Beruflich Dokumente
Kultur Dokumente
Bipolar Membrane Prepared by Grafting and Plasma Polymerization
Hochgeladen von
saadiisCopyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Bipolar Membrane Prepared by Grafting and Plasma Polymerization
Hochgeladen von
saadiisCopyright:
Verfügbare Formate
Journal of Membrane Science 219 (2003) 113
Bipolar membrane prepared by grafting and plasma
polymerization
Chan-Li Hsueh
a
, Yu-Jen Peng
a
, Cheng-Chien Wang
b,
, Chuh-Yung Chen
a,
a
Department of Chemical Engineering, National Cheng-Kung University, Tainan 70101, Taiwan
b
Department of Chemical Engineering, Southern Taiwan University of Technology, Tainan 710, Taiwan
Received 9 September 2002; received in revised form 10 December 2002; accepted 20 February 2003
Abstract
Ce
4+
-initiated and plasma-induced grafting polymerization was used to prepare bipolar membranes. The former method
used the membrane of copoly(2-hydroxylethylene-methacrylate (2-HEMA)/n-butylacrylate (BA)/glycidyl-methacrylate
(GMA)) on the surfaces of which were grafted acrylic acid (AA: COO
) and 4-vinyl pyridine (4VP: NH
+
Cl
), to prepare
the bipolar membrane. The latter method utilized the porous PVDF membrane as a substrate, onto one side of which AA (or
sodium vinyl sulfonate, SVS) monomer was grafted, and onto the other of which, 4-vinyl pyridine (or N
,N-dimethyl amino
ethyl acrylate, DMAEA) monomer was grafted. The contact angle of bipolar membranes dramatically decreased when the
ionic polymer covered the surface of the membranes by plasma-grafting polymerization. Meanwhile, the swelling level of
bipolar membranes was in the range of 2550%. Furthermore, the cross-section SEM photographs of bipolar membranes
further illustrated a sandwich structure that consisted of anionic polymer, cationic polymer and PVDF substrate. The limiting
current increased with the diffusion coefcient and the concentration of electrolysis; however, the critical voltage was inde-
pendent of the concentration and the kind of electrolysis. The 963 of AAPVDFDMAEA bipolar membrane exhibited the
best ratio of dissociation rate constants (k
d
/k
0
d
) of all membranes. Results concerning the efciency of the current showed
plasma-induced preparation of a bipolar membrane is better than Ce
4+
-initiated preparation.
2003 Elsevier Science B.V. All rights reserved.
Keywords: Bipolar membrane; Plasma; Limiting current
1. Introduction
A bipolar ion-exchange membrane (BM) is a poly-
meric layered structure composed of a cation ion-
exchange layer joined in series to an anion ion-exchange
layer. As the electric current passed, the cations mi-
grated through the cation-exchange membrane to the
cathode and the anions permeated the anion-exchange
membrane to the anode. Water splitting did not oc-
cur within the bipolar membrane until the current
Corresponding authors.
E-mail address: cc7@ccmail.ncku.edu.tw (C.-C. Wang).
intensity reached the critical value (limiting current
density). The current versus voltage curves and the
limiting current density of water splitting indicated
the performance of the bipolar membrane [15]. The
proton (H
+
) and hydroxide (OH
) ions generated
by the splitting of the water migrated to the anode
and cathode, respectively. Then, an alkaline solution
was formed on the anion-exchange side and an acidic
solution was formed on the cation-exchange side
of the bipolar membrane. A bipolar membrane has
been produced bonding heterogeneous membranes,
containing strongly acidic groups and strongly basic
groups, together by pressing and heating (Filter Corp.,
0376-7388/$ see front matter 2003 Elsevier Science B.V. All rights reserved.
doi:10.1016/S0376-7388(03)00106-6
2 C.-L. Hsueh et al. / Journal of Membrane Science 219 (2003) 113
New York, USA). The use of bipolar membranes to
produce the protons and hydroxyl ions thus became
an interesting and economically attractive process
[610], especially to control pollution and regenerate
acids and alkalis in the chemical industry. For exam-
ple, Allied-Signal Co. Ltd. developed the Aquatech
[1113] system, in which the splitting of water via a
bipolar membrane was used for industrial applications,
including deacidication, regeneration of waste liquid
in photography, surface treatment of metals, recycling
of metal waste water, separation and renement of
organic substance and removal of waste gas and pollu-
tant, and others. However, the high electric resistance
of the membrane limited its development. Krsy and
Zeigerson [14] suggested the use of a mono casting
sheet to overcome the shortcoming of high electric
resistance. Accordingly, the sandwich-like bipolar
membranes with both an anion-exchange sheet and
a cation-exchange sheet were prepared. Yokoyama
et al. [15] synthesized a bipolar membrane from a sin-
gle membrane, by grafting monomers with opposite
electric charges onto the porous polymer membrane
using plasma-induced graft polymerization.
With respect to commercial applications, a bipo-
lar membrane has high selectivity, low potential drop,
acid and base resistance, solvent resistance, a wide pH
range and good mechanical strength. However, even
though its electric conductivity can be increased by in-
creasing exchange capacity and the hydrophilicity of
the membrane, the dilation of the membrane is limited
by the mechanical properties and chemical stability of
the membrane.
