)
1,1,2,2 - tetrachloroethane
(1,1,2,2-TeCA)
trichloroethene
(TCE)
perchloroethene
(PCE)
Figure 2.1. Chemical structures of some common chlorinated solvents.
30 D.M. Cwiertny and M.M. Scherer
(e.g., tetrachloroethene contains four chlorine substituents). The same is true for the acronyms
commonly applied to this solvent class, only this time the last letter in all cases is E, which
represents ethenes. The lone exception to this convention for acronyms is vinyl chloride,
which is typically abbreviated as VC.
Additional nomenclature is necessary in order to distinguish the possible isomers of
dichloroethene. As with 1,1,1-TCA and 1,1,2-TCA, dichloroethene (DCE) can exist as either of
two structural isomers (1,1-DCE and 1,2-DCE). In addition, the p-bond system in chlorinated
ethenes differs from the single carbon-carbon bond in chlorinated ethanes because it does not
allow the halogen substituents to rotate freely in the plane perpendicular to the direction of the
p-bond. Consequently, there are multiple spatial orientations for the two chloride substituents
in 1,2-dichloroethene (Figure 2.2). One possibility is for the chlorine atoms to arrange them-
selves on the same side of the carbon-carbon double bond in a configuration known as cis.
Alternatively, the chlorine atoms can be located on the opposite side of the p-bond system in a
configuration known as trans. These two dichloroethenes, which are structurally identical but
differ in the spatial arrangement of their chlorine substituents, are called conformational
isomers (or simply conformers) (Vollhardt and Schore, 1994).
Table 2.1. Nomenclature for Selected Chlorinated Solvents
IUPAC Name Common Name Abbreviation/Acronym Molecular Formula
Chlorinated Methanes
tetrachloromethane carbon tetrachloride CT CCl
4
trichloromethane chloroform CF CHCl
3
dichloromethane methylene chloride DCM CH
2
Cl
2
chloromethane methyl chloride CM CH
3
Cl
Chlorinated Ethanes
hexachloroethane perchloroethane HCA C
2
Cl
6
pentachloroethane ---- PCA C
2
HCl
5
1,1,1,2-tetrachloroethane ---- 1,1,1,2-TeCA C
2
H
2
Cl
4
1,1,2,2-tetrachloroethane ---- 1,1,2,2-TeCA C
2
H
2
Cl
4
1,1,2-trichloroethane ---- 1,1,2-TCA C
2
H
3
Cl
3
1,1,1-trichloroethane methyl chloroform 1,1,1-TCA C
2
H
3
Cl
3
1,2-dichloroethane ---- 1,2-DCA C
2
H
4
Cl
2
1,1-dichloroethane ---- 1,1-DCA C
2
H
4
Cl
2
chloroethane ---- CA C
2
H
5
Cl
Chlorinated Ethenes
tetrachloroethene perchloroethene PCE C
2
Cl
4
trichloroethene ---- TCE C
2
HCl
3
cis-1,2-dichloroethene cis-dichloroethene cis-DCE C
2
H
2
Cl
2
trans-1,2-dichloroethene trans-dichloroethene trans-DCE C
2
H
2
Cl
2
1,1-dichloroethene vinylidene chloride 1,1-DCE C
2
H
2
Cl
2
chloroethene vinyl chloride VC C
2
H
3
Cl
Chlorinated Solvent Chemistry: Structures, Nomenclature and Properties 31
Chlorinated methanes, ethanes and ethenes clearly do not encompass all types of chlori-
nated solvents that may be encountered at hazardous waste sites. For instance, chlorinated
propanes, which possess three carbon atoms joined by single bonds, can represent important
groundwater pollutants. Some examples of chlorinated propanes include 1,2-dichloropropane,
which is regulated in drinking water by the U.S. Environmental Protection Agency (USEPA)
(2003). Another example is 1,2,3-trichloropropane, which has been detected at more than 20
National Priorities List sites identified by the USEPA (ATSDR, 1992). Although such species
are not the focus of subsequent portions of this chapter, the physical and chemical principles
developed for chlorinated methanes, ethanes and ethenes can easily be extended to include
these additional chlorinated solvents.
