Beruflich Dokumente
Kultur Dokumente
CARA 2Q14 Research Report (Janney)
Hochgeladen von
KipWellsCopyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
CARA 2Q14 Research Report (Janney)
Hochgeladen von
KipWellsCopyright:
Verfügbare Formate
Chiara Russo 617-557-2984
crusso@janney.com
Cara Therapeutics Inc.
CARA - BUY
August 8, 2014
Specialty Pharmaceuticals
Cara Therapeutics Inc.
(CARA) - BUY
Price: $10.80
Fair Value Estimate: $25.00
52-Week Range: $10.40-$23.25
Market Cap (MM): $244
Shr.O/S-Diluted (mm): 22.6
Average Daily Volume: 81,498
Dividend: NA
Book Value: $1.33
FYE: Dec 2013A 2014E 2015E
EPS: $(0.74)A $(0.84)E $(1.06)E
Prior EPS: $(1.20) $(1.52)
P/E: NA NA NA
Quarterly EPS:
Q1 $(0.42)A $(0.22)A --
Q2 $(0.38)A $(0.16)A --
Q3 $(0.38)A $(0.22)E --
Q4 $(0.49)A $(0.22)E --
FYE: Dec 2013A 2014E 2015E
Revenue (M): $11.9A $2.0E $0.0E
Quarterly Revenue (M):
Q1 $0.1A $0.2A --
Q2 $10.1A $1.0A --
Q3 $0.8A $0.2E --
Q4 $1.0A $0.7E --
Equity Research
Note
CARA: 2Q14 IV CR845
Preparing for Phase 3 Initiation
End of 2014 Full of Catalysts
INVESTMENT CONCLUSION:
Last night after the close, CARA reported 2Q14 earnings with an EPS of ($0.16),
compared to Janney estimates of ($0.27). On the call, management continued to speak
positively about the clinical progress of its lead candidate CR845 (IV administrated
kappa-opioid for acute pain) as in late July, CARA initiated its Human Abuse Liability
(HAL) study with top-line data expected in 4Q14. These likability studies will help CARA
better understand the potential for abuse of the product and even more importantly, where
it may be evaluated as a scheduled or non-scheduled product. CARA continues to manage
its burn rate in a cost effective manner, which is always a plus in our book. Late 2014 also
brings several key data points that should solidify the value of CR845 program. Maintain
Buy rating, $25 fair value.
KEY POINTS:
Managing the cash burn: CARA reported 2Q14 earnings after the close of the
market last night with an EPS of ($0.16) compared to a Janney estimate of ($0.27).
The two main differences were the $500K Maruishi milestone payment and higher
than expected R&D costs. These costs should continue to accelerate as clinical
development continues into 2H14. G&A was in-line with estimates. CARA finished
2Q14 with ~$63M in cash, enough for operations through 2015.
IV CR845 continues to progress with HAL up and running: In late July, CARA
initiated the likeability studies for IV CR845. Though originally thought to be
measured against morphine, a schedule II pain killer, the study will be conducted,
per FDA guidance with pentazocine, a mixed-action kappa and mu opioid receptor
that is a schedule IV substance. Management feels (and we concur) that if IV CR845
has a lower likeability score than pentazocine an easier argument can be made for
an approval as a schedule V or even unscheduled opioid given the starting point of a
schedule IV versus the original starting point of a schedule II. Data for the HAL study
is expected in 4Q14. Phase 3 initiation expected to start shortly after in 1Q15.
Got to have a tablet with an IV: In June, CARA began the single- and multiple dose
ascending Phase 1 trials. A tablet formulation will allow for a more commercially
viable product as patients can "step-down" from the IV and take home the tablets.
Top-line results expected in 4Q14.
Dialysis itch? IV CR845 could scratch it: CARA has also initiated a Phase 2a Proof-
of-Concept study of IV CR845 in uremic pruritus (UP) which is very common side
of kidney dialysis. Though initially focusing on UP, if successful the program could
expand to a more broad and general anti-itch agent. Data is expected 1H15.
Maintain Buy rating, $25 fair value: We value CARA at $25/share based on a sum-
of-the-parts with CR845 sales of at $22.50/share based on a 4x multiple of 2019 US
sales discounted 5 years at 20% to account for the risks remaining in this program.
Our remaining $2.50/share value is based on cash (end 2014) and technology value.
Research Analyst Certifications and Important Disclosures
are on pages 4 - 5 of this report
EXHIBIT 1:
- 2 -
EXHIBIT 2:
- 3 -
IMPORTANT DISCLOSURES
Research Analyst Certification
I, Chiara Russo, the Primarily Responsible Analyst for this research report, hereby certify that all of the views expressed in
this research report accurately reflect my personal views about any and all of the subject securities or issuers. No part of my
compensation was, is, or will be, directly or indirectly, related to the specific recommendations or views I expressed in this research
report.
Janney Montgomery Scott LLC ("Janney") Equity Research Disclosure Legend
Cara Therapeutics Inc. currently is, or during the past 12 months was, a Janney Montgomery Scott LLC client. Janney Montgomery
Scott LLC, provided investment banking related services.
Janney Montgomery Scott LLC managed or co-managed a public offering of securities for Cara Therapeutics Inc. in the past 12
months.
Janney Montgomery Scott LLC received compensation for investment banking services from Cara Therapeutics Inc. in the past
12 months.
Janney Montgomery Scott LLC intends to seek or expects to receive compensation for investment banking services from Cara
Therapeutics Inc. in the next three months.
The research analyst is compensated based on, in part, Janney Montgomery Scott's profitability, which includes its investment
banking revenues.