Bipolar membranes were thus designed to opti-
mize ion-exchange capacity, dilation and mechanical
strength. Therefore, two methods, grafting polymer-
ization and plasma-induced grafting polymerization,
to prepare bipolar membranes in this study. The for-
mer method used the copolymer of n-butylacrylate
(BA), 2-hydroethylene-methacrylate (2-HEMA) and
glycidyl-methacrylate (GMA) as the matrix of the
membrane, acrylic acid (AA) was grafted onto one
side, and 4-vinyl pyridine (4VP) onto the other side
of the membranes to prepare bipolar membranes. The
second method used plasma-induced polymerization
to graft the AA (or sodium vinyl sulfonate, SVS) and
4-vinyl pyridine (or N
,N-dimethyl amino ethyl acry-
late, DMAEA) onto the surface of the porous PVDF
membrane.
2. Experimental
2.1. Preparing bipolar membrane by solution
grafting polymerization
Acrylic acid, 2-hydroxylethylene-methacrylate,
n-butylacrylate, and glycidyl-methacrylate were
puried by distillation before use. Reagent-grade
azoisobutylnitrile (AIBN) and dimethylformamide
(DMF) (Fluka) were used without further purication.
Reagent-grade water was obtained from a Waters
Millipore purifying system. The reaction temperature
was well controlled using a thermostat and water
bath. The substrate of the membrane was synthe-
sized from l2.5 g 2-HEMA, 1.25 g GMA, and 12.5 g
BA with 0.7 g AIBN initiator in DMF solution. The
prepared aqueous solution with 0.5 g AA monomer,
0.1 g Ce
4+
activator and 10 ml water was poured onto
the top of the polymer membrane, to prepare the
cation-exchange membrane, by grafting polymeriza-
tion at 60
C for 6 h. Then, the anion-exchange mem-
brane was prepared similarly by pouring the aqueous
solution of 0.5 g 4VP monomer and 0.1 g Ce
4+
(am-
monium cerium(IV) nitrate: Merck) activator onto the
other side of the membrane.
2.2. Preparing bipolar membrane by plasma-induced
grafting polymerization
Fig. 1 schematically the equipment employed in the
experiment. A porous polyvinylidene uoride mem-
brane (Gelman Sciences 66477, PALL Co. Ltd., with a
pore size of 0.2 m, and a diameter of 47 mm) was po-
sitioned and activated in the plasma reaction chamber.
The AA monomer was subsequently grafted onto the
surface of PVDF by introducing them into the plasma
chamber. Similarly, 4VP monomer was grafted on
the other surface of PVDF using the plasma method.
Table 1 shows the experimental conditions.
2.3. Characteristic bipolar membrane
The characteristic functional groups and polarity on
bipolar membrane surface were recorded by Infrared
Attenuated Total Reection spectra (IR-ATR: JASCO
ATR61), and contact angle measurement (GAKU Co.
Ltd.), respectively. The degree of grafting was deter-
mined from the weight difference between the nal
C.-L. Hsueh et al. / Journal of Membrane Science 219 (2003) 113 3
Fig. 1. The plasma reactor.
and initial membrane according to
grafting (%) =
W
f
W
i
W
i
100 (1)
and the results are listed in Table 2. The degree of
swelling was determined from the weight difference
between the wet and dry membrane according to
Table 1
Reaction condition for plasma-induced graft polymerization
Membrane Flow gas Frequency
(MHz)
Power
(W)
Time
(min)
Plasma
PVDF Ar 13.56 50 5
Monomer Temperature
(
C)
Reaction
time (h)
Graft polymerization
AA 70 4
SVS 60 2
4VP 50 1
DMAEA 70 4
swelling (%) =
W
wet
W
dry
W
dry
100 (2)
Before measurement, the bipolar membrane was im-
mersed in the distilled water for 24 h under ambient
temperature to reach the balance of dilation.
2.4. Electrochemistry properties of bipolar
membrane
The ion-exchange capacity of bipolar membrane
was determined by titration method. The anion (4VP,
DMAEA)/cation (AA, SVS) exchange capacity of
Table 2
The degree of grafting by different kind of monomers
Monomer Plasma-induced
grafting (%)
Ce
4+
-initiated
grafting (%)
AA 18.3 14.3
SVS 28.9
4VP 15.1 19.1
DMAEA 32.1
4 C.-L. Hsueh et al. / Journal of Membrane Science 219 (2003) 113
the bipolar membranes were respectively (1.43,
1.86)/(2.54, 2.22) meq./g.
The bipolar membrane was placed in electric cells
with 0.1 M KCl solution at 25
C. The voltage be-
tween the bipolar membrane was measured by po-
tentiostate/galvanostat (EG&G, Model 273, Princeton
Applied Research, USA).
According to the Wiens second effect, the ratio of
the dissociation rate constant under the inuence of
an electric eld, k
d
, to that without an electrical eld,
k
0
d
, can be determined by [16,17]:
k
d
k
0
d
= 1 + b +
b
2
3
+
b
3
18
+
b
4
180
+
b
5
2700
+
b
6
56700
+ (3)
with
b = 0.09636
E
r
T
2
(4)
where
r
is the relative permittivity of the bipolar junc-
tion and T the temperature. E represents the electric
eld density, can be determined by [10]:
E =
2F
0
(V)
X
P
X
N
X
P
+ X
N
1/2
(5)
where
0
is the electric permittivity of free space. X
N
and X
P
denote the xed charge concentrations in the
cation and anion layers, respectively. V stands for the
applied voltage.
Alternately, when system is recorded in the high
electrical eld intensities (E > 10
8
V/m), the effect of
the electric eld on the dissociation rate constant can
be determined by:
k
d
k
0
d
=
1/2
(8b)
3/4
e
(8b)
1/2
(6)
On the other hands, the current efciency () is given
by the equation:
=
c v F
i t
(7)
where c is the difference of mole concentration of
H
+
. v and F denote the volume of the electrolyte so-
lution and the Faraday constant. i and t represent the
current intensity and time.