Although this chapter is devoted to treatment strategies for chlorinated solvents, solvents
with other halogen substituents (such as bromine or fluorine) are also frequently encountered in
contaminated groundwater. A common example is 1,2-dibromoethane (also known as ethylene
dibromide [EDB]), which was used as an additive in leaded gasoline (Baird and Cann, 2005).
Methanes, ethanes and ethenes with mixed halogen substituents can represent important
environmental pollutants as well, as is the case for common disinfection byproducts bromodi-
chloromethane (CHBrCl
2
) and dibromochloromethane (CHBr
2
Cl). When necessary, key differ-
ences in the behavior and environmental fate of halogenated solvents with chlorine, bromine
and fluorine substituents will be noted.
2.3 PROPERTIES
The behavior of chlorinated solvents in the subsurface is controlled to a large extent by their
physical and chemical properties. The properties considered most relevant to chlorinated
solvent fate and transport in the subsurface are summarized in Table 2.2. In order to maintain
some consistency among the values presented, the majority of the values were obtained from
Mackay et al. (1993), one of the very few sources that contain data for all of the chlorinated
methanes, ethanes and ethenes. In general, there is reasonable agreement between these values
and several other summary tables available (e.g., Pankow and Cherry, 1996; Fetter, 1999;
Schwarzenbach et al., 2003; Chapter 1 of this volume). Table 2.2 is provided for purposes of
discussion with regards to relevant trends in behavior and properties and is not intended as a set
of values selected from a critical review of the literature. For a review of the primary literature,
Pankow and Cherry (1996) is recommended because it provides a detailed review of the
chlorinated solvent properties discussed herein as well as an excellent discussion of the history
of production and industrial uses of chlorinated solvents.
The following discussion of chemical and physical properties is organized around the major
processes that impact the fate and transport of chlorinated solvents in the subsurface, starting
with the process by which pure phase chlorinated solvents dissolve into groundwater, followed
by their partitioning between the three phases present in the subsurface: aquifer solids, water
and air. An overview linking these partitioning processes to the relevant chlorinated solvent
properties is provided in Figure 2.3. The discussion concludes with an introduction to trans-
formation reactions, which are discussed in greater detail in Chapters 3 and 4.
H
Cl Cl
H
C C
H
Cl H
Cl
C C
cis-DCE trans-DCE
Figure 2.2. Conformational isomers of 1,2-dichloroethene.
32 D.M. Cwiertny and M.M. Scherer
2.3.1 Dissolution
At room temperature (25 degrees Celsius [
C]), most chlorinated solvents are colorless
liquids with densities (r) greater than that of water (r
solvent
> 1 gram per liter [g/L]).
Table 2.2. Summary of Some Physical and Chemical Properties of Chlorinated Organic Solvents at
25DegreesCelsius(
C). Unlessotherwisenoted, all valueshavebeentakenfromMackayet al. (1993).
Species
Formula
Weight
(g/mol)
Carbon
Oxidation
State
a
Density
(r) (g/mL)
Solubility (S)
(mg/L)
Vapor
Pressure
(p
o
) (torr)
Henrys
Law
Constant
(K
H
) ( 10
-3
atmm
3
/mol)
Log
(K
ow
)
Log
(K
oc
)
b
MCL
c
(mg/L)
Chlorinated Methanes
CT 153.8 +IV 1.59 800 153.8 28.9 2.64 1.9 0.005
CF 119.4 +III 1.49 8,200 196.8 3.8 1.97 1.52 0.10
d
DCM 84.9 +II 1.33 13,200 415 1.7 1.25 ---- 0.005
CM 50.5 +I 0.92 5,235 4,275 9.6 0.91 ---- NR
e
Chlorinated Ethanes
HCA 236.7 +III 2.09 50 0.38
f
---- 3.93 ---- NR
PCA 202.3 +II 1.68 500 4.7 2.5 2.89 ---- NR
1122-TeCA 167.9 +I 1.60 2,962 5.9 0.44 2.39 1.9 NR
1112-TeCA 167.9 +I 1.54 1,100 11.9 2.4 ---- ---- NR
111-TCA 133.4 0 1.35 1,495 123.8 14.5 2.49 2.25 0.2
112-TCA 133.4 0 1.44 4,394 24.2 0.96 2.38 ---- 0.005
12-DCA 99.0 -I 1.25 8,606 79.0 1.2 1.48 1.52 0.005
11-DCA 99.0 -I 1.17 4,676 227 6.2 1.79 ---- NR
CA 64.5 -II 0.90 5,700 120 1.8 1.43 ---- NR
Chlorinated Ethenes
PCE 165.8 +II 1.63 150 18.1 26.3 2.88 2.29 0.005
TCE 131.4 +I 1.46 1,100 74.2 11.7 2.53 1.53 0.005
cis-DCE 96.9 0 1.28 3,500 203 7.4 1.86 ---- 0.07
trans-DCE 96.9 0 1.26 6,260 333 6.8 1.93 ---- 0.1
11-DCE 96.9 0 1.22 3,344 604 23.0 2.13 ---- 0.007
VC 62.5 -I 0.91 2,763 2,660 79.2 1.38 ---- 0.002
a
Average value calculated using oxidation states for H I and Cl I.
