Beruflich Dokumente
Kultur Dokumente
Flash Concepts
Hochgeladen von
Juan Camilo HenaoOriginalbeschreibung:
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Flash Concepts
Hochgeladen von
Juan Camilo HenaoCopyright:
Verfügbare Formate
temperature of one or more components in the mixture, Prausnitz
et al. use a Henrys law constant H
i, M
in place of the product
i
L
i
L
in
Eq. (13-4). Otherwise
i
L
is evaluated from vapor pressure data with
a Poynting saturated-vapor fugacity correction. When the total pres-
sure is less than about 202.6 kPa (2 atm) and all components in the
mixture have a critical temperature that is greater than the system
temperature, then
i
L
= P
i
sat
/P and
i
V
= 1.0. Equation (13-4) then
reduces to
K
i
= (13-10)
i
L
P
i
sat
P
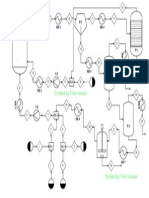
The simplest continuous distillation process is the adiabatic single-
stage equilibrium flash process pictured in Fig. 13-16. Feed tempera-
ture and the pressure drop across the valve are adjusted to vaporize
the feed to the desired extent, while the drum provides disengaging
space to allow the vapor to separate from the liquid. The expansion
across the valve is at constant enthalpy, and this fact can be used to cal-
culate T
2
(or T
1
to give a desired T
2
).
A degrees-of-freedom analysis indicates that the variables subject
to the designers control are C + 3 in number. The most common way
to use these is to specify the feed rate, composition, and pressure
(C + 1 variables) plus the drum temperature T
2
and pressure P
2
. This
operation will give one point on the equilibrium flash curve shown in
Fig. 13-17. This curve shows the relation at constant pressure between
the fraction V/F of the feed flashed and the drum temperature. The
temperature at V/F = 0.0 when the first bubble of vapor is about to
form (saturated liquid) is the bubble point temperature of the feed
mixture, and the value at V/F = 1.0 when the first droplet of liquid is
about to form (saturated vapor) is the dew point temperature.
BUBBLE POINT AND DEW POINT
For a given drum pressure and feed composition, the bubble and dew
point temperatures bracket the temperature range of the equilibrium
flash. At the bubble point temperature, the total vapor pressure
exerted by the mixture becomes equal to the confining drum pressure,
and it follows that y
i
= 1.0 in the bubble formed. Since y
i
= K
i
x
i
and since the x
i
s still equal the feed compositions (denoted by z
i
), cal-
culation of the bubble point temperature involves a trial-and-error
search for the temperature which, at the specified pressure, makes
K
i
z
i
= 1.0. If instead the temperature is specified, one can find the
bubble point pressure that satisfies this relationship.
At the dew point temperature y
i
still equals z
i
and the relationship
x
i
= z
i
K
i
= 1.0 must be satisfied. As in the case of the bubble point,
a trial-and-error search for the dew point temperature at a specified
pressure is involved. Or, if the temperature is specified, the dew point
pressure can be calculated.
ISOTHERMAL FLASH
The calculation for a point on the flash curve that is intermediate
between the bubble point and the dew point is referred to as an
isothermal flash calculation because T
2
is specified. Except for an ideal
binary mixture, procedures for calculating an isothermal flash are
iterative. A popular and recommended method is the following, due to
Rachford and Rice [J. Pet. Technol., 4(10), sec. 1, p. 19, and sec. 2, p. 3
(October 1952)]. The component mole balance (Fz
i
= Vy
i
+ Lx
i
), phase
distribution relation (K
i
= y
i
/x
i
), and total mole balance (F = V + L) can
be combined to give
x
i
= (13-12)
y
i
= (13-13)
Since x
i
y
i
= 0,
f
=
i
= 0 (13-14)
Equation (13-14) is solved iteratively for V/F, followed by the calcula-
tion of values of x
i
and y
i
from Eqs. (13-12) and (13-13) and L from the
total mole balance. Any one of a number of numerical root-finding
procedures such as the Newton-Raphson, secant, false-position, or
bisection method can be used to solve Eq. (13-14). Values of K
i
are
constants if they are independent of liquid and vapor compositions.
