Beruflich Dokumente
Kultur Dokumente
Ali and Ards
Hochgeladen von
AdeRohmana0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
29 Ansichten5 SeitenThis document provides an overview of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). It discusses the definition and diagnosis of ALI, noting the criteria established in 1994. It also covers the epidemiology, etiology, pathophysiology, investigations, and management of ALI. The key points are that ALI is associated with high mortality, correct diagnosis is important to identify underlying causes, management includes protective ventilation strategies and treating the underlying condition.
Originalbeschreibung:
mangga
Copyright
© © All Rights Reserved
Verfügbare Formate
PDF, TXT oder online auf Scribd lesen
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenThis document provides an overview of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). It discusses the definition and diagnosis of ALI, noting the criteria established in 1994. It also covers the epidemiology, etiology, pathophysiology, investigations, and management of ALI. The key points are that ALI is associated with high mortality, correct diagnosis is important to identify underlying causes, management includes protective ventilation strategies and treating the underlying condition.
Copyright:
© All Rights Reserved
Verfügbare Formate
Als PDF, TXT herunterladen oder online auf Scribd lesen
0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
29 Ansichten5 SeitenAli and Ards
Hochgeladen von
AdeRohmanaThis document provides an overview of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). It discusses the definition and diagnosis of ALI, noting the criteria established in 1994. It also covers the epidemiology, etiology, pathophysiology, investigations, and management of ALI. The key points are that ALI is associated with high mortality, correct diagnosis is important to identify underlying causes, management includes protective ventilation strategies and treating the underlying condition.
Copyright:
© All Rights Reserved
Verfügbare Formate
Als PDF, TXT herunterladen oder online auf Scribd lesen
Sie sind auf Seite 1von 5
Acute lung injury and acute
respiratory distress syndrome
A Mackay MB ChB FRCA
M Al-Haddad MB ChB FRCA EDIC
Acute lung injury (ALI) is a common condition
that is characterized by acute severe hypoxia
that is not due to left atrial hypertension. The
term ALI encompasses a continuum of clinical
and radiographic changes that affect the lungs
with the acute respiratory distress syndrome
(ARDS) representing the more severe end of
this continuum. Despite advances in our under-
standing of the pathophysiology and manage-
ment of ALI, it is still associated with a high
mortality.
The aim of this review is to provide an over-
view of ALI and present the best available evi-
dence for its management.
Denition and diagnosis
ALI is a condition that is diagnosed clinically
and radiologically based on the presence of
non-cardiogenic pulmonary oedema and respir-
atory failure in a critically ill patient. It was
rst described in 12 patients in 1967 and
termed respiratory distress syndrome.
The denition in current use was described
in 1994 at the AmericanEuropean Consensus
Conference on ARDS. The aim of the de-
nition was to improve the diagnostic consist-
ency of ARDS and ALI allowing comparison
of data relating to epidemiological and treat-
ment studies. The criteria for diagnosing ALI
are listed in Table 1.
Strengths of these denition criteria are that
they are clinically relevant and easy to use,
making them ideal for use both in the clinical
setting and for research purposes.
Weaknesses are that the radiological criteria
are non-specic and are subject to inter-
observer variability. These radiological criteria
might be the only way in which ALI is distin-
guished from other pulmonary conditions such
as pneumonia. It is therefore possible that a
patient with the same condition is diagnosed
with pneumonia by one clinician and with ALI
(secondary to pneumonia) by another.
There is also no agreed standard ventilatory
strategy for patients before meeting the criteria
for hypoxaemia. It is possible, for example, to
improve the Pa
O
2
/FI
O
2
ratio of patients meeting
the diagnostic criteria by increasing PEEP. In
addition, patients with high atrial hypertension
from other causes, for example, left ventricular
failure or mitral regurgitation, are not immune
from developing ALI, but it is unclear how to
make the diagnosis in these cases.
Inconsistencies in the application of these
criteria could explain some of the variability in
the reported incidence of ALI. Universal appli-
cation of the diagnostic criteria without search-
ing for other causes of hypoxaemia may lead to
over-diagnosis of the condition. It is essential
to exclude conditions such as uid overload
before the diagnosis is made.
In summary, the diagnosis of ALI requires
the application of the consensus criteria by a
clinician who is able to take into account all
the factors mentioned above.
Epidemiology
The incidence of ALI is quoted to be between
17 and 34 per 100 000 patients per year.
1
About 16% of all patients ventilated in the ICU
for 24 h or more develop ALI. These patients
have a high mortality. This is a reection of the
severity of the primary pathology causing ALI,
other associated organ failures, and compli-
cations associated with critical illness. The
extent to which ALI contributes to the mor-
tality of patients is unknown, but most patients
die due to other organ failures rather than
hypoxaemia. There is some evidence, though,
that mechanical ventilation might contribute to
the failure of other organs as mentioned below.
Aetiology
ALI is a multifactorial disease process that
occurs due to an environmental trigger on the
background of a genetic predisposition. The
environmental causes of ALI can be classied
as direct or indirect depending on the mechan-
ism of injury to the lung. The most common
Key points
Acute lung injury (ALI) is a
common condition that is
associated with a high
mortality.