b
When available, log(K
oc
) values were obtained from Nguyen et al. (2005).
c
Source: USEPA (2003).
d
MCL for total trihalomethanes, which is defined as the summed concentration of chloroform, bromoform (CHBr
3
),
bromodichloromethane (CHBrCl
2
), and dibromochloromethane (CHBr
2
Cl).
e
NR Not regulated.
f
Reported vapor pressure for solid-phase hexachloroethane.
Notes: atm -- atmosphere; g -- gram; K
ow
-- octanol/water partitioning coefficient; K
oc
-- soil organic carbon/water
partitioning coefficient; L -- liter; MCL -- maximum contaminant level; mg -- milligram; mL -- milliliter; mol -- mole.
Water
Soil Air
K
H
p
S, K
OW
, K
OC
p
S
@
Figure 2.3. The three major phases present in the subsurface and the properties of chlorinated
solvents that govern the partitioning between these phases.
Chlorinated Solvent Chemistry: Structures, Nomenclature and Properties 33
Chlorinated solvents are typically discharged into the environment as pure organic liquids or as
mixtures of several organic liquids. The process through which these organic phases are
gradually released into groundwater is referred to as dissolution.
For a chlorinated solvent, the extent of dissolution is controlled by the solvents aqueous
solubility (S), defined as the maximum amount of a chlorinated solvent that will partition
into water at a given temperature (Lyman, 1982). Also referred to as saturation concentra-
tions (Schwarzenbach et al., 2003), aqueous solubilities are typically reported with units of
moles of chlorinated solvent per liter of water (molarity or M) or milligrams of chlorinated
solvent per liter of water (mg/L, which is equivalent to parts per million [ppm]). Most
chlorinated solvents can be classified as sparingly soluble in water, with aqueous solubilities
generally on the order of several tens to hundreds of mg/L (Table 2.2). However, their
aqueous solubilities are high relative to their established USEPA MCLs (Pankow and Cherry,
1996), which contributes to their prominence as groundwater pollutants. Another conse-
quence of their limited solubility is their tendency to occur in the subsurface as a separate
liquid phase at the base of an aquifer commonly referred to as dense nonaqueous phase
liquid (DNAPL).
Table 2.2 reveals the general solubility trend among chlorinated solvents- as the number
of chlorine atoms on a compound increases, the aqueous solubility of that species decreases.
This inverse relationship illustrates the influence that molecular size (specifically molar
volume [Horvath et al., 1999]) exerts on the miscibility of a chlorinated solvent in water.
Environmental variables also can influence chlorinated solvent solubility. One such variable is
temperature, although changes in the solubility of most chlorinated solvents are relatively
minor over environmentally relevant temperature ranges (Horvath, 1982). Another important
variable is salinity; an increased concentration of dissolved salts results in a moderate
decrease in chlorinated solvent solubility (Lyman, 1982). The presence of other organic
chemicals (referred to as co-solutes) also can increase the saturation concentration of
chlorinated solvents in water, behavior that is utilized for the treatment of chlorinated
solvents during surfactant-enhanced aquifer remediation (SEAR) (e.g., Pennell et al., 1994;
Fountain et al., 1996).
2.3.2 Solid-Water Partitioning
Partitioning of chlorinated solvents between aquifer solids and water plays an important
role in contaminant fate and treatability because it affects the rate of transport in the
subsurface. As a class, chlorinated solvents can be considered moderately hydrophobic;
although they partition (or sorb) onto aquifer solids, their affinity for such processes is not
as great as that for other organic pollutants such as polycyclic aromatic hydrocarbons (PAHs)
or polychlorinated biphenyls (PCBs).