3. Results and discussion
3.1. 2-HEMABAGMA bipolar membrane
Bipolar membranes, containing COO
and
NH
+
Cl
, were prepared by 2-HEMABAGMA
copolymer-grafting AA and 4VP polymers, respec-
tively. Hydroxyl-containing copoly(HEMABA
GMA) undergoes a redox reaction with ceric ions or
other oxidants to form polymer radicals [18,19] that
can initiate grafting polymerization as follows:
The reaction between the polymer radicals and
AA (or 4VP) led to the grafting of poly(AA) (or
poly(4VP)) onto the 2-HEMABAGMA copoly-
mer. The copolymer membrane, with 2-HEMA as
the main component of the matrix, not only con-
tains a CH
2
OH group as a grafting active site, but
also is a hydrogel material. The hydrogel material
unfortunately has low resistance to water molec-
ular migration, so the mechanical strength of the
hydrogel is generally unavailable in solution. Thus,
the highly hydrophobic matrix of BA and GMA
monomer is introduced inside the copolymer to in-
crease the mechanical strength of the membrane in
solution.
Fig. 2 displays the IR spectra (ATR technique) of
the coply(2HEMABAGMA) bipolar membrane sur-
face before and after it was grafted by poly(AA) and
poly(4VP). The characteristic absorption peaks of AA
functional groups appear at l558 cm
1
(
as
COO
)
and 1380 cm
1
(
as
COO
) in Fig. 2b. Meanwhile,
Fig. 2c shows the AIRspectra of poly(4VP), grafted on
the other side of copoly(2-HEMABAGMA) mem-
brane. The spectra exhibit the characteristic IR peaks
of 4VP at 3050 cm
1
(
s
CH), 16201330 cm
1
(
as
C=C, C=N in the pyridine ring) and 920720 cm
1
(
as
CH) all in the same gure. The IR-ATR
spectra clearly demonstrate that poly(AA) and
poly(4VP) were successfully grafted onto dif-
ferent sides of the copoly(2-HEMABAGMA)
membrane.
C.-L. Hsueh et al. / Journal of Membrane Science 219 (2003) 113 5
Fig. 2. Surface IR-ATR spectra of: (a) poly(BAHEMAGMA) membrane; (b) AA grafting poly(BAHEMAGMA) polar membrane; (c)
4VP grafting poly(BAHEMAGMA) polar membrane.
The contact angle was measured to determine the
surface properties of the bipolar copolymer mem-
brane. Table 3 lists contact angles of the substrate
(2-HEMABAGMA) and the bipolar copolymer
membrane (AA/2-HEMABAGMA/4VP). After
AA and 4VP were grafted onto the surface of the
polymer substrate, their contact angles decreased be-
cause of the highly hydrophilic nature of COO
and NH
4
+
Cl
. Table 3 shows the thickness and
swelling of the bipolar membrane. The thickness
and swelling were, respectively, 135 m and 87.8%.
The anion/cation-exchange capacity of the bipolar
membrane was 1.82/1.98 meq./g after isothermal ad-
sorption/desorption measurement.
Table 3
The contact angle of bipolar membranes on distilled water
Sample name Contact angle of distilled water
Cation
a
(
) Anion
a
(
)
2-HEMABAGMA 7.8
AA/2-HEMA
BAGMA/4VP
0 0
PVDF 124.8
AAPVDF4VP 3.0 8.5
AAPVDFDMAEA 3.5 11.5
SVSPVDF4VP 7.5 8.5
SVSPVDFDMAEA 8.0 12.0
a
Cation: AA or SVS; anion: 4VP or DMAEA.
6 C.-L. Hsueh et al. / Journal of Membrane Science 219 (2003) 113
3.2. Preparing the membrane with PVDF as matrix
by plasma-induced grafting polymerization
Belljar type with internal electrode high-frequency
wave plasma was used as a reactor in this study. The
high-frequency wave generator was quartz-oscillation
controller (13.56 MHz) with a maximum power out
put of 1000 W. Plasma treatment was performed in a
near-vacuum at 1.333224 Pa (10
2
Torr) and argon is
used as the ow gas. Two stages of plasma-induced
grafting polymerization method were used to prepare
bipolar membranes. The plasma-induced procedure
rst activated the surface of PVDF membrane and
then monomers were directly introduced into the re-
action chamber to induce post-graft polymerization.
The degree of grafting polymerization depended on the
power and duration of the plasma treatment, as well as
the temperature and the reaction time of post-grafting
polymerization. Table 1 presents the power of the
plasma and the duration of the treatment in preparing
the bipolar membrane.
Fig. 3 shows FTIR spectra of the original PVDF
membrane, poly(AA)-grafted copolymer membrane,
poly(4VP)-grafted copolymer membrane, poly(SVS)-
grafted copolymer membrane and poly(DMAEA)-
grafted copolymer membrane. The characteristic
absorptive bands of the functional groups of poly(AA)
and poly(4VP) are similar to those obtained above,
as shown in Fig. 3b and c. The absorptive peaks at
1351 cm
1
(
as
O=S=O), 1176 cm
1
(
s
O=S=O),
and 1000769 cm
1
(strong stretching broadband
absorption of SOC) correspond to the functional
groups of poly(SVS) (Fig. 3d). Fig. 3e shows that
the absorption peak at 1760 cm
1
corresponds to the
stretching absorptive band of C=O for poly(DMAEA).
All spectra in Fig. 3 clearly indicate that the ionic
polymers were successfully grafted onto the surface
of the PVDF membrane.