Correct diagnosis of ALI is
important because other
causes of hypoxaemia may
be more easily treated.
Identication and treatment
of the underlying condition
is paramount.
Management includes
standard ICU supportive
care and a tidal volume
limited to 6 ml kg
21
.
A conservative uid
management strategy might
have some benets.
A Mackay MB ChB FRCA
Specialist Registrar in Anaesthesia and
Intensive Care Medicine
Glasgow Royal Inrmary
84 Castle Street
Glasgow G4 0SF
UK
M Al-Haddad MB ChB FRCA EDIC
Consultant in Anaesthesia and Intensive
Care Medicine
Western Inrmary
Dumbarton Rd
Glasgow G11 6NT
UK
Tel: 44 141 211 2291
Fax: 44 141 211 1966
E-mail: malhaddad@doctors.org.uk
(for correspondence)
152
doi:10.1093/bjaceaccp/mkp028 Advance Access publication 30 August, 2009
Continuing Education in Anaesthesia, Critical Care & Pain | Volume 9 Number 5 2009
& The Author [2009]. Published by Oxford University Press on behalf of the British Journal of Anaesthesia.
All rights reserved. For Permissions, please email: journals.permissions@oxfordjournal.org
b
y
g
u
e
s
t
o
n
J
u
l
y
1
3
,
2
0
1
3
h
t
t
p
:
/
/
c
e
a
c
c
p
.
o
x
f
o
r
d
j
o
u
r
n
a
l
s
.
o
r
g
/
D
o
w
n
l
o
a
d
e
d
f
r
o
m
cause of ALI in the UK is sepsis which may be either intra- or
extrapulmonary. Other important causes are listed in Table 2.
The precise genetic basis of ALI remains elusive due to several
factors: the diversity of environmental stimuli involved, the
complex geneenvironment interaction, the heterogeneous nature
of the illness itself, and the presence of varying comorbidity.
Nevertheless, genomic markers have been identied that are
associated with a phenotype that predisposes to ALI.
2
Other genes
have been identied which may inuence the severity of the
illness, providing multiple potential targets for novel therapies to
be developed.
Pathophysiology
Patients with ALI and ARDS progress through a similar pathophy-
siological process irrespective of whether the damage is a direct
effect on the alveolar epithelial cells by an external stimulus or an
indirect process resulting from a more distant systemic inamma-
tory process mediated via cytokines.
The course of ALI can be described in three overlapping
phases. The acute or exudative phase starts early, lasts for up to 7
days from onset and is characterized by the development of hypox-
aemia, inltrates on the chest radiograph, and a reduction in pul-
monary compliance. These clinical changes are accompanied by
leakage of protein-rich uid in the alveoli, haemorrhage, and
diffuse neutrophilic alveolar inltrate with resultant endothelial
and epithelial injury. These activated neutrophils that have been
sequestered in the alveoli can be seen on microscopy of bronchoal-
veolar lavage uid.
The subacute or proliferative phase of ALI can occur from day
5 onwards. It is characterized by persistent hypoxaemia, increased
dead space, and reduced lung compliance. This is accompanied by
interstitial brosis, proliferation of type 2 alveolar cells, and dis-
ruption of capillary function due to microvascular thrombus for-
mation. In some patients, these changes resolve and clinical
improvement follows whereas other patients progress into the
chronic or brotic stage. This stage results from widespread pul-
monary brosis and loss of the normal lung structure leading to
worsening lung compliance and an increase in dead space. This
manifests clinically as a reduction in carbon dioxide excretion
which may be accompanied by an improvement in oxygenation.
This chronic stage is not clearly dened temporally but may start
as early as 14 days and last for many weeks.
The pathophysiological changes described above do not occur
homogenously in the lungs. High-resolution computed tomography
(CT) of the chest demonstrates this heterogeneity of alveoli
involvement.
Investigations
ALI is primarily a clinical diagnosis, although a chest X-ray
(CXR) and arterial blood gas analysis are also required to make
the diagnosis. Table 3 lists typical ndings on CXR that may
differentiate ALI from other conditions. These characteristic
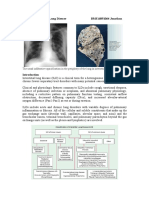
changes can be seen in Figure 1. It should be remembered,
however, that there are no pathognomonic radiographic ndings
for ALI and it is often difcult to differentiate it from other con-
ditions purely on the basis of radiographic ndings.
CT of the chest may give useful information in the patient with
ALI and typically demonstrates the heterogeneous nature of the
consolidation seen in ALI. It is predominantly visible in dependent
areas and has a patchy appearance with air bronchograms. CT
may also be useful to demonstrate the sequelae of ARDS such as
pneumothoraces and brosis. The risks of transferring a ventilated
patient to the CT scanner have to be considered carefully.