A practical measure of a compounds hydrophobicity is the octanol-water partitioning
coefficient (K
ow
). For a two-phase system containing octanol and water, values of K
ow
are
defined as the equilibrium concentration of the chlorinated solvent in octanol relative to its
equilibrium concentration in water (Equation 2.1).
K
ow
C
octanol
C
water
(Eq. 2.1)
For laboratory investigations of hydrophobicity, octanol is chosen as a convenient reference
solvent because it is immiscible with water. By definition, large values of K
ow
correspond to
hydrophobic chemicals that are expected to sorb to soils and sediments more readily.
34 D.M. Cwiertny and M.M. Scherer
More pertinent for describing processes in the subsurface are values of K
oc
, which
represent a measure of a chemicals equilibrium partitioning between water and the organic
carbon fraction of aquifer solids (Equation 2.2).
K
oc
C
organic carbon
C
water
(Eq. 2.2)
Accordingly, a key factor controlling the extent of chlorinated solvent sorption is the
organic carbon content of the subsurface material and the dissolved organic matter. Often
times, values of K
oc
can be estimated using linear correlations developed between log(K
ow
) and
log(K
oc
) for a given pollutant class.
In Table 2.2, values of both K
ow
and K
oc
generally increase as the number of chlorine
substituents on a compound increases. These larger values of solid-water partitioning coeffi-
cients will result in slower rates of subsurface transport. An inverse relationship between
aqueous solubility and K
ow
(or K
oc
) values is also observed in Table 2.2; chemicals with limited
aqueous solubilities generally prefer to partition into a phase such as octanol or soil organic
matter rather than associate with water.
2.3.3 Air-Water Partitioning
Chlorinated solvents are relatively volatile compounds. Accordingly, air-water partitioning
is expected to take place when contaminated groundwater comes into contact with air, as is the
case in unsaturated subsurface zones (e.g., the vadose zone). In such instances, the equilibrium
partitioning between air and water is typically described by Henrys Law, which is applicable to
dilute solutions of a chlorinated solvent in water. The Henrys Law constant, K
H
, relates the
equilibrium concentration of the chlorinated solvent in air to its equilibrium concentration in
water (Equation 2.3).
K
H
C
air
C
water
(Eq. 2.3)
By definition, large K
H
values indicate a chemicals preference to partition from water into
air, although additional chemical properties and several environmental factors will also influ-
ence the volatility of a species (Thomas, 1982a).
In Table 2.2, K
H
values are reported with units of atmm
3
/mol, but K
H
values also are
commonly reported with alternative units that depend upon the conventions used to report the
chlorinated solvents concentrations in air and water. Unlike reported values of S, K
ow
and K
oc
,
the K
H
values presented in Table 2.2 do not reveal any significant trends within or across the
different classes of chlorinated solvents.
2.3.4 Solid-Air Partitioning
The last chlorinated solvent partitioning process to consider is that between aquifer solids
and air, a topic covered in detail by Thomas (1982b). As with volatilization between air and
water, several chemical and environmental factors are at play in solid-air partitioning processes
(Thomas, 1982b), but our mechanistic understanding of this process is rather limited. One
noteworthy variable is the vapor pressure (p
) of a chlorinated solvent, which represents the
maximum attainable concentration of a chlorinated solvent in air (Schwarzenbach et al., 2003).
Compounds with high values of p
(which has units of torr or atm) tend to partition more
Chlorinated Solvent Chemistry: Structures, Nomenclature and Properties 35
readily between air and sediments (and similarly, between air and water), and empirical
relationships have been developed to estimate the rates at which such partitioning processes
occur (Thomas, 1982b). Values of p
tend to decrease with increasing chlorination, although
exceptions to this generalization are frequently observed (e.g., compare the p
values for
chloroethane and 1,1,2-trichloroethane in Table 2.2).