The contact angles of the PVDF bipolar membrane
can further specify the polymer-grafting level of the
PVDF membrane surface, due to the discrepancy
between the hydrophobia of the ionic polymer and
that of PVDF. A smaller angle between the PVDF
membranes surface and the corresponds to the graft-
ing of more ionic polymer on the PVDF membranes
surface. The mean contact angles, which are the aver-
age of measurements taken at ve different points on
the PVDF membranes surface, for all of the PVDF
membranes, clearly decline from 124.8 to ca. 8 after
grafting polymerization, as shown in Table 2. (This
result indicates that the surface of PVDF membranes
was covered with the ionic polymer layer.) SEM pho-
tographs of the cross-section of PVDF membranes in
Figs. 4 and 5 clearly show the grafted polymer layer,
further demonstrating that the polyanion or polyca-
tion had grafted onto the surface of PVDF. The PVDF
membranes surface had the same pore size and
porosity before grafting polymerization, as shown in
Fig. 4a. However, almost all of the pores on the surface
were covered with poly(AA), poly(4VP), poly(SVS),
and/or poly(DMAEA) layers, after the PVDF surface
were plasma-treated and grafted by AA, 4VP, SVS,
and DMAEA, as shown in Fig. 4be. Meanwhile, in
Fig. 5, the SEM photographs of the cross-section of
PVDF membranes present an obvious tri-layer struc-
ture. Independently of the use of AAPVDF4VP
BM, AAPVDFDMAEA BM, SVSPVDF4VP
BM or SVSPVDFDMAEA BM are, all the bipolar
membranes have sandwich structures. The thickness
of middle layers of all PVDF substrate less than
20m, according to the SEM photographs. Shrinking
the middle zone of the bipolar membranes decreases
the resistance to ionic migration in solution.
Table 4 lists the thickness and swelling of all bipo-
lar membranes generated in this investigation. The
thickness and the swelling of A, prepared by grat-
ing AA/4VP onto 2-HEMABAGMA copolymers
exceeds those of the other membranes. In fact, the
swelling of the bipolar membrane affected the elec-
trochemical properties of the bipolar membrane in the
electrobath. Sufcient water must be contained in the
bipolar membrane to keep the system functional be-
cause water molecules are split into H
+
and OH
by
the electric eld during electrodialysis. The optimum
swelling is generally in the range of 2550%. Conse-
quently, preparing bipolar membranes with favorable
mechanical properties depends on sufcient swelling
of water. Among the bipolar membranes generated in
this work, BE membranes exhibit adequate swelling.
The material of the middle layer in the BE mem-
branes is the high-strength but porous PVDF, which
supplies the mechanical strength of the bipolar mem-
branes during electrodialysis.
Restated, the structure and properties of a bipolar
membrane prepared by plasma-grafting were better
than those obtained grafting copolymerization in this
C.-L. Hsueh et al. / Journal of Membrane Science 219 (2003) 113 7
Fig. 3. Surface IR spectra of: (a) original PVDF membrane; (b) AA grafting PVDF membrane; (c) 4VP grafting PVDF membrane; (d)
SVS grafting PVDF membrane; (e) DMAEA grafting PVDF membrane.
Table 4
Properties of bipolar membrane
Symbol Membrane Swelling
(%)
Thickness
(m)
Critical
voltage (V)
Limiting current
density (mA/cm
2
)
Electrical
eld (V/m)
Water dissociation
ratio (K
d
/K
0
d
)
A AA/2-HEMA
BAGMA/4VP
87.8 135 0.95 1.48 2.18 10
8
434
B AAPVDF4VP 45.3 89 0.93 0.65 2.34 10
8
584
C AAPVDFDMAEA 39.2 92 0.92 0.84 2.62 10
8
963
D SVSPVDF4VP 42.5 91 0.89 0.73 2.24 10
8
487
E SVSPVDFDMAEA 37.6 89 0.87 0.92 2.48 10
8
751
8 C.-L. Hsueh et al. / Journal of Membrane Science 219 (2003) 113
Fig. 4. Scanning electron micrographs of: (a) original PVDF membrane; (b) AA grafting PVDF membrane; (c) 4VP grafting PVDF
membrane; (d) SVS grafting PVDF membrane; (e) DMAEA grafting PVDF membrane.
investigation. The thickness and swelling level of the
A membranes are too large for electrodialysis. Thus,
the limiting current density of A is obviously higher
than that of the other membranes, as shown in Table
4. Such a currentvoltage property is discussed below.
3.3. Currentvoltage curves of bipolar membranes
Fig. 6 plots the current versus voltage curves of
the various bipolar membranes. The current versus
voltage curves and the limiting current density due
C.-L. Hsueh et al. / Journal of Membrane Science 219 (2003) 113 9
Fig. 5. Cross-section of the scanning electron micrographs of: (a) original PVDF membrane; (b) AAPVDF4VP membrane; (c)
AAPVDFDMAEA membrane; (d) SVSPVDF4VP membrane; (e) SVSPVDFDMAEA membrane.
10 C.-L. Hsueh et al. / Journal of Membrane Science 219 (2003) 113
Fig. 6. Currentvoltage curves for the ve bipolar membranes in an aqueous solution of concentration = 0.1 M KCl. All measurements
were carried out at T = 298 K.
to water splitting have been typically used to evalu-
ate the performance of a bipolar membrane [1217].