Management
General care
The principles of treating ALI are providing good supportive care
and maintaining oxygenation while diagnosing and treating the
underlying cause. It is important to emphasize that if the patient
fails to respond to therapy in the expected manner, then it should
Table 2 Causes of ALI
Direct Indirect
Pneumonia Sepsis
Aspiration Blood transfusion
Drowning Pancreatitis
Pulmonary embolism Trauma
Pulmonary contusion Burns
Inhalation injury Drugs
Reperfusion injury
Table 1 Diagnostic criteria for ALI
Acute onset
Presence of bilateral inltrates on CXR consistent with oedema
PAWP ,18 mm Hg or clinical absence of left atrial hypertension
Hypoxaemia with Pa
O
2
/FI
O
2
,40 (if Pa
O
2
/FI
O
2
,27, the term ARDS is used)
Table 3 Chest radiograph abnormalities. LVF, left ventricular failure
Diagnosis CXR ndings
ALI Bilateral peripheral asymmetrical consolidation
Normal width of vascular pedicle
Air bronchograms
Pneumonia Consolidationdiffuse patchy or focal
Diffuse oedema
Air bronchograms
Pleural effusion
LVF Upper lobe venous diversion (may not be apparent in supine patient)
Peribronchial cufng
Distension of interlobular septaeKerley A and B lines
Cardiomegaly
ALI and ARDS
Continuing Education in Anaesthesia, Critical Care & Pain j Volume 9 Number 5 2009 153
b
y
g
u
e
s
t
o
n
J
u
l
y
1
3
,
2
0
1
3
h
t
t
p
:
/
/
c
e
a
c
c
p
.
o
x
f
o
r
d
j
o
u
r
n
a
l
s
.
o
r
g
/
D
o
w
n
l
o
a
d
e
d
f
r
o
m
raise the suspicion that the original cause has not been treated
adequately or has been misdiagnosed.
Supportive care should include glycaemic control, deep venous
thrombosis and stress ulcer prophylaxis, preventing ventilator-
associated pneumonia and catheter-related bloodstream infection,
and early enteral feeding. No studies exist to support these
measures in patients with ALI specically, although they are con-
sidered standards of care in ICU.
Mechanical ventilation
Mechanical ventilation is conventionally delivered as positive
pressure ventilation with PEEP via a tracheal tube. The principle
of ventilation in patients with ALI is to maintain adequate gas
exchange until the cellular damage resolves without causing
ventilator-induced lung injury. The latter condition encompasses
the tetrad of barotrauma, volutrauma, atelectrauma, and biotrauma
and poses a signicant risk to all patients receiving mechanical
ventilation.
The ARDSnet (National Heart, Lung, and Blood Institutes
Acute Respiratory Distress Syndrome Clinical Trials Network)
tidal volume study
3
showed a signicant reduction in mortality by
utilizing a low-volume ventilatory strategy based on predicted
body weight (6 ml kg
21
and peak pressures ,30 cm H
2
O vs
12 ml kg
21
and peak pressures ,50 cm H
2
O). Traditionally, high
volumes were used in an effort to normalize the patients arterial
blood gases. These large volumes are thought to damage the
remaining areas of healthy lung by over-ination due to the rela-
tively normal compliance of these areas compared with the seg-
ments affected by ALI. This more conservative ventilatory strategy
is also associated with a signicantly lower level of circulating
cytokines, the cause of biotrauma, and distant organ damage.
PEEP improves oxygenation in ALI by maintaining the patency
of injured alveoli and allowing an improvement in ventilation/
perfusion matching and a reduction of intrapulmonary shunt. It
also prevents atelectrauma caused by the repeated opening and
closing of alveoli. These factors have led investigators to try to
dene an optimal level of PEEP in ALI/ARDS. The ARDSnet
group conducted a large randomized controlled trial
4
that did not
demonstrate a difference in outcome between patients ventilated
with a lower level of PEEP (8 cm H
2
O) and those ventilated with
a higher level of PEEP (14 cm H
2
O). Two recent large randomized
controlled trials also studied the effect of elevated levels of PEEP
and failed to show any reduction in mortality.
5 6
Recruitment manoeuvres have been used for many years to
improve oxygenation by opening up collapsed alveoli. Notwith-
standing recent advances in the monitoring of recruitment
manoeuvres using CT and electrical impedance tomography, no
study has demonstrated an improvement in survival using these
measures.
Despite the many different ventilator modes available, there is
no evidence to date to suggest that any method improves survival,
provided the above limitations to tidal volume and peak pressure
are adhered to. With regard to weaning from mechanical venti-
lation, no method has been proven to be superior to any other.
Fluid management
Owing to the altered capillary permeability in ALI, excessive uid
administration leads to a deterioration in gas exchange. This is
because excess uid increases the capillary hydrostatic pressure,
which in turn causes pulmonary oedema and worsening oxygen-
ation and carbon dioxide elution. On the other hand, the benets
of a more liberal uid strategy are an increase in cardiac output
with a possible improvement in non-pulmonary organ perfusion.
The benet of uid restriction was partially demonstrated by
the Fluids and Catheters Treatment Trial (FACTT) study carried
out by the ARDSnet group,
7
which compared a conservative
(2163 ml in rst 7 days) with a liberal (6992 ml in rst 7 days)
uid strategy. Patients who were in the conservative uid group
had a reduction in duration of ventilation and ICU stay, but no
reduction in mortality. Notably, there was no increase in any clini-
cally signicant adverse event, including renal failure. On this
basis, we would recommend a conservative uid regime as a
low-cost, low-risk intervention that could lead to improvement in
clinically important outcomes.