2.3.5 Transformation Reactions
Not included in Figure 2.3 is an additional critical pathway that impacts chlorinated solvent
fate in groundwater, that of transformation reactions. Rates and products of transformation
reactions will depend upon many of the chemical and physical properties discussed above, as
well as the average oxidation state of carbon in the chlorinated solvent (Table 2.2). The carbon
oxidation state is a measure of the number of electrons associated with the carbon atoms
in a chlorinated solvent; this value ranges from I to +IV for the chlorinated solvents listed in
Table 2.2. The more negative the oxidation state, the more electrons associated with the carbon
atom. A positive oxidation state (e.g., carbon tetrachloride with a +IV) corresponds to a species
in a highly oxidized form that is prone to reduction (gaining electrons). On the other hand,
chlorinated solvents with more reduced carbon centers, such as vinyl chloride (C oxidation state
of I), are more susceptible to being oxidized (losing electrons).
From a practical sense, transformation reactions are often classified as either biotic or
abiotic. Biotic reactions are typically those that involve microbial processes associated with
bacterial metabolism, whereas abiotic reactions are defined as those processes that involve
another chemical species. The distinction, however, can become blurred when discussing
chemicals such as biological exudates or minerals formed as a direct result of microbial activity
or as an indirect result of biological modification of a chemical environment.
The classification does, however, provide a convenient organizational structure for dis-
cussing the principles of chlorinated solvent remediation, and it has been adopted for use by the
authors in Chapter 4. Chapter 3 discusses microbially driven processes, including cometabolic
reductive reactions, oxidative metabolism, and dehalorespiration. Chapter 4 describes the impor-
tant abiotic processes for chlorinated solvents, including sorption, volatilization and transforma-
tion reactions such as substitution, elimination, oxidation and reduction. Chapter 5 examines the
practical challenges for site remediation that result from the properties and behavior of
chlorinated solvents.
REFERENCES
ATSDR (Agency for Toxic Substances and Disease Registry). 1992. Toxicological profile
for 1,2,3-trichloropropane. U.S. Department of Health and Human Services ATSDR
Public Health Service, Atlanta, GA, USA. http://www.atsdr.cdc.gov/toxprofiles/tp57.pdf.
Accessed January 11, 2010.
Baird C, Cann M. 2005. Environmental Chemistry, 3rd ed. W.H. Freeman and Company,
New York, NY, USA. 652 p.
Fetter CW. 1999. Contaminant Hydrogeology, 2nd ed. Prentice-Hall, Inc., Upper Saddle River,
NJ, USA. 500 p.
Fountain JC, Starr RC, Middleton T, Beikirch M, Taylor C, Hodge D. 1996. A controlled field
test of surfactant-enhanced aquifer remediation. Ground Water 34:910916.
Horvath AL. 1982. Halogenated Hydrocarbons: Solubility-Miscibility with Water. Marcel
Dekker, New York, NY, USA. 889 p.
36 D.M. Cwiertny and M.M. Scherer
Horvath AL, Getzen FW, Maczynska Z. 1999. IUPAC-NIST solubility data series 67.
Halogenated ethanes and ethenes with water. J Phys Chem Ref Data 28:395628.
Lyman WJ. 1982. Solubility in Water. In Lyman WJ, Reehl WF, Rosenblatt DH, eds, Handbook
of Chemical Property Estimation Methods: Environmental Behavior of Organic
Compounds. McGraw-Hill, New York, NY, USA, pp 151.
Mackay D, Shiu WY, Ma KC. 1993. Illustrated Handbook of Physical-Chemical Properties and
Environmental Fate for Organic Chemicals. Lewis Publishers, Chelsea, MI, USA.
Nguyen TH, Goss K, Ball WP. 2005. Polyparameter linear free energy relationships for
estimating the equilibrium partition of organic compounds between water and the natural
organic matter in soils and sediments. Environ Sci Technol 39:913924.
Pankow JF, Cherry JA. 1996. Dense Chlorinated Solvents and Other DNAPLs in Groundwater:
History, Behavior, and Remediation. Waterloo Press, Portland, OR, USA. 525 p.
Pennell KD, Jin M, Abriola LM, Pope GA. 1994. Surfactant enhanced remediation of soil
columns contaminated by residual tetrachloroethylene. J Contam Hydrol 16:3553.
Schwarzenbach RP, Gschwend PM, Imboden DM. 2003. Environmental Organic Chemistry.
John Wiley & Sons, Inc., Hoboken, NJ, USA. 1313 p.
Thomas RG. 1982a. Volatilization from Water. In Lyman WJ, Reehl WF, Rosenblatt DH, eds,
Handbook of Chemical Property Estimation Methods: Environmental Behavior of Organic
Compounds. McGraw-Hill, New York, NY, USA, pp 15.115.34.