Then the resulting calculations are straightforward. Otherwise, the K
i
values must be periodically updated for composition effects, perhaps
z
i
(1 K
i
)
1 + (V/F)(K
i
1)
V
F
K
i
z
i
1 + (V/F)(K
i
1)
z
i
1 + (V/F)(K
i
1)
which is referred to as a modified Raoults law K value. If, furthermore,
the liquid phase is ideal, then
i
L
= 1.0 and
K
i
= (13-11)
which is referred to as a Raoults law K value that is dependent solely
on the vapor pressure P
i
sat
of the component in the mixture. The
UNIFAC method is being periodically updated with new group con-
tributions; e.g., see Hansen et al. [Ind. Eng. Chem. Res., 30, 2352
(1991)].
P
i
sat
P
SINGLE-STAGE EQUILIBRIUM FLASH CALCULATIONS 13-15
SINGLE-STAGE EQUILIBRIUM FLASH CALCULATIONS
FIG. 13-16 Equilibrium flash separator. FIG. 13-17 Equilibrium flash curve.
0.0
V/F
Constant Pressure
1.0
T
e
m
p
e
r
a
t
u
r
e
,
F
after each iteration, using prorated values of x
i
and y
i
from Eqs. (13-12)
and (13-13). Generally the iterations are continued until the change in
the absolute value of V/F is sufficiently small and until the absolute
value of the residual f(V/F) is close to zero. When converged, x
i
and
y
i
will each be very close to a value of 1, and, if desired, T
1
can be
computed from an energy balance around the valve if no heat
exchanger is used. Alternatively, if T
1
is fixed, as mentioned earlier, a
heat exchanger must be added before, after, or in place of the valve
with the required heat duty being calculated from an energy balance.
The limits of applicability of Eqs. (13-12) to (13-14) are the bubble
point, at which V = 0 and x
i
= z
i
, and the dew point, at which L = 0 and
y
i
= z
i
. At these limits Eq. (13-14) reduces to the bubble point equation
i
K
i
x
i
= 1 (13-15)
and the dew point equation, respectively,
i
= 1 (13-16)
For a binary feed, specification of the flash drum temperature and
pressure fixes the equilibrium-phase compositions, which are related
to the K values by
x
1
=
K
1
1
K
K
2
2
and y
1
=
The mole balance can be rearranged to
=
If K
1
and K
2
are functions of temperature and pressure only (ideal
solutions), the flash curve can be calculated directly without iteration.
ADIABATIC FLASH
In Fig. 13-16, if P
2
and the feed-stream conditions (that is, F, z
i
, T
1
, P
1
)
are known, then the calculation of T
2
, V, L, y
i
, and x
i
is referred to as
an adiabatic flash. In addition to Eqs. (13-12) to (13-14) and the total
mole balance, the following energy balance around both the valve and
the flash drum combined must be included:
H
F
F = H
V
V + H
L
L (13-17)
By taking a basis of F = 1.0 mol and eliminating L with the total mole
balance, Eq. (13-17) becomes
f
2
{V, T
2
} = H
F
V(H
V
H
L
) H
L
= 0 (13-18)
With T
2
now unknown, Eq. (13-14) becomes
f
1
{V, T
2
} =
i
= 0 (13-19)
A number of iterative procedures have been developed for solving
Eqs. (13-18) and (13-19) simultaneously for V and T
2
. Frequently, and
especially if the feed contains components of a narrow range of volatil-
ity, convergence is rapid for a tearing method in which a value of T
2
is
assumed, Eq. (13-19) is solved iteratively by the isothermal flash pro-
cedure, and, using that value of V, Eq. (13-18) is solved iteratively for
a new approximation of T
2
, which is then used to initiate the next cycle
z
i
(1 K
i
)
1 + V(K
i
1)
z
1
(K
1
K
2
)(1 K
2
) 1
K
1
1
V
F
K
1
K
2
K
1
K
2
K
1
y
i
K
i
until T
2
and V converge. However, if the feed contains components of
a wide range of volatility, it may be best to invert the sequence and
assume a value for V, solve Eq. (13-19) for T
2
, solve Eq. (13-18) for V,
and then repeat the cycle. If the K values and/or enthalpies are sensi-
tive to the unknown phase compositions, it may be necessary to solve
Eqs. (13-18) and (13-19) simultaneously by a Newton or other suitable
iterative technique. Alternatively, the two-tier method of Boston and
Britt [Comput. Chem. Eng., 2, 109 (1978)], which is also suitable for
difficult isothermal flash calculations, may be applied.