The currentvoltage curves of all bipolar mem-
branes exhibit the typical behavior associated with
eld-enhanced dissociation of water, caused by a
chemical reaction. Table 3 presents the critical volt-
age and limiting current density of the various bipolar
membranes. The results reveal that the limiting cur-
rent density of AA/2-HEMABAGMA/4VP bipolar
membrane exceeds that of the other plasma-induced
grafting polymerization bipolar membranes. The
limiting current is dened as that at which water
molecules start to dissociate into H
+
and OH
in-
side the bipolar membrane. A lower limiting current
corresponds to more economically efcient bipolar
membrane applications. The thickness, especially for
water-swelling of bipolar membranes prepared by
plasma-grafting polymerization is clearly smaller than
Ce
4+
-initiated grafting polymerization, correspond-
ing to lower resistance and limiting current in elec-
trodialysis. Table 4 also shows the electrical eld and
water dissociation ratio determined by Eqs. (4)(6).
The bipolar membrane prepared by plasma-induced
grafting polymerization is better than that prepared by
Ce
4+
-initiated grafting polymerization. Additionally,
the membrane onto which was grafted AA was more
effective than that onto which was grafted SVS. Sim-
ilarly, grafting with DMAEA yielded a more effective
membrane than grafting with 4VP. Furthermore, the
characters of the anion-exchange zone inuence the
water dissociation ratio more than does that of the
cation-exchange zone. However, the water molecules
are arranged more regularly in the anion-exchange
zone than in the cation-exchange zone, accord-
ing to the chemical reaction model [20,21]. The
AAPVDFDMAEA bipolar membrane has ratio of
dissociation rate constants (k
d
/k
0
d
) of 963, better than
all the others.
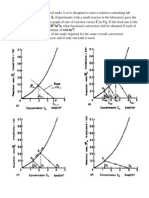
Figs. 7 and 8 plot the current versus voltage curves
of the AAPVDF4VP membrane at various concen-
trations of KCl and for different kinds of electrolyte,
respectively. Tables 5 and 6 list the limiting current
and critical voltage, respectively. The results show that
Table 5
The value of critical voltage and limiting current density for the
AAPVDF4VP membrane in an aqueous solution of different
concentration of KCl
Concentration (M) Critical voltage (V) Limiting current
density (mA/cm
2
)
0.2 0.93 0.41
0.1 0.93 0.65
0.05 0.93 1.18
C.-L. Hsueh et al. / Journal of Membrane Science 219 (2003) 113 11
Fig. 7. Currentvoltage curves for the AAPVDF4VP membrane in an aqueous solution of different concentration of KCl.
the limiting current increased with the diffusion co-
efcient (KCl = 1.87 10
9
m
2
/s, NaCl = 1.45
10
9
m
2
/s, LiCl = 1.14 10
9
m
2
/s) [22] and the con-
centration of the electrolyte. However, the critical volt-
age is independent of the concentration and the kind
of electrolysis. Under reverse bias, the ions come out
Fig. 8. Currentvoltage curves for the AAPVDF4VP membrane in an aqueous solution of different kinds of electrolyte of
concentration = 0.1 M.
of the BM and form a depleted electric double layer
that instantly resists causes resistance the production
of the limiting current. Then, the lower concentration
of the electrolyte promotes the production of a de-
pleted electric double layer and causes the current to
reach the limit earlier.
12 C.-L. Hsueh et al. / Journal of Membrane Science 219 (2003) 113
Fig. 9. The current efciency for the ve bipolar membranes.
3.4. Current efciency of bipolar membranes
Eq. (7) determines the efciency of the current in
all bipolar membranes. Fig. 9 shows these efcien-
cies. The efciency of the current approaches a steady
state value after the limiting current is reached. That
is the limiting current was the minimum value at
which water dissociated. The efciency of the current
of AA/2-HEMABAGMA/4VP BM was lower than
that of other plasma-induced graft polymerization
bipolar membranes, since the thicker AA/2-HEMA
BAGMA/4VP bipolar membrane consumes more
energy than other, thinner membranes. This result is
consistent with the aforementioned description that
the bipolar membrane prepared by plasma-induced
Table 6
The value of critical voltage and limiting current density for the
AAPVDF4VP membrane in an aqueous solution of different
kinds of electrolyte (concentration = 0.1 M)
Electrolyte Critical voltage (V) Limiting current
density (mA/cm
2
)
KCl 0.93 0.69
NaCl 0.93 0.61
LiCl 0.93 0.42
grafting polymerization is better than that prepared
by Ce
4+
-initiated grafting polymerization.
4. Conclusion
The FTIR spectra, the difference of contact an-
gle and SEM photographs all indicate that a bipolar
membrane can be prepared by chemical grafting or
plasma-grafting polymerization. The ratio of the dis-
sociation rate constants of plasma-induced grafting
exceeded that of Ce
4+
-initiated polymerization in this
study. The AAPVDFDMAEA bipolar membrane
was the best one in terms of this ratio. The limit-
ing current of the bipolar membranes increased with
the diffusion coefcient and the concentration of the
electrolyte. The tendency for water dissociation de-
pended only on the kind of bipolar membranes, and
was independent of the concentration and the species
of the electrolyte. However, the membrane grafted
by AA was more effective than that grafted by SVS.
Similarly, the DMAEA membrane was more effective
than the 4VP membrane. Additionally, the character
of the anion-exchange zone inuenced the water dis-
sociation ratio more strongly than did the character
of the cation-exchange zone. The limiting current and
C.-L. Hsueh et al. / Journal of Membrane Science 219 (2003) 113 13
the efciency of the current showed that the bipolar
membrane prepared by the plasma-induced method
is better than that produced by the Ce
4+
-initiated
method.