Thomas RG. 1982b. Volatilization from Soil. In Lyman WJ, Reehl WF, Rosenblatt DH, eds,
Handbook of Chemical Property Estimation Methods: Environmental Behavior of Organic
Compounds, McGraw-Hill, New York, NY, USA, pp 16.116.50.
USEPA (U.S. Environmental Protection Agency). 2003. Ground Water and Drinking Water.
National Primary Drinking Water Standards. EPA-816-F-03-016. USEPA, Office of Water,
Washington, DC, USA.
Vollhardt KPC, Schore NE. 1994. Organic Chemistry. W.H. Freeman and Company, New York,
NY, USA. 1156 p.
Chlorinated Solvent Chemistry: Structures, Nomenclature and Properties 37
http://www.springer.com/978-1-4419-1400-2
Das könnte Ihnen auch gefallen
- Schaum's Easy Outline of Organic Chemistry, Second EditionVon EverandSchaum's Easy Outline of Organic Chemistry, Second EditionBewertung: 3.5 von 5 Sternen3.5/5 (2)
- IB Topic 10: Organic Chemistry Practice QuestionsDokument36 SeitenIB Topic 10: Organic Chemistry Practice Questionshunarsandhu50% (2)
- Organic Chem Part 1 - Nomenclature ReadDokument5 SeitenOrganic Chem Part 1 - Nomenclature Readngcoboandiswa573Noch keine Bewertungen
- Organic Chemistry: Muh. Yanis Musdja The Study of The Compounds of CarbonDokument58 SeitenOrganic Chemistry: Muh. Yanis Musdja The Study of The Compounds of CarbonAgung Nugroho OteNoch keine Bewertungen
- Naming Organic CompoundsDokument22 SeitenNaming Organic CompoundsNovira ChandisaNoch keine Bewertungen
- Simple IUPAC NomenclatureDokument15 SeitenSimple IUPAC Nomenclatureapi-3757218100% (6)
- Types of HydrocarbonsDokument19 SeitenTypes of HydrocarbonsMoniko ShinneNoch keine Bewertungen
- New Microsoft Office Word DocumentDokument9 SeitenNew Microsoft Office Word DocumentPrince JoseNoch keine Bewertungen
- Naming Organic CompoundsDokument9 SeitenNaming Organic CompoundsRonikeNoch keine Bewertungen
- Organic NomenclatureDokument41 SeitenOrganic NomenclatureInventyourselfNoch keine Bewertungen
- Introduction To Organic ChemistryDokument12 SeitenIntroduction To Organic ChemistryPaul Nathaniel GwapoNoch keine Bewertungen
- Nomenclature Part 2Dokument27 SeitenNomenclature Part 2gcxnhmzNoch keine Bewertungen
- Naming Organic Compounds 1Dokument27 SeitenNaming Organic Compounds 1Vince C.Noch keine Bewertungen
- Alkanes To Alkynes and AromaticsDokument48 SeitenAlkanes To Alkynes and AromaticswallabooNoch keine Bewertungen
- Lecture 3 Organic ChemistryDokument10 SeitenLecture 3 Organic ChemistryAhmed ShakerNoch keine Bewertungen
- Chapter 15 Intro To OrganicDokument8 SeitenChapter 15 Intro To OrganicLisa DentonNoch keine Bewertungen
- Halogenated Hydrocarbon Structure and Chemistry: Jack DeruiterDokument10 SeitenHalogenated Hydrocarbon Structure and Chemistry: Jack DeruiterfreeeebooksNoch keine Bewertungen
- 12 Intro To OrganicDokument129 Seiten12 Intro To OrganicSyamil AdzmanNoch keine Bewertungen
- A2AS CHEM REVISED Support 20632Dokument4 SeitenA2AS CHEM REVISED Support 20632Cosmescu Mario FlorinNoch keine Bewertungen
- Functional Group Nomenclature & ReactionsDokument106 SeitenFunctional Group Nomenclature & Reactionsdang minh nhutNoch keine Bewertungen
- What Are The Essential Properties of An Ideal Refrigerant and Why Freon 12 Was BannedDokument5 SeitenWhat Are The Essential Properties of An Ideal Refrigerant and Why Freon 12 Was BannedAlexander MugabeNoch keine Bewertungen