OTHER FLASH SPECIFICATIONS
Flash drum specifications in addition to (P
2
, T
2
) and (P
2
, adiabatic) are
possible but must be applied with care, as discussed by Michelsen
[Comp. Chem. Engng., 17, 431 (1993)]. Most computer-aided process
design and simulation programs permit a wide variety of flash specifi-
cations.
THREE-PHASE FLASH
Single-stage equilibrium flash calculations become considerably more
complex when an additional liquid phase can form, as in mixtures of
water with hydrocarbons, water with ethers, and water with higher alco-
hols (containing four or more carbon atoms). Procedures for computing
such situations are referred to as three-phase flash methods, which are
given for the general case by Henley and Rosen (Material and Energy
Balance Computations, Wiley, New York, 1968, chap. 8). When the two
liquid phases are almost mutually insoluble, they can be considered sep-
arately and relatively simple procedures apply, as discussed by Smith
(Design of Equilibrium Stage Processes, McGraw-Hill, New York, 1963).
Condensation of such mixtures may result in one liquid phase being
formed before the other. Computer-aided process design and simulation
programs all contain a Gibbs free-energy routine that can compute a
three-phase flash by minimization of the Gibbs free energy. Many
important and subtle aspects of three-phase flash calculations are dis-
cussed by Michelsen [Fluid Phase Equil., 9, 1, 21 (1982)], McDonald
and Floudas [AIChE J., 41, 1798 (1995)], and Wasylkiewicz et al. [Ind.
Eng. Chem. Research, 35, 1395 (1996)].
COMPLEX MIXTURES
Feed analyses in terms of component compositions are usually not
available for complex hydrocarbon mixtures with a final normal boiling
point above about 38C (100F) (n-pentane). One method of handling
such a feed is to break it down into pseudocomponents (narrow-boiling
fractions) and then estimate the mole fraction and Kvalue for each such
component. Edmister [Ind. Eng. Chem., 47, 1685 (1955)] and Maxwell
(Data Book on Hydrocarbons, Van Nostrand, Princeton, N.J., 1958)
give charts that are useful for this estimation. Once K values are avail-
able, the calculation proceeds as described above for multicomponent
mixtures. Another approach to complex mixtures is to obtain an Ameri-
can Society for Testing and Materials (ASTM) or true-boiling point
(TBP) curve for the mixture and then use empirical correlations to con-
struct the atmospheric-pressure equilibrium flash vaporization (EFV)
curve, which can then be corrected to the desired operating pressure. A
discussion of this method and the necessary charts is presented in a later
subsection Petroleum and Complex-Mixture Distillation.
13-16 DISTILLATION
GRAPHICAL METHODS FOR BINARY DISTILLATION
Multistage distillation under continuous, steady-state operating con-
ditions is widely used in practice to separate a variety of mixtures.