Steroids
Steroids exert an anti-inammatory effect by inhibiting arachidonic
acid metabolism and reducing eosinophil activity. As ALI is
associated with an increase in both serum and bronchoalveolar
lavage levels of cytokines and chemokines, it is tempting to think
that the anti-inammatory effects of steroids would be benecial.
For this reason, their use has been investigated as a preventive
measure and also a therapeutic one in all phases of the condition.
A recent meta-analysis of the use of steroids in the early exudative
phase of ALI conrmed that steroids confer no survival benet.
In the subacute phase, the alveoli are inltrated by myobro-
blasts and collagen deposits. Following a study that showed a
Fig 1 Chest radiograph of a patient with ARDS.
ALI and ARDS
154 Continuing Education in Anaesthesia, Critical Care & Pain j Volume 9 Number 5 2009
b
y
g
u
e
s
t
o
n
J
u
l
y
1
3
,
2
0
1
3
h
t
t
p
:
/
/
c
e
a
c
c
p
.
o
x
f
o
r
d
j
o
u
r
n
a
l
s
.
o
r
g
/
D
o
w
n
l
o
a
d
e
d
f
r
o
m
signicant reduction in this process after the administration of
methylprednisolone,
8
the ARDSnet group performed a large multi-
centre randomized controlled trial using the same trial protocol.
9
This new larger study failed to show a difference in hospital mor-
tality. On the basis of these studies, the routine use of methylpred-
nisolone is not recommended in patients with ALI.
Prone positioning
Prone positioning has been demonstrated to enhance oxygenation
by improving alveolar ventilation/perfusion matching. It improves
lung mechanics by increasing compliance and recruitment of
atelectatic basal regions in addition to improving clearance of
respiratory secretions.
By achieving the same Pa
O
2
at lower airway pressures, it was
postulated that prone positioning might reduce the occurrence of
ventilator-induced lung injury. Despite improving the Pa
O
2
/FI
O
2
ratio, reducing the incidence of ventilator-associated pneumonia,
and not being associated with major adverse airway compli-
cations,
10
routine prone positioning does not improve survival or
reduce the duration of mechanical ventilation in patients with ALI/
ARDS. On this basis, its routine use is not recommended;
however, it may be a useful temporary measure in a patient who is
critically hypoxic.
Other therapies
Nitric oxide
A recent systematic review of the use of iNO in ALI
11
showed no
improvement in clinical outcome, despite a short-lived (2448 h)
improvement in oxygenation and a reduction in pulmonary vascu-
lar resistance. On this basis, its routine use for patients with
ARDS/ALI cannot be recommended.
Extracorporeal lung support
Extracorporeal membrane oxygenation (ECMO) has been
suggested as a therapy for ALI/ARDS, as it allows gas exchange
to continue while preventing further damage to the lung by venti-
lation. The recently completed Conventional ventilation vs ECMO
in Severe Acute Respiratory failure (CESAR) trial suggests that
the use of ECMO results in improved survival without severe dis-
ability with a number needed to treat of 6 and thus may prove to
be cost-effective when compared with conventional ventilation.
This study was not published in a peer-reviewed journal at the
time this manuscript was written. The use of ECMO is also con-
ned to a few specialist centres in the UK. Arteriovenous extracor-
poreal CO
2
removal (AVECCO
2
R) has also been proposed as a
rescue therapy in severe ARDS in order to treat hypercarbia.
Current evidence is based on case series and results from these are
promising. Uncoupling of respiratory functions allows carbon
dioxide clearance while maintaining oxygenation by low-frequency
positive pressure ventilation or apnoeic oxygenation, effectively
preventing further ventilator-induced injury. Both ECMO and
AVECCO
2
R have interventional procedure guidance available
from the National Institute for Health and Clinical Excellence.
Physiotherapy and positioning
The role of physiotherapy in ARDS is uncertain and little evidence
exists concerning its use. Aims of physiotherapy in ARDS are
similar to those in most ICU patientsto promote the removal of
retained secretions and to encourage active and passive movements
in patients who are often bed bound for a long period of time.
Methods used to achieve these aims include:
positioning to enhance the removal of secretions and to
improve gas exchange;
percussion and vibration;
tracheal suction;
continuous rotational therapy.
Sitting ventilated patients up to 308 may reduce the risk of aspira-
tion and subsequent ventilator-associated pneumonia and can be
recommended on the basis of the available evidence.
Outcome
A large European study in 2004 demonstrated that ICU and hospi-
tal mortality associated with ALI were 45.8% and 54.7%, respect-
ively.
12
Mortality was signicantly worse for patients with ARDS
(ICU mortality 49.4% and hospital mortality 57.9%) compared
with those who had ALI but not ARDS (ICU mortality 22.6% and
hospital mortality 32.7%).
Independent factors associated with an increase in mortality
include:
advanced age;
immunocompromise;
high APACHE-II/SAPS-II/SOFA score;
H
over 50 nmol litre
21
;
early barotrauma (air leak within rst 2 days).