Acknowledgements
The authors would like to thank the National
Science Council of the Republic of China for nan-
cially supporting this research under Contract No.
NSC90-2216-E-006-04.
Nomenclature
c difference of mole concentration of H
+
E electric eld density
F Faraday constant
i current intensity
k
d
under the inuence of an electric eld
k
0
d
without an electrical eld
t time
T temperature
v volume of the electrolyte solution
V applied voltage
X
N
xed charge concentrations in the cation
X
P
xed charge concentrations in the anion
Greek letters
0
electric permittivity of free space
r
relative permittivity of the
bipolar junction
current efciency
References
[1] P.C. Rieke, N.E. Vanderborgh, Thin lm electrode arrays for
mapping the currentvoltage distributions in proton exchange
membrane fuel cells, J. Electrochem. Soc. 134 (5) (1987)
1099.
[2] J. Kassotis, H.P. Gregor, Electrodialytic water spling, J.
Electrochem. Soc. 131 (12) (1984) 2810.
[3] B. Bauer, F.J. Gerner, H. Strathmann, Development of bipolar
membranes, Desalination 68 (1988) 279.
[4] N. Onishi, T. Osaki, M. Minagawa, A. Tanioka, Alcohol
splitting in a bipolar membrane and analysis of the product,
J. Electroanal. Chem. 506 (2001) 34.
[5] F.G. Wilhelm, N.F.A. van der Vegt, M. Wessling, H.
Strathmann, Chronopotentiometry for the advanced current
voltage characterization of bipolar membranes, J. Electroanal
Chem. 502 (2001) 152.
[6] R. Simons, Electric eld effects on proton transfer between
ionizable groups and water in ion exchange membranes,
Electrochim. Acta 29 (2) (1984) 151.
[7] R. Simons, A novel method for preparing bipolar membranes,
Electrochim. Acta 31 (9) (1986) 1175.
[8] K.N. Mani, F.P. Chlanda, C.H. Byszewski, Aquatech
membrane technology for recovery of acid/base values from
salt streams, Desalination 68 (1988) 149.
[9] F.P. Chlanda, Y.C. Chiao, Water splitting efciency of bipolar
membranes, AIChE Symp. Ser. 84 (261) (1987) 64.
[10] H. Holdik, A. Alcaraz, P. Ramirez, S. Maf, Electric eld
enhanced water dissociation at the bipolar membrane junction
from ac impedance spectra measurements, J. Electroanal.
Chem. 442 (12) (1998) 13.
[11] K.J. Liu, F.P. Chlanda, K. Nagasubramanian, Application of
bipolar membrane technology: a novel process for control of
sulfur dioxide from ue gases, J. Membr. Sci. 3 (1978) 57.
[12] K.J. Liu, K. Nagasubramanian, F.P. Chlanda, Membrane
electrodialysis process for recovery of sulfur dioxide from
power plant stack gases, J. Membr. Sci. 3 (1978) 71.
[13] K. Nagasubramanian, F.P. Chlanda, K.J. Liu, Use of bipolar
membranes for generation of acid and basean engineering
and economic analysis, J. Membr. Sci. 2 (1977) 109.
[14] F.D. Krsy, E. Zeigerson, Bipolar membranes made of a
single polyolephine sheet, Israel J. Chem. 9 (1971) 483.
[15] Y. Yokoyama, A. Tanioka, K. Miyasaka, Preparation of
a single bipolar membranes by plasma-induced graft
polymerization, J. Membr. Sci. 43 (1989) 165.
[16] L. Onsager, Deviations from Ohms law in week electrolytes,
J. Chem. Phys. 2 (1934) 599.
[17] H. Strathmann, J.J. Krol, H.J. Rapp, G. Eigenberger, Limiting
current density and water dissociation in bipolar membranes,
J. Membr. Sci. 125 (1) (1997) 123.
[18] G. Odian, J.H.T. Kho, Ceric ion initiated graft polymerization
onto poly(vinyl alcohol), J. Macromol. Sci. Chem. A4 (2)
(1970) 317.
[19] A. Higuchi, T. Nakagawa, Membrane potential and
permeation of salts across bipolar membranes, J Membr. Sci.
32 (1987) 267.
[20] V.V. Umnov, N.V. Sheldeshov, V.I. Zabolotskii, Structure
of the spacecharge region at anionitecationite interface in
bipolar membranes, Russ. J. Electrochem. 450 (1999) 35.
[21] V.V. Umnov, N.V. Sheldeshov, V.I. Zabolotskii, Current
voltage curve for the space charge region of a bipolar
membranes, Russ. J. Electrochem. 982 (1999) 35.
[22] V.M.M. Lobo, Handbook of Electrolyte Solutions, Elsevier,
New York, 1989, pp. 828 (Part A); 1200, 1611 (Part B).