Table 13-5, taken from the study of Mix, Dweck, Weinberg, and
Armstrong [AIChE Symp. Ser. 76, 192, 10 (1980)] lists key compo-
nents along with typical stage requirements to perform the separation
for 27 industrial distillation processes. The design of multistage
columns can be accomplished by graphical techniques when the feed
mixture contains only two components. The x-y diagram method
developed by McCabe and Thiele [Ind. Eng. Chem., 17, 605 (1925)]
uses only phase equilibrium and mole balance relationships. The
method assumes an adiabatic column (no heat losses through the col-
umn walls) and constant latent heat for the binary mixture at all com-
positions (which requires, among other things, equal latent heat for
both components). The method is exact only for those systems in
which energy effects on vapor and liquid rates leaving the stages are
negligible. However, the approach is simple and gives a useful first
estimate of the column design which can be refined by using the
enthalpy composition diagram method of Ponchon [Tech. Mod., 13,
Das könnte Ihnen auch gefallen
- Partes de Cameras FotograficasDokument2 SeitenPartes de Cameras FotograficasejmelchiorsNoch keine Bewertungen
- Lab5 First DraftDokument5 SeitenLab5 First DraftLe VoyageurNoch keine Bewertungen
- Design and Operation of Fractionating ColumnsDokument6 SeitenDesign and Operation of Fractionating ColumnsJuan Camilo HenaoNoch keine Bewertungen
- 1987 - Multiphase Isenthalpic and Isentropic Flash Algorithms - MichelsenDokument15 Seiten1987 - Multiphase Isenthalpic and Isentropic Flash Algorithms - MichelsenhakeemniyasNoch keine Bewertungen
- Naphtali & Sandholm - Multicomponent Separation Calculations by Linearization PDFDokument6 SeitenNaphtali & Sandholm - Multicomponent Separation Calculations by Linearization PDFCarlos PlazasNoch keine Bewertungen
- A Comparison of The Peng-Robinson and Soave-Redlich-Kwong Equations of StateDokument18 SeitenA Comparison of The Peng-Robinson and Soave-Redlich-Kwong Equations of StateVettidog100% (1)
- CHF LUT 2006 Lookup TableDokument14 SeitenCHF LUT 2006 Lookup Tablefaisal58650Noch keine Bewertungen
- Phase Oil Equilibria of Oil-Water-Brine - Yaun Kun LiDokument11 SeitenPhase Oil Equilibria of Oil-Water-Brine - Yaun Kun LiRonald NgueleNoch keine Bewertungen
- Cubic Equations of State-Which Is BestDokument17 SeitenCubic Equations of State-Which Is BestvenkieeNoch keine Bewertungen
- High-Pressure Fluid Phase Equilibria: Phenomenology and ComputationVon EverandHigh-Pressure Fluid Phase Equilibria: Phenomenology and ComputationNoch keine Bewertungen
- A Quasi-Newton Algorithm For Solving Multi Phase Equilibrium Flash ProblemsDokument22 SeitenA Quasi-Newton Algorithm For Solving Multi Phase Equilibrium Flash ProblemsDaniel MedeirosNoch keine Bewertungen
- Newton-Raphson Vs BroydenDokument6 SeitenNewton-Raphson Vs Broydencorreita77Noch keine Bewertungen
- Flash Calc 1flash Calc 185Dokument18 SeitenFlash Calc 1flash Calc 185Mohamed MamdouhNoch keine Bewertungen
- Computation of Phase and Chemical Equilibrium IDokument9 SeitenComputation of Phase and Chemical Equilibrium IThou KanshieNoch keine Bewertungen
- Keyword Manual PDFDokument953 SeitenKeyword Manual PDFYves-donald MakoumbouNoch keine Bewertungen
- A Improving Predictions of Equation of State by Modifying Its Parameters For Super Critical Components of Hydrocarbon Reservoir FluidsDokument17 SeitenA Improving Predictions of Equation of State by Modifying Its Parameters For Super Critical Components of Hydrocarbon Reservoir Fluids13670319Noch keine Bewertungen