Although respiratory function is often normal by 12 months after
admission to the ICU for patients who survive ALI, long-term
sequelae are common. These include neurocognitive impairment,
psychological morbidity, and muscle weakness that often delay the
return to a normal quality of life and sometimes lead to permanent
disability.
Conclusion
ALI is a common condition in patients admitted to the ICU.
Despite recent advances, ALI is still associated with signicant
morbidity and mortality. Basic management should include good
supportive care and treatment of the underlying cause. The only
specic management strategy shown to have a survival benet is
restrictive ventilation with a tidal volume of 6 ml kg
21
and a
plateau pressure of ,30 cm H
2
O. A conservative uid manage-
ment strategy has been shown to reduce duration of mechanical
ventilation and ICU stay. Other measures may be considered in
ALI and ARDS
Continuing Education in Anaesthesia, Critical Care & Pain j Volume 9 Number 5 2009 155
b
y
g
u
e
s
t
o
n
J
u
l
y
1
3
,
2
0
1
3
h
t
t
p
:
/
/
c
e
a
c
c
p
.
o
x
f
o
r
d
j
o
u
r
n
a
l
s
.
o
r
g
/
D
o
w
n
l
o
a
d
e
d
f
r
o
m
individual cases, but there is insufcient evidence to recommend
their widespread use for all patients.
References
1. McCallum NS, Evans TW. Epidemiology of acute lung injury. Curr Opin
Crit Care 2005; 11: 439
2. Kamp R, Sun X, Garcia JG. Making genomics functional: deciphering the
genetics of acute lung injury. Proc Am Thorac Soc 2008; 5: 34853
3. The Acute Respiratory Distress Syndrome Network. Ventilation with
lower tidal volumes as compared with traditional tidal volumes for acute
lung injury and the acute respiratory distress syndrome. N Engl J Med
2000; 342: 13018
4. The Acute Respiratory Distress Syndrome Network. Higher versus
lower positive end-expiratory pressures in patients with the acute respir-
atory distress syndrome. N Engl J Med 2004; 351: 32736
5. Meade MO, Cook DJ, Guyatt GH et al. Ventilation strategy using low
tidal volumes, recruitment maneuvers, and high positive end-expiratory
pressure for acute lung injury and acute respiratory distress syndrome: a
randomized controlled trial. J Am Med Assoc 2008; 299: 63745
6. Mercat A, Richard J-CM, Vielle B. Positive end-expiratory pressure
setting in adults with acute lung injury and acute respiratory distress syn-
drome: a randomized controlled trial. J Am Med Assoc 2008; 299:
64655
7. The Acute Respiratory Distress Syndrome Network. Comparison of
two uid-management strategies in acute lung injury. N Engl J Med 2006;
354: 256475
8. Meduri GU, Headley AS, Golden E et al. Effect of prolonged methylpred-
nisolone therapy in unresolving acute respiratory distress syndrome: a
randomized controlled trial. J Am Med Assoc 1998; 280: 15965
9. The Acute Respiratory Distress Syndrome Network. Efcacy and Safety
of corticosteroids for persistent acute respiratory distress syndrome. N
Engl J Med 2006; 354: 167184
10. Abroug F, Ouanes-Besbes L, Elatrous S, Brochard L. The effect of prone
positioning in acute respiratory distress syndrome or acute lung injury:
a meta-analysis. Areas of uncertainty and recommendations for
research. Intensive Care Med 2008; 34: 100211
11. Adhikari NK, Burns KE, Freidrich JO, Granton JT, Cook DJ, Meade
MO. Effect of nitric oxide on oxygenation and mortality in acute lung
injury: systematic review and meta analysis. Br Med J 2007; 334:
77982
12. Brun-Buisson C, Minelli C, Bertolini G et al. ALIVE Study Group.
Epidemiology and outcome of acute lung injury in European intensive
care units. Results from the ALIVE study. Intensive Care Med 2004; 30:
5161
Please see multiple choice questions 1315
ALI and ARDS
156 Continuing Education in Anaesthesia, Critical Care & Pain j Volume 9 Number 5 2009
b
y
g
u
e
s
t
o
n
J
u
l
y
1
3
,
2
0
1
3
h
t
t
p
:
/
/
c
e
a
c
c
p
.
o
x
f
o
r
d
j
o
u
r
n
a
l
s
.