Das könnte Ihnen auch gefallen
- Shoe Dog: A Memoir by the Creator of NikeVon EverandShoe Dog: A Memoir by the Creator of NikeBewertung: 4.5 von 5 Sternen4.5/5 (537)
- Grit: The Power of Passion and PerseveranceVon EverandGrit: The Power of Passion and PerseveranceBewertung: 4 von 5 Sternen4/5 (587)
- Isolation of Aspergillus NigerDokument1 SeiteIsolation of Aspergillus NigersaadiisNoch keine Bewertungen
- Furnace FiguresDokument1 SeiteFurnace FiguressaadiisNoch keine Bewertungen
- TodayDokument9 SeitenTodaysaadiisNoch keine Bewertungen
- Bio Reactor Citric Acid Surface Fermentaion226-230Dokument5 SeitenBio Reactor Citric Acid Surface Fermentaion226-230saadiisNoch keine Bewertungen
- Using Ode 45 in MatlabDokument15 SeitenUsing Ode 45 in MatlabsaadiisNoch keine Bewertungen
- Constants For DaubertDokument1 SeiteConstants For DaubertsaadiisNoch keine Bewertungen
- Amylolytic Enzymes Produced by The Yeast Saccharomycopsis FibuligeraDokument5 SeitenAmylolytic Enzymes Produced by The Yeast Saccharomycopsis FibuligerasaadiisNoch keine Bewertungen
- What Is The Best Way To Harvest Spores of ADokument4 SeitenWhat Is The Best Way To Harvest Spores of AsaadiisNoch keine Bewertungen
- What Is The Best Way To Harvest Spores of ADokument4 SeitenWhat Is The Best Way To Harvest Spores of AsaadiisNoch keine Bewertungen
- Citric Acid ProductionDokument20 SeitenCitric Acid ProductionDiana MuñozNoch keine Bewertungen
- Kmol/m xl0 M /S, What Fractional Conversion Will Be Obtained If Each of M ?Dokument2 SeitenKmol/m xl0 M /S, What Fractional Conversion Will Be Obtained If Each of M ?saadiisNoch keine Bewertungen
- Fired Heater DesignDokument47 SeitenFired Heater DesignMarcel100% (4)
- A Novel Solid State Fermentation Coupled With Gas Stripping Enhancing The SweetDokument23 SeitenA Novel Solid State Fermentation Coupled With Gas Stripping Enhancing The SweetsaadiisNoch keine Bewertungen
- Citric Acid ProductionDokument20 SeitenCitric Acid ProductionDiana MuñozNoch keine Bewertungen
- Citric Acid ProductionDokument20 SeitenCitric Acid ProductionDiana MuñozNoch keine Bewertungen
- Lecture 6Dokument23 SeitenLecture 6siegherrNoch keine Bewertungen
- Matlab Guide BookDokument26 SeitenMatlab Guide BookKingchemNoch keine Bewertungen
- Fischer Tropsch Synthesis Activity and SelectivityDokument13 SeitenFischer Tropsch Synthesis Activity and SelectivitysaadiisNoch keine Bewertungen
- 1 s2.0 S0016236110000670 MainDokument8 Seiten1 s2.0 S0016236110000670 MainsaadiisNoch keine Bewertungen
- NernstequationderivDokument2 SeitenNernstequationderivaravind_elec5654Noch keine Bewertungen
- Sample Exam 1 SolDokument4 SeitenSample Exam 1 SolsaadiisNoch keine Bewertungen
- Control SystemsDokument29 SeitenControl SystemsFırat ErdoganNoch keine Bewertungen
- CP302 Example 02 OKDokument4 SeitenCP302 Example 02 OKsaadiis100% (1)
- Chart of Shell Sidew Heat Transfer CoeffDokument9 SeitenChart of Shell Sidew Heat Transfer CoeffsaadiisNoch keine Bewertungen
- Chpman Onskoog Theory, Eyring TheoryDokument11 SeitenChpman Onskoog Theory, Eyring TheorysaadiisNoch keine Bewertungen
- Chapter 9 Mass TransferDokument64 SeitenChapter 9 Mass TransfersaadiisNoch keine Bewertungen
- Malir River Pollution 1027Dokument445 SeitenMalir River Pollution 1027samoonibrahimNoch keine Bewertungen
- NSPE Code of EthicsDokument2 SeitenNSPE Code of EthicsRizka WidyariniNoch keine Bewertungen
- HAZOP Case Studies For Workshop SessionDokument4 SeitenHAZOP Case Studies For Workshop Sessionsaadiis100% (2)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceVon EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceBewertung: 4 von 5 Sternen4/5 (894)
- The Yellow House: A Memoir (2019 National Book Award Winner)Von EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Bewertung: 4 von 5 Sternen4/5 (98)
- The Little Book of Hygge: Danish Secrets to Happy LivingVon EverandThe Little Book of Hygge: Danish Secrets to Happy LivingBewertung: 3.5 von 5 Sternen3.5/5 (399)
- On Fire: The (Burning) Case for a Green New DealVon EverandOn Fire: The (Burning) Case for a Green New DealBewertung: 4 von 5 Sternen4/5 (73)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeVon EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeBewertung: 4 von 5 Sternen4/5 (5794)
- Never Split the Difference: Negotiating As If Your Life Depended On ItVon EverandNever Split the Difference: Negotiating As If Your Life Depended On ItBewertung: 4.5 von 5 Sternen4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureVon EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureBewertung: 4.5 von 5 Sternen4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryVon EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryBewertung: 3.5 von 5 Sternen3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerVon EverandThe Emperor of All Maladies: A Biography of CancerBewertung: 4.5 von 5 Sternen4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreVon EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreBewertung: 4 von 5 Sternen4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyVon EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyBewertung: 3.5 von 5 Sternen3.5/5 (2219)