- Rachford Ride EquationDokument15 SeitenRachford Ride EquationEstuardo Javier Gan RodríguezNoch keine Bewertungen
- Inside-Out Algo - Boston 1974Dokument12 SeitenInside-Out Algo - Boston 1974Jeremy HernandezNoch keine Bewertungen
- Flash Calculation Stability PDFDokument12 SeitenFlash Calculation Stability PDFAndre BecNoch keine Bewertungen
- Jacobian Matrix PDFDokument23 SeitenJacobian Matrix PDFRajesh MalikNoch keine Bewertungen
- SPE 63158 Inflow Performance Relationships For Gas CondensatesDokument14 SeitenSPE 63158 Inflow Performance Relationships For Gas Condensatesabnou_223943920Noch keine Bewertungen
- Applications of Equations of StateDokument17 SeitenApplications of Equations of StateJatin RamboNoch keine Bewertungen
- Unsteady State Conduction in a Solid ExperimentDokument7 SeitenUnsteady State Conduction in a Solid ExperimentBilenNoch keine Bewertungen
- Flash Calc 3Dokument16 SeitenFlash Calc 3Mohamed MamdouhNoch keine Bewertungen
- Modelling of Continuous Distillation ColumnDokument4 SeitenModelling of Continuous Distillation ColumnKate MayerNoch keine Bewertungen
- Extension of Peng-Robinson For Complex MixturesDokument18 SeitenExtension of Peng-Robinson For Complex MixturesMandy NelsonNoch keine Bewertungen
- Michelsen The Isothermal Flash Problem 2 PhasDokument20 SeitenMichelsen The Isothermal Flash Problem 2 Phasgggggg82Noch keine Bewertungen
- 4528 - CourseManual DynamicDokument362 Seiten4528 - CourseManual DynamicMuntaser YousifNoch keine Bewertungen
- Combustion Science and Technology PDFDokument2 SeitenCombustion Science and Technology PDFJordanNoch keine Bewertungen
- Intro To Finite Element Modeling and COMSOLDokument18 SeitenIntro To Finite Element Modeling and COMSOLSCR_010101Noch keine Bewertungen
- Finite Volume Data PresentationDokument35 SeitenFinite Volume Data PresentationTarık YılmazNoch keine Bewertungen
- Collocated FVMDokument17 SeitenCollocated FVMapoorvs75Noch keine Bewertungen
- Particle Image VelocimetryDokument57 SeitenParticle Image VelocimetryNathan Zammit MumaNoch keine Bewertungen
- Phase Equilibria Gernert Et Al Fpe 2014Dokument10 SeitenPhase Equilibria Gernert Et Al Fpe 2014RoseJauneNoch keine Bewertungen
- Group-Contribution Method for Predicting Activity CoefficientsDokument14 SeitenGroup-Contribution Method for Predicting Activity CoefficientsArun EbenezerNoch keine Bewertungen
- CAPE-OPEN.. Interoperability in Industrial Flowsheet Simulation Software PDFDokument13 SeitenCAPE-OPEN.. Interoperability in Industrial Flowsheet Simulation Software PDFMedardo AnibalNoch keine Bewertungen
- Correlation of entrainment for annular flow in horizontal pipesDokument24 SeitenCorrelation of entrainment for annular flow in horizontal pipesJohn Doe100% (1)
- AIChE Journal Volume 23 Issue 6 1977 (Doi 10.1002/aic.690230602) Karl Gardner Jerry Taborek - Mean Temperature Difference - A ReappraisalDokument10 SeitenAIChE Journal Volume 23 Issue 6 1977 (Doi 10.1002/aic.690230602) Karl Gardner Jerry Taborek - Mean Temperature Difference - A Reappraisalneozero2006Noch keine Bewertungen
- Rheology Notes Introduction To RheologyDokument30 SeitenRheology Notes Introduction To RheologyDoug AmatoNoch keine Bewertungen
- Documents - Tips Multicomponent Distillation Column Design A Semi Rigorous ApproachDokument16 SeitenDocuments - Tips Multicomponent Distillation Column Design A Semi Rigorous ApproachPriyanshiVadaliaNoch keine Bewertungen