o
r
g
/
D
o
w
n
l
o
a
d
e
d
f
r
o
m
Das könnte Ihnen auch gefallen
- "Imitators" of The ARDS Implications For Diagnosis and Treatment Acute Lung Injury (ALI) and ARDS (ALI/ARDS)Dokument22 Seiten"Imitators" of The ARDS Implications For Diagnosis and Treatment Acute Lung Injury (ALI) and ARDS (ALI/ARDS)Odett EzzatNoch keine Bewertungen
- ID Faktor Faktor Yang Berhubungan Dengan Buang Air Besar Di Jamban Di Desa GunungsaDokument14 SeitenID Faktor Faktor Yang Berhubungan Dengan Buang Air Besar Di Jamban Di Desa GunungsaRSU DUTA MULYANoch keine Bewertungen
- Geriatrics PT Presentation by Group 2Dokument33 SeitenGeriatrics PT Presentation by Group 2Dr-Muhammad QasimNoch keine Bewertungen
- SDL 18 Interstitial Lung Disease BMS16091064Dokument8 SeitenSDL 18 Interstitial Lung Disease BMS16091064Jonathan YeohNoch keine Bewertungen
- Acute Lung InjuryDokument5 SeitenAcute Lung InjuryKinanti Devia LarasatiNoch keine Bewertungen
- Gold Criteria For COPDDokument8 SeitenGold Criteria For COPDmrppserodio7856Noch keine Bewertungen
- 1 s2.0 S0025712522000244 MainDokument13 Seiten1 s2.0 S0025712522000244 Mainlmra89Noch keine Bewertungen
- Acute Exacerbation of COPD Nursing Application of Evidence-Based GuidelinesDokument17 SeitenAcute Exacerbation of COPD Nursing Application of Evidence-Based GuidelinesCandice ChengNoch keine Bewertungen
- Comprehensive Review of Current Diagnostic and Treatment Approaches To Interstitial Lung Disease Associated With Rheumatoid ArthritisDokument10 SeitenComprehensive Review of Current Diagnostic and Treatment Approaches To Interstitial Lung Disease Associated With Rheumatoid ArthritisskoamdaskondiajosNoch keine Bewertungen
- FPI Progn TratDokument13 SeitenFPI Progn TratTurcu AndreeaNoch keine Bewertungen
- Sdra Reconocimiento Actual 2021Dokument16 SeitenSdra Reconocimiento Actual 2021NATHALY JOVANA CONTRERAS HERRERANoch keine Bewertungen
- ARDSDokument6 SeitenARDSppdsbedahumum24Noch keine Bewertungen
- Chronic Obstructive Pulmonary Disease in Elderly PatientsDokument14 SeitenChronic Obstructive Pulmonary Disease in Elderly PatientsFranz MiguelNoch keine Bewertungen
- Transfusion-Related Acute Lung Injury: HistoryDokument20 SeitenTransfusion-Related Acute Lung Injury: HistoryBladimir CentenoNoch keine Bewertungen
- Copd Review 2002 ChestDokument8 SeitenCopd Review 2002 ChestLeonardo GarciaNoch keine Bewertungen
- 2010 Hemorragia Alveolar DifusaDokument8 Seiten2010 Hemorragia Alveolar DifusaCecilia Requejo TelloNoch keine Bewertungen
- TRALI - A Review.: TRALI Is Transfusion Related Acute Lung Injury Due To Immune MediatedDokument6 SeitenTRALI - A Review.: TRALI Is Transfusion Related Acute Lung Injury Due To Immune MediatedArun KumarNoch keine Bewertungen
- Manifestaciones Sistemicas EPOCDokument9 SeitenManifestaciones Sistemicas EPOCAlejandra OrtegaNoch keine Bewertungen
- ABIGAIL 2010 - Diffuse Alveolar HemorrhageDokument10 SeitenABIGAIL 2010 - Diffuse Alveolar HemorrhageRafael JustinoNoch keine Bewertungen
- COPD and Pulmonary Thromboembolism (For Galley Proof)Dokument6 SeitenCOPD and Pulmonary Thromboembolism (For Galley Proof)Ram AdhikariNoch keine Bewertungen
- Fluid Therapy and Acute Respiratory Distress Syndrome: Jisoo Lee,, Keith Corl,, Mitchell M. LevyDokument9 SeitenFluid Therapy and Acute Respiratory Distress Syndrome: Jisoo Lee,, Keith Corl,, Mitchell M. LevyLuis HernándezNoch keine Bewertungen
- Chronic Obstructive Pulmonary DiseaseDokument8 SeitenChronic Obstructive Pulmonary DiseaseGeozel VivienneNoch keine Bewertungen
- Srda 2016 PDFDokument14 SeitenSrda 2016 PDFGeibel Reis JrNoch keine Bewertungen
- Transfusion-Related Acute Lung Injury - StatPearls - NCBI BookshelfDokument5 SeitenTransfusion-Related Acute Lung Injury - StatPearls - NCBI Bookshelfindahmsafitri3Noch keine Bewertungen
- Acute Respiratory Distress Syndrome: Clinical ReviewDokument6 SeitenAcute Respiratory Distress Syndrome: Clinical ReviewVijeyachandhar DorairajNoch keine Bewertungen
- The Asthma-COPD Overlap Syndrome: LT Col John F. Freiler, MDDokument5 SeitenThe Asthma-COPD Overlap Syndrome: LT Col John F. Freiler, MDIan HuangNoch keine Bewertungen
- Acute Respiratory Distress SyndromeDokument21 SeitenAcute Respiratory Distress Syndromemisseve252100% (1)