- ALKANESDokument73 SeitenALKANESChona TuyNoch keine Bewertungen
- Greensfelder - Catalytic and Thermal Cracking of Pure Hydrocarbons (1949)Dokument12 SeitenGreensfelder - Catalytic and Thermal Cracking of Pure Hydrocarbons (1949)Thomas ChenNoch keine Bewertungen
- Trichloroisocyanuric Acid ApplicationsDokument10 SeitenTrichloroisocyanuric Acid Applicationsashbhave100% (2)
- Ter/atomic and Molecular Properties/Intermolecular Forces/Van Der Waals ForcesDokument6 SeitenTer/atomic and Molecular Properties/Intermolecular Forces/Van Der Waals ForcesIana Jane BuronNoch keine Bewertungen
- Chapter 4 - Carbon and Its CompoundsDokument15 SeitenChapter 4 - Carbon and Its Compoundsnorthamericaffid31Noch keine Bewertungen
- CH 03Dokument24 SeitenCH 03Ummi Khairani UrfaNoch keine Bewertungen
- Lech 201Dokument34 SeitenLech 201Roshan M DavNoch keine Bewertungen
- Organic ChemistryDokument44 SeitenOrganic ChemistryKushashwa Ravi ShrimaliNoch keine Bewertungen
- Chemistry 2Dokument77 SeitenChemistry 2Victor MutugiNoch keine Bewertungen
- HydrocarbonsDokument11 SeitenHydrocarbonsCornellius KurniawanNoch keine Bewertungen
- Module 5 Review of Basic Organic CompoundsDokument18 SeitenModule 5 Review of Basic Organic CompoundsBig BrotherNoch keine Bewertungen
- BASIC PRINCIPLES OF ORGANIC CHEMISTRY-1207010003142-phpapp02Dokument51 SeitenBASIC PRINCIPLES OF ORGANIC CHEMISTRY-1207010003142-phpapp02ayankhan207242786Noch keine Bewertungen
- Chapter 3 Alkanes and Their Stereochemistry-1-1Dokument23 SeitenChapter 3 Alkanes and Their Stereochemistry-1-1eas111Noch keine Bewertungen
- Ib Notes For Organic ChemistryDokument12 SeitenIb Notes For Organic ChemistryNazish AltafNoch keine Bewertungen
- ALKANADokument37 SeitenALKANAKhoirun NisyakNoch keine Bewertungen
- Naming Organic Compounds: The IUPAC Systematic Approach To NomenclatureDokument10 SeitenNaming Organic Compounds: The IUPAC Systematic Approach To NomenclatureMabelle DucusinNoch keine Bewertungen
- Chapter 15Dokument8 SeitenChapter 15Tilak K CNoch keine Bewertungen
- Samiran Pptonhydrocarbonforupload 150623185215 Lva1 App6891Dokument89 SeitenSamiran Pptonhydrocarbonforupload 150623185215 Lva1 App6891Israel EsmileNoch keine Bewertungen
- Naming Organic CompoundsDokument15 SeitenNaming Organic CompoundsEdward Estrella GuceNoch keine Bewertungen
- Grade 12 Chemistry Organic Chemistry I HydrocarbonsDokument92 SeitenGrade 12 Chemistry Organic Chemistry I Hydrocarbonsraadumar02Noch keine Bewertungen
- Alkanes, Alkenes, AlkynesDokument11 SeitenAlkanes, Alkenes, AlkynesRosalia JasmineNoch keine Bewertungen
- Chemguide - Answers: H-1 NMR: High ResolutionDokument2 SeitenChemguide - Answers: H-1 NMR: High ResolutionKhondokar TarakkyNoch keine Bewertungen
- Alkanes and CycloalkanesDokument17 SeitenAlkanes and CycloalkanesPeter ParkerNoch keine Bewertungen
- Essential Organic Chemistry: An Introduction To Organic CompoundsDokument101 SeitenEssential Organic Chemistry: An Introduction To Organic CompoundsJane Limsan PaglinawanNoch keine Bewertungen
- Topic 3 - AlkenesDokument16 SeitenTopic 3 - AlkenesRichard WalkerNoch keine Bewertungen