- Wong Sandler (1992)Dokument10 SeitenWong Sandler (1992)Anonymous PO7VwbBnNoch keine Bewertungen
- Riazi Daubert 1980Dokument6 SeitenRiazi Daubert 1980Jeiel FrançaNoch keine Bewertungen
- Mixing Rule For Cubic Equations of StateDokument9 SeitenMixing Rule For Cubic Equations of StateMundo LepalcaNoch keine Bewertungen
- Ahmad, Linnhoff, Smith - Design of Multipass Heat Exchangers - An Alternative Approach (ASME) PDFDokument6 SeitenAhmad, Linnhoff, Smith - Design of Multipass Heat Exchangers - An Alternative Approach (ASME) PDFjdgh1986Noch keine Bewertungen
- Schulkes - Slug Frequencies Revisited PDFDokument15 SeitenSchulkes - Slug Frequencies Revisited PDFneverwolfNoch keine Bewertungen
- A New Generalized Alpha Function For A Cubic Equation of StateDokument11 SeitenA New Generalized Alpha Function For A Cubic Equation of StateJenn QuintoNoch keine Bewertungen
- Binary Interaction Parameters in Cubic-ValderramaDokument6 SeitenBinary Interaction Parameters in Cubic-Valderramaflavio_cordero_1Noch keine Bewertungen
- Azeotropic Systems in DistillationDokument19 SeitenAzeotropic Systems in DistillationHummel JohnsonNoch keine Bewertungen
- 1992 BenderDokument12 Seiten1992 BenderJohn PACHON MORALESNoch keine Bewertungen
- MA3004 Part 3: Computational Fluid Dynamics (CFD) : Martin SkoteDokument46 SeitenMA3004 Part 3: Computational Fluid Dynamics (CFD) : Martin SkotedavidbehNoch keine Bewertungen
- The Liquid Film and The Core Region Velocity Profiles in Annular Two-Phase FlowDokument14 SeitenThe Liquid Film and The Core Region Velocity Profiles in Annular Two-Phase FlowNishant ManepalliNoch keine Bewertungen
- Improvement in Patel Teja Eqn of StatesDokument10 SeitenImprovement in Patel Teja Eqn of StatesSumukh VermaNoch keine Bewertungen
- Flash CheguideDokument16 SeitenFlash Cheguidesok_splNoch keine Bewertungen
- Development of Liquid Slug Length in Gas-Liquid Slug Flow Along Horizontal Pipeline: Experiment and SimulationDokument8 SeitenDevelopment of Liquid Slug Length in Gas-Liquid Slug Flow Along Horizontal Pipeline: Experiment and SimulationAzizNoch keine Bewertungen
- OTC 24437 A Comprehensive Analysis On Gas-Liquid Slug Flows in Horizontal PipesDokument12 SeitenOTC 24437 A Comprehensive Analysis On Gas-Liquid Slug Flows in Horizontal PipesbabyweiNoch keine Bewertungen
- Launder1974 - Application of The Energy-Dissipation Model of Turbulence To The Calculation of Flow Near A Spinning Disc PDFDokument7 SeitenLaunder1974 - Application of The Energy-Dissipation Model of Turbulence To The Calculation of Flow Near A Spinning Disc PDFThePriusNoch keine Bewertungen
- Je300655b PDFDokument60 SeitenJe300655b PDF方琳 徐Noch keine Bewertungen
- Development of A Generalized Quartic Equation of State For Pure FDokument171 SeitenDevelopment of A Generalized Quartic Equation of State For Pure FFelipe Miguel Sánchez ClementsNoch keine Bewertungen
- Fundamentals of Valve Sizing For Liquids d350408x012Dokument10 SeitenFundamentals of Valve Sizing For Liquids d350408x012twins19564839100% (1)
- Modeling of Buried Natural Gas Pipeline Decompression: X. L. Zhou G. G. KingDokument8 SeitenModeling of Buried Natural Gas Pipeline Decompression: X. L. Zhou G. G. Kingmatrix69Noch keine Bewertungen
- Gases and Vacua: Handbook of Vacuum PhysicsVon EverandGases and Vacua: Handbook of Vacuum PhysicsA. H. BeckNoch keine Bewertungen
- Childhood Dream Job - AstronautDokument1 SeiteChildhood Dream Job - AstronautJuan Camilo HenaoNoch keine Bewertungen