- Transfusion-Related Acute Lung Injury (Trali) : Description and IncidenceDokument9 SeitenTransfusion-Related Acute Lung Injury (Trali) : Description and IncidenceNanda SilvaNoch keine Bewertungen
- COPD2019Dokument9 SeitenCOPD2019ClintonNoch keine Bewertungen
- 2° Artículo de EpocDokument10 Seiten2° Artículo de EpocveroarujogonzalesNoch keine Bewertungen
- Idiopathic Pulmonary Fibrosis: Interstitial Lung DiseaseDokument5 SeitenIdiopathic Pulmonary Fibrosis: Interstitial Lung DiseaseAmjaSaudNoch keine Bewertungen
- ARDS EngDokument25 SeitenARDS EngTofik ShehajNoch keine Bewertungen
- The Acute Respiratory Distress Syndrome in 2013: Review Open AccessDokument6 SeitenThe Acute Respiratory Distress Syndrome in 2013: Review Open Accessindriyanti natasya ayu utami kottenNoch keine Bewertungen
- Coagulation Dysfunction in Patients With AECOPD and ItsDokument6 SeitenCoagulation Dysfunction in Patients With AECOPD and ItsAndreea MoalesNoch keine Bewertungen
- Enfermedad Pulmonar ObstructivaDokument9 SeitenEnfermedad Pulmonar ObstructivaRomi Vanessa GutiérrezNoch keine Bewertungen
- Congenital Lobar EmphysemaDokument3 SeitenCongenital Lobar Emphysemamadimadi11Noch keine Bewertungen
- DR Syed Aqeel Gilani Department of Pulmonology Ayub Teaching Hospital AbbottabadDokument49 SeitenDR Syed Aqeel Gilani Department of Pulmonology Ayub Teaching Hospital AbbottabadAnka EremiaNoch keine Bewertungen
- The Acute Respiratory Distress Syndrome: Review SeriesDokument10 SeitenThe Acute Respiratory Distress Syndrome: Review Seriesadek07Noch keine Bewertungen
- Sarcoidosis Review. JAMADokument9 SeitenSarcoidosis Review. JAMARafael ReañoNoch keine Bewertungen
- Pulmonary Overlap Syndromes, With A Focus On COPD and ILDDokument15 SeitenPulmonary Overlap Syndromes, With A Focus On COPD and ILDsavvy_as_98-1Noch keine Bewertungen
- 127+Layout+JR+8 (2) +DR +Budi+Yanti+ (P 87-93) +Dokument7 Seiten127+Layout+JR+8 (2) +DR +Budi+Yanti+ (P 87-93) +Julian HutabaratNoch keine Bewertungen
- Pulmonary Tuberculosis Presenting With Acute Respiratory Distress Syndrome (Ards) : A Case Report and Review of LiteratureDokument5 SeitenPulmonary Tuberculosis Presenting With Acute Respiratory Distress Syndrome (Ards) : A Case Report and Review of Literatureamelya asryNoch keine Bewertungen
- Thorax00193 0009 PDFDokument10 SeitenThorax00193 0009 PDFhotmart ventasNoch keine Bewertungen
- Asthma Diagnosis and TreatmentDokument11 SeitenAsthma Diagnosis and TreatmentginaNoch keine Bewertungen
- Fisiopatologia de SDRADokument19 SeitenFisiopatologia de SDRARuben HerediaNoch keine Bewertungen
- Copd Exacerbations in EdDokument23 SeitenCopd Exacerbations in EdEzequiel MenesesNoch keine Bewertungen
- Ali 01Dokument10 SeitenAli 01Juan Fernando Garcia RobledoNoch keine Bewertungen
- Evolvingepidemiology Anddefinitionsofthe Acuterespiratorydistress Syndromeandearlyacute LunginjuryDokument16 SeitenEvolvingepidemiology Anddefinitionsofthe Acuterespiratorydistress Syndromeandearlyacute LunginjuryVijay KumarNoch keine Bewertungen
- Interstitial Lung Changes in Sjögren's Syndrome: Report of 3 CasesDokument5 SeitenInterstitial Lung Changes in Sjögren's Syndrome: Report of 3 CasesRyl WonNoch keine Bewertungen
- Imaging in Chronic Obstructive Pulmonary DiseaseDokument9 SeitenImaging in Chronic Obstructive Pulmonary DiseaseHarizNoch keine Bewertungen
- 2019 - Chronic Obstructive Pulmonary Disease andDokument6 Seiten2019 - Chronic Obstructive Pulmonary Disease andAndreea MoalesNoch keine Bewertungen
- Acute Lung InjuryDokument20 SeitenAcute Lung Injurymelissa silvaNoch keine Bewertungen
- Utilidad Del Lavado Broncoalveolar en La Evaluación de La Enfermedad Pulmonar IntersticialDokument8 SeitenUtilidad Del Lavado Broncoalveolar en La Evaluación de La Enfermedad Pulmonar IntersticialAlexis Guevara QuijadaNoch keine Bewertungen
- Cardiogenic Pulmonary Edema in Emergency Medi - 231130 - 222540Dokument19 SeitenCardiogenic Pulmonary Edema in Emergency Medi - 231130 - 222540Dayana OviedoNoch keine Bewertungen
- Hepatic Encephalopathy Novel Insights Into Classification, Pathophysiology and TherapyDokument22 SeitenHepatic Encephalopathy Novel Insights Into Classification, Pathophysiology and TherapyMichelle RdgzNoch keine Bewertungen