- 20.3 Aldehydes, Ketones, Carboxylic Acids, and EstersDokument5 Seiten20.3 Aldehydes, Ketones, Carboxylic Acids, and EstersAcieNoch keine Bewertungen
- Alkane-Full Notes FazliDokument47 SeitenAlkane-Full Notes Fazlijokowi123Noch keine Bewertungen
- Introduction To Organic ChemistryDokument27 SeitenIntroduction To Organic ChemistrySuryani JumatNoch keine Bewertungen
- OrganicDokument93 SeitenOrganicPatel MswaziNoch keine Bewertungen
- AlkenesDokument52 SeitenAlkeneszaharanuraaNoch keine Bewertungen
- 2: Alkanes and Cycloalkanes: PreviewDokument26 Seiten2: Alkanes and Cycloalkanes: PreviewDORINA MANTUNoch keine Bewertungen
- Alkanes, Alkenes, AlkynesDokument7 SeitenAlkanes, Alkenes, AlkynesMuhammad Hasnain AliNoch keine Bewertungen
- CHEM 332 Carbonyl Cpds Keto and AldsDokument16 SeitenCHEM 332 Carbonyl Cpds Keto and Aldsoyamo markNoch keine Bewertungen
- Nomenclature of AlcoholsDokument7 SeitenNomenclature of AlcoholsJuselle Faith AtaNoch keine Bewertungen
- Introduction To Organic Chemistry NotesDokument30 SeitenIntroduction To Organic Chemistry Notesمریم کیانی100% (1)
- Chem CHPT 6 Learning Module 2Dokument57 SeitenChem CHPT 6 Learning Module 2Patrick Joshua GregorioNoch keine Bewertungen
- Gas Hydrates 1: Fundamentals, Characterization and ModelingVon EverandGas Hydrates 1: Fundamentals, Characterization and ModelingDaniel BrosetaNoch keine Bewertungen
- Selected Constants: Oxidation–Reduction Potentials of Inorganic Substances in Aqueous SolutionVon EverandSelected Constants: Oxidation–Reduction Potentials of Inorganic Substances in Aqueous SolutionNoch keine Bewertungen
- Physical Chemistry of Polyelectrolyte SolutionsVon EverandPhysical Chemistry of Polyelectrolyte SolutionsMitsuru NagasawaNoch keine Bewertungen
- Impact of CovidDokument1 SeiteImpact of CovidpromitiamNoch keine Bewertungen
- Economic Performance of States in Post-Reforms Period: Pecial ArticlesDokument12 SeitenEconomic Performance of States in Post-Reforms Period: Pecial ArticlespromitiamNoch keine Bewertungen
- 6daysplit 0 PDFDokument1 Seite6daysplit 0 PDFSaul Antonio Espino VenturaNoch keine Bewertungen
- Pi Kit - Iim RaipurDokument48 SeitenPi Kit - Iim RaipurpromitiamNoch keine Bewertungen
- RegistrationDetail PDFDokument1 SeiteRegistrationDetail PDFpromitiamNoch keine Bewertungen
- ch2 3edDokument30 Seitench2 3edsaad aliNoch keine Bewertungen
- Pubdet Pumdet PaperDokument46 SeitenPubdet Pumdet PaperpromitiamNoch keine Bewertungen
- QP HY 09-10 - Class - 10Dokument59 SeitenQP HY 09-10 - Class - 10promitiamNoch keine Bewertungen
- SYMBIDokument146 SeitenSYMBIpromitiamNoch keine Bewertungen
- PaperSet Symbiosis Entrance Test (SET) Quantitative AptitudeDokument4 SeitenPaperSet Symbiosis Entrance Test (SET) Quantitative Aptitudepromitiam100% (1)
- PositionalPlay ExcerptDokument12 SeitenPositionalPlay ExcerptpromitiamNoch keine Bewertungen
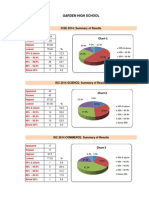
- Garden High School: ICSE 2014: Summary of ResultsDokument1 SeiteGarden High School: ICSE 2014: Summary of ResultspromitiamNoch keine Bewertungen
- Development of Tourism in India PDFDokument10 SeitenDevelopment of Tourism in India PDFpromitiam69% (13)
- English LanguageDokument21 SeitenEnglish LanguagepromitiamNoch keine Bewertungen