- Nitrogen BlanketingDokument7 SeitenNitrogen Blanketing3bandhuNoch keine Bewertungen
- Dow Filmtec BW30XFR 400 34iDokument2 SeitenDow Filmtec BW30XFR 400 34iJuan Camilo HenaoNoch keine Bewertungen
- Parker Tank Blanketing White Paper PDFDokument4 SeitenParker Tank Blanketing White Paper PDFshashi kant kumarNoch keine Bewertungen
- Algebra Angel Cap9Dokument66 SeitenAlgebra Angel Cap9Ariel GomezNoch keine Bewertungen
- Batista 2013Dokument13 SeitenBatista 2013Juan Camilo HenaoNoch keine Bewertungen
- Purge With NitrogenDokument5 SeitenPurge With Nitrogendeion29100% (1)
- Uniteolstates Patent Office: "133.2535.lftii?iiii'tfiiiw 7 'Dokument2 SeitenUniteolstates Patent Office: "133.2535.lftii?iiii'tfiiiw 7 'Juan Camilo HenaoNoch keine Bewertungen
- Dow Filmtec BW30XFR 400 34iDokument2 SeitenDow Filmtec BW30XFR 400 34iJuan Camilo HenaoNoch keine Bewertungen
- Learn English Podcasts Elementary 01 01 Support Pack TranscriptDokument22 SeitenLearn English Podcasts Elementary 01 01 Support Pack Transcriptpramithbuddika100% (1)
- Creative Science & Research - Screen Printing Press (2004)Dokument21 SeitenCreative Science & Research - Screen Printing Press (2004)amrandconan100% (2)
- Extreme Independence - All Combinations of 4 LimbsDokument3 SeitenExtreme Independence - All Combinations of 4 LimbsJuan Camilo HenaoNoch keine Bewertungen
- Papper Co2Dokument15 SeitenPapper Co2Juan Camilo HenaoNoch keine Bewertungen
- IT H Unit IGDokument19 SeitenIT H Unit IGJuan Camilo HenaoNoch keine Bewertungen
- 1 PBDokument11 Seiten1 PBJuan Camilo HenaoNoch keine Bewertungen
- CLASS 300 Welding Neck & Slip-On Flanges: ASTM A-105 & A181-CLASS 60Dokument3 SeitenCLASS 300 Welding Neck & Slip-On Flanges: ASTM A-105 & A181-CLASS 60Juan Camilo HenaoNoch keine Bewertungen
- Created by Trial Version: HE-3 HE-4Dokument1 SeiteCreated by Trial Version: HE-3 HE-4Juan Camilo HenaoNoch keine Bewertungen
- 02bfe50dacb1fe39e0000000 PDFDokument50 Seiten02bfe50dacb1fe39e0000000 PDFJuan Camilo HenaoNoch keine Bewertungen
- Aspen HYSYS DynamicsDokument3 SeitenAspen HYSYS DynamicsJuan Camilo HenaoNoch keine Bewertungen
- Benzoguanamine: Product Specification SheetDokument1 SeiteBenzoguanamine: Product Specification SheetJuan Camilo HenaoNoch keine Bewertungen
- Zirconia-Supported Vanadium Oxide Catalysts For Ammoxidation and Oxidation of Toluene: A Characterization and Activity StudyDokument22 SeitenZirconia-Supported Vanadium Oxide Catalysts For Ammoxidation and Oxidation of Toluene: A Characterization and Activity StudyJuan Camilo HenaoNoch keine Bewertungen
- Poços de CaldasDokument8 SeitenPoços de CaldasAnninha FlavioNoch keine Bewertungen
- Ie00064a001 PDFDokument9 SeitenIe00064a001 PDFJuan Camilo HenaoNoch keine Bewertungen
- AbsorptionStripping PDFDokument25 SeitenAbsorptionStripping PDFJuan Camilo HenaoNoch keine Bewertungen
- United States' Patent Office: Patented July 20, 1948Dokument5 SeitenUnited States' Patent Office: Patented July 20, 1948Juan Camilo HenaoNoch keine Bewertungen
- Dowtherm A PropertiesDokument2 SeitenDowtherm A Propertiesflashiitm100% (1)
- Benzoguanamine: Product Specification SheetDokument1 SeiteBenzoguanamine: Product Specification SheetJuan Camilo HenaoNoch keine Bewertungen
- Answer Key p2 p1Dokument95 SeitenAnswer Key p2 p1Nafisa AliNoch keine Bewertungen
- Brochure Personal CareDokument38 SeitenBrochure Personal CarechayanunNoch keine Bewertungen