- Acute Respiratory Distress Syndrome: Diagnosis and ManagementDokument7 SeitenAcute Respiratory Distress Syndrome: Diagnosis and ManagementCharly GradosNoch keine Bewertungen
- P 6 B 06 Sample 01Dokument15 SeitenP 6 B 06 Sample 01adjoaaframd12Noch keine Bewertungen
- Distinguishing TRALI and TACODokument6 SeitenDistinguishing TRALI and TACOl1o2stNoch keine Bewertungen
- Tsuchiya 2019Dokument11 SeitenTsuchiya 2019Olivia Chandra DeviNoch keine Bewertungen
- Hydrochlorothiazide Versus Chlorthalidone: Brief ReviewDokument6 SeitenHydrochlorothiazide Versus Chlorthalidone: Brief ReviewKrishna PrasadNoch keine Bewertungen
- OET Practice Reading Test Part A:: JourneyDokument9 SeitenOET Practice Reading Test Part A:: JourneyPrasoon PremrajNoch keine Bewertungen
- My Personal Health and Wellness PlanDokument2 SeitenMy Personal Health and Wellness PlanLeighsen VillacortaNoch keine Bewertungen
- Population Pyramid IndiaDokument4 SeitenPopulation Pyramid India18maneeshtNoch keine Bewertungen
- Research IDokument3 SeitenResearch Iapi-3730987Noch keine Bewertungen
- Unit 11: Develop Professional Supervision Practice in Health and Social Care or Children and Young People's Work SettingsDokument4 SeitenUnit 11: Develop Professional Supervision Practice in Health and Social Care or Children and Young People's Work SettingsMalgorzata MarcinkowskaNoch keine Bewertungen
- Traumatic Brain InjuryDokument50 SeitenTraumatic Brain InjuryDavide LeeNoch keine Bewertungen
- Risk Assessment For International Dissemination of COVID-19 To The ASEAN RegionDokument16 SeitenRisk Assessment For International Dissemination of COVID-19 To The ASEAN RegionLouieNoch keine Bewertungen
- Community Health Nursing Approach: Mrs - Neethu Vincent Asst Professor KVM College of NursingDokument21 SeitenCommunity Health Nursing Approach: Mrs - Neethu Vincent Asst Professor KVM College of NursingNeethu VincentNoch keine Bewertungen
- HIV - LancetDokument13 SeitenHIV - LancetcristhianldsNoch keine Bewertungen
- Exploring TheDokument20 SeitenExploring TheMiroslava DjordjevicNoch keine Bewertungen
- Queen - My Melancholy Blues (Piano Sheet Music)Dokument27 SeitenQueen - My Melancholy Blues (Piano Sheet Music)Kamil Iżykowski0% (3)
- The Children Act 1989 Guidance and RegulationDokument186 SeitenThe Children Act 1989 Guidance and RegulationkvisaNoch keine Bewertungen
- UntitledDokument2 SeitenUntitledapi-76999604Noch keine Bewertungen
- Sheet 1Dokument30 SeitenSheet 1ahmed amiraliNoch keine Bewertungen
- 3a. Antisocial Personality DisorderDokument19 Seiten3a. Antisocial Personality DisorderspartanNoch keine Bewertungen
- Curriculum VitaeDokument2 SeitenCurriculum VitaeHORT SroeuNoch keine Bewertungen
- Science EssayDokument2 SeitenScience EssayRehoboth EljakyNoch keine Bewertungen
- Types of Hospitals: Gynecology Services & Other Facilities Pharmacy LaboratoryDokument6 SeitenTypes of Hospitals: Gynecology Services & Other Facilities Pharmacy LaboratoryMaham ShahidNoch keine Bewertungen
- BBLK Surabaya: National Tuberculosis Reference Laboratory For Culture/Drug Susceptibility TestingDokument12 SeitenBBLK Surabaya: National Tuberculosis Reference Laboratory For Culture/Drug Susceptibility TestingMas Paijo PeloukNoch keine Bewertungen
- RRLDokument12 SeitenRRLEmilyne Joy Mendoza CabayaNoch keine Bewertungen
- 30 Days To Better Habits - Examples DatabaseDokument9 Seiten30 Days To Better Habits - Examples DatabaseAlysia SiswantoNoch keine Bewertungen
- Case Study 12Dokument1 SeiteCase Study 12Kaitlyn BretoiNoch keine Bewertungen
- Hyperbaric Oxygen Theray (HBOT) Service StandardsDokument30 SeitenHyperbaric Oxygen Theray (HBOT) Service StandardsalviaNoch keine Bewertungen
- Bosh Training ManualDokument287 SeitenBosh Training ManualYouls TorNoch keine Bewertungen
- Omeprazole Drug StudyDokument8 SeitenOmeprazole Drug StudyJe Michelle LoayonNoch keine Bewertungen
- Procedural Dermatology CmeDokument3 SeitenProcedural Dermatology CmePadmapriya SrinivasanNoch keine Bewertungen
- RejectedDokument2 SeitenRejectedAutismeyeNoch keine Bewertungen
- Complete Cardiac DiagnosisDokument2 SeitenComplete Cardiac DiagnosisAria Jean MostajoNoch keine Bewertungen
- List of Selected Sanjeevini Combinations SSCDokument1 SeiteList of Selected Sanjeevini Combinations SSCsatyr7020% (1)