- Team of Rivals: The Political Genius of Abraham LincolnVon EverandTeam of Rivals: The Political Genius of Abraham LincolnBewertung: 4.5 von 5 Sternen4.5/5 (234)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersVon EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersBewertung: 4.5 von 5 Sternen4.5/5 (344)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaVon EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaBewertung: 4.5 von 5 Sternen4.5/5 (265)
- The Unwinding: An Inner History of the New AmericaVon EverandThe Unwinding: An Inner History of the New AmericaBewertung: 4 von 5 Sternen4/5 (45)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Von EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Bewertung: 4.5 von 5 Sternen4.5/5 (119)
- Her Body and Other Parties: StoriesVon EverandHer Body and Other Parties: StoriesBewertung: 4 von 5 Sternen4/5 (821)
- B. Tech. - I (All Branches) ASP102ABC (T) : Engineering Physics (Theory) L T P C 3 0 2 4Dokument2 SeitenB. Tech. - I (All Branches) ASP102ABC (T) : Engineering Physics (Theory) L T P C 3 0 2 4Harikrishna ShenoyNoch keine Bewertungen
- MSDS - Sulphur 90%: Section 1. Product InformationDokument3 SeitenMSDS - Sulphur 90%: Section 1. Product InformationsahilchemNoch keine Bewertungen
- Patchouli Oil Isolation and Identification of Chemical Components Using GC-MSDokument7 SeitenPatchouli Oil Isolation and Identification of Chemical Components Using GC-MSjuggernautzNoch keine Bewertungen
- A Presentation On ChemistryDokument18 SeitenA Presentation On ChemistryBimal DasNoch keine Bewertungen
- Linear Thermal Expansion of Solid Materials With A Vitreous Silica DilatometerDokument7 SeitenLinear Thermal Expansion of Solid Materials With A Vitreous Silica Dilatometerluis_may22Noch keine Bewertungen
- PT SSJ Corporate ProfileDokument8 SeitenPT SSJ Corporate ProfileYohanest ChandraNoch keine Bewertungen
- Diffusion and Osmosis LabDokument11 SeitenDiffusion and Osmosis Labapi-255906283Noch keine Bewertungen
- 152 TOP Thermodynamics Mechanical Engineering Multiple Choice Questions and Answers List MCQs Preparation For Engineering Competitive Exams PDFDokument17 Seiten152 TOP Thermodynamics Mechanical Engineering Multiple Choice Questions and Answers List MCQs Preparation For Engineering Competitive Exams PDFIlhariri Muhammad IrlisNoch keine Bewertungen
- High-Performance Concrete Characteristics and PropertiesDokument16 SeitenHigh-Performance Concrete Characteristics and PropertiesChukwuma OgbonnaNoch keine Bewertungen
- Chemical Analysis of FoodDokument12 SeitenChemical Analysis of Foodprince1900100% (1)
- Phase Diagrams For Metallic SystemsDokument5 SeitenPhase Diagrams For Metallic SystemsZesi Villamor Delos SantosNoch keine Bewertungen
- VLSI Epitaxy and Thin Film DepositionDokument20 SeitenVLSI Epitaxy and Thin Film DepositionRubel RiadNoch keine Bewertungen
- Superabsorbent PolymerDokument21 SeitenSuperabsorbent PolymerIsha MeshramNoch keine Bewertungen
- Frank Girgsdies Phase Analysis and Structure Refinement 131129Dokument91 SeitenFrank Girgsdies Phase Analysis and Structure Refinement 131129MegaTypers100% (1)
- Valvulas de Bola 2Dokument20 SeitenValvulas de Bola 2claudio godinezNoch keine Bewertungen
- Human Digestive SystemDokument13 SeitenHuman Digestive SystemEvansNoch keine Bewertungen
- New Microsoft Word DocumentDokument10 SeitenNew Microsoft Word DocumentNawnit KumarNoch keine Bewertungen
- Aluminum Handbook: Properties and SpecificationsDokument106 SeitenAluminum Handbook: Properties and Specificationsxaaabbb_550464353Noch keine Bewertungen
- Cellular Respiration LabDokument6 SeitenCellular Respiration Labluckyduck2869100% (1)
- Sikaflex®-400 Fire: Product Data SheetDokument4 SeitenSikaflex®-400 Fire: Product Data SheetLA BoiserNoch keine Bewertungen
- Effect of The Use of Ceramic Filters in Steel CastingDokument6 SeitenEffect of The Use of Ceramic Filters in Steel CastingJavier Escalante VillanuevaNoch keine Bewertungen
- Absorption of Carbon Dioxide Into Aqueous Blends of Diethanolamine and MethyldiethanolamineDokument11 SeitenAbsorption of Carbon Dioxide Into Aqueous Blends of Diethanolamine and MethyldiethanolamineAnkit KumarNoch keine Bewertungen
- Lab 6 (Soaps & Detergents)Dokument21 SeitenLab 6 (Soaps & Detergents)AmeerRashidNoch keine Bewertungen
- Tablas de AlcoholesDokument13 SeitenTablas de AlcoholesHugo miranda86% (7)
- UNPAD Applied Chemistry Course Teaches Entrepreneurship SkillsDokument5 SeitenUNPAD Applied Chemistry Course Teaches Entrepreneurship SkillsYolanda VitriNoch keine Bewertungen
- PSA Oxygen Generator SystemDokument8 SeitenPSA Oxygen Generator SystemSudhakar ShresthaNoch keine Bewertungen
- Buried Pipe Calculation Report: Title Name Date Report NameDokument3 SeitenBuried Pipe Calculation Report: Title Name Date Report NameDenis OhwofadaNoch keine Bewertungen
- Synthesis of Fluorescein Dye UsingDokument6 SeitenSynthesis of Fluorescein Dye UsingMoises BarraganNoch keine Bewertungen
- G7 Learning Activities 1ST Quarter Sy 2020-2021Dokument13 SeitenG7 Learning Activities 1ST Quarter Sy 2020-2021Zet Gutierrez0% (1)
- Bolts, threaded parts and rivets allowable loadsDokument2 SeitenBolts, threaded parts and rivets allowable loadsMary MarasiganNoch keine Bewertungen