- Material and Energy Balance: PN Husna Binti ZulkiflyDokument108 SeitenMaterial and Energy Balance: PN Husna Binti ZulkiflyFiras 01Noch keine Bewertungen
- Tutorial On The ITU GDokument7 SeitenTutorial On The ITU GCh RambabuNoch keine Bewertungen
- What Is DSP BuilderDokument3 SeitenWhat Is DSP BuilderĐỗ ToànNoch keine Bewertungen
- 2018-04-12 List Mold TVSDokument5 Seiten2018-04-12 List Mold TVSFerlyn ValentineNoch keine Bewertungen
- LSUBL6432ADokument4 SeitenLSUBL6432ATotoxaHCNoch keine Bewertungen
- F-16c.1 Ginkgo Ginkgolic AcidDokument2 SeitenF-16c.1 Ginkgo Ginkgolic AcidNarongchai PongpanNoch keine Bewertungen
- DENSO COMMON RAIL INJECTOR REPAIR GUIDEDokument22 SeitenDENSO COMMON RAIL INJECTOR REPAIR GUIDEMarcoNoch keine Bewertungen
- Pitch Manual SpecializedDokument20 SeitenPitch Manual SpecializedRoberto Gomez100% (1)
- Direct From: 1St Quarter 2020Dokument23 SeitenDirect From: 1St Quarter 2020JeanNoch keine Bewertungen
- Laser Surface Treatment ProcessesDokument63 SeitenLaser Surface Treatment ProcessesDIPAK VINAYAK SHIRBHATENoch keine Bewertungen
- Datasheet PDFDokument6 SeitenDatasheet PDFAhmed ElShoraNoch keine Bewertungen
- Discuss The Challenges For Firms To Operate in The Hard-Boiled Confectionery Market in India?Dokument4 SeitenDiscuss The Challenges For Firms To Operate in The Hard-Boiled Confectionery Market in India?harryNoch keine Bewertungen
- Patent for Fired Heater with Radiant and Convection SectionsDokument11 SeitenPatent for Fired Heater with Radiant and Convection Sectionsxyz7890Noch keine Bewertungen
- Front Wheel Steering System With Movable Hedlights Ijariie5360Dokument6 SeitenFront Wheel Steering System With Movable Hedlights Ijariie5360Ifra KhanNoch keine Bewertungen
- Plate-Load TestDokument20 SeitenPlate-Load TestSalman LakhoNoch keine Bewertungen
- Sri Radhakrishna SwamijiDokument43 SeitenSri Radhakrishna SwamijiNarayana IyengarNoch keine Bewertungen
- Who will buy electric vehicles Segmenting the young Indian buyers using cluster analysisDokument12 SeitenWho will buy electric vehicles Segmenting the young Indian buyers using cluster analysisbhasker sharmaNoch keine Bewertungen
- Compare Blocks - ResultsDokument19 SeitenCompare Blocks - ResultsBramantika Aji PriambodoNoch keine Bewertungen
- RPG-7 Rocket LauncherDokument3 SeitenRPG-7 Rocket Launchersaledin1100% (3)
- Hypophosphatemic Rickets: Etiology, Clinical Features and TreatmentDokument6 SeitenHypophosphatemic Rickets: Etiology, Clinical Features and TreatmentDeysi Blanco CohuoNoch keine Bewertungen
- Reinforced Concrete Beam DesignDokument13 SeitenReinforced Concrete Beam Designmike smithNoch keine Bewertungen
- A Compilation of Thread Size InformationDokument9 SeitenA Compilation of Thread Size Informationdim059100% (2)
- Lec9-Rock Cutting ToolsDokument35 SeitenLec9-Rock Cutting ToolsAmraha NoorNoch keine Bewertungen
- Maintenance Handbook On Compressors (Of Under Slung AC Coaches) PDFDokument39 SeitenMaintenance Handbook On Compressors (Of Under Slung AC Coaches) PDFSandeepNoch keine Bewertungen
- O2 Orthodontic Lab Catalog PDFDokument20 SeitenO2 Orthodontic Lab Catalog PDFplayer osamaNoch keine Bewertungen
- Metal Framing SystemDokument56 SeitenMetal Framing SystemNal MénNoch keine Bewertungen
- Space DynamicsDokument37 SeitenSpace Dynamicspurushottam KashyapNoch keine Bewertungen
- Reflection 2: WHAT DOES It Mean To Be A Pacific Islander Today and in The Future To Me?Dokument5 SeitenReflection 2: WHAT DOES It Mean To Be A Pacific Islander Today and in The Future To Me?Trishika NamrataNoch keine Bewertungen