Definition of Ratings
BUY: Janney expects that the subject company will appreciate in value. Additionally, we expect that the subject company will
outperform comparable companies within its sector.
NEUTRAL: Janney believes that the subject company is fairly valued and will perform in line with comparable companies within
its sector. Investors may add to current positions on short-term weakness and sell on strength as the valuations or fundamentals
become more or less attractive.
SELL: Janney expects that the subject company will likely decline in value and will underperform comparable companies within
its sector.
Price Charts
- 4 -
Janney Montgomery Scott Ratings Distribution as of 6/30/14
IB Serv./Past 12 Mos.
Rating Count Percent Count Percent
BUY [B] 207 53.80 53 25.60
NEUTRAL [N] 176 45.70 28 15.90
SELL [S] 2 0.50 0 0.00
*Percentages of each rating category where Janney has performed Investment Banking services over the
past 12 months.
Other Disclosures
Janney Montgomery Scott LLC, is a U.S. broker-dealer registered with the U.S. Securities and Exchange Commission and a
member of the New York Stock Exchange, the Financial Industry Regulatory Authority and the Securities Investor Protection Corp.
This report is for your information only and is not an offer to sell or a solicitation of an offer to buy the securities or instruments
named or described in this report. Interested parties are advised to contact the entity with which they deal or the entity that provided
this report to them, should they desire further information. The information in this report has been obtained or derived from sources
believed by Janney Montgomery Scott LLC, to be reliable. Janney Montgomery Scott LLC, however, does not represent that
this information is accurate or complete. Any opinions or estimates contained in this report represent the judgment of Janney
Montgomery Scott LLC at this time and are subject to change without notice.
Investment opinions are based on each stock's 6-12 month return potential. Our ratings are not based on formal price targets,
however, our analysts will discuss fair value and/or target price ranges in research reports. Decisions to buy or sell a stock should
be based on the investor's investment objectives and risk tolerance and should not rely solely on the rating. Investors should read
carefully the entire research report, which provides a more complete discussion of the analyst's views. Supporting information
related to the recommendation, if any, made in the research report is available upon request.
- 5 -
Das könnte Ihnen auch gefallen
- JMP Expects U.S. Approval of Contrave Reiterates Outperform and $12 PTDokument6 SeitenJMP Expects U.S. Approval of Contrave Reiterates Outperform and $12 PTMayTepperNoch keine Bewertungen
- Aurobindo Pharma Ltd.-1Dokument4 SeitenAurobindo Pharma Ltd.-1Dhiren DesaiNoch keine Bewertungen
- GuggenheimDokument5 SeitenGuggenheimRochester Democrat and ChronicleNoch keine Bewertungen
- Prescience Point - LKQ Corp - 2014.01.15Dokument122 SeitenPrescience Point - LKQ Corp - 2014.01.15Wall Street WanderlustNoch keine Bewertungen
- Stock Fundamental Analysis Mastery: Unlocking Company Stock Financials for Profitable TradingVon EverandStock Fundamental Analysis Mastery: Unlocking Company Stock Financials for Profitable TradingNoch keine Bewertungen
- United Spirits JPM Sep 12Dokument5 SeitenUnited Spirits JPM Sep 12preet13Noch keine Bewertungen
- Nifty: Trading StrategiesDokument2 SeitenNifty: Trading StrategiesAbhishek KumarNoch keine Bewertungen
- JP Morgan BacheletDokument4 SeitenJP Morgan BacheletDiario ElMostrador.clNoch keine Bewertungen
- Nestlé India (NESIND) : Royalty To Increase To 4.5% by CY18Dokument1 SeiteNestlé India (NESIND) : Royalty To Increase To 4.5% by CY18drsivaprasad7Noch keine Bewertungen
- Jana q1 2014 LetterDokument10 SeitenJana q1 2014 LetterangadsawhneyNoch keine Bewertungen
- Exide Industries (EXIIND) : Finally, Margins Surprise "Positively"Dokument1 SeiteExide Industries (EXIIND) : Finally, Margins Surprise "Positively"prince1900Noch keine Bewertungen
- Initiating Coverage Ruchi Soya Industries LTDDokument20 SeitenInitiating Coverage Ruchi Soya Industries LTDShruti SharmaNoch keine Bewertungen
- ACB Reports Q4/FY17 Results Below ExpectationsDokument2 SeitenACB Reports Q4/FY17 Results Below ExpectationsAnonymous oHRghBVNoch keine Bewertungen
- Sterne Agee On Brinker - EATDokument5 SeitenSterne Agee On Brinker - EATlehighsolutionsNoch keine Bewertungen
- Nifty: Trading StrategiesDokument2 SeitenNifty: Trading StrategiesAbhishek KumarNoch keine Bewertungen
- Table of Contains SL No Contains Page NoDokument8 SeitenTable of Contains SL No Contains Page NoRasel SarderNoch keine Bewertungen
- Pershing Square Management's Letter To InvestorsDokument7 SeitenPershing Square Management's Letter To InvestorsDealBook100% (1)
- Craig Johnson - TechnicalAnalysisDokument29 SeitenCraig Johnson - TechnicalAnalysisanalyst_anil14Noch keine Bewertungen
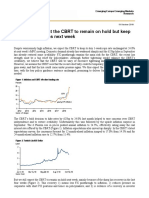
- JPM Turkey We Expect The CBRT To Remain On Hold But Keep The Ti 2018-10-19 2804995Dokument5 SeitenJPM Turkey We Expect The CBRT To Remain On Hold But Keep The Ti 2018-10-19 2804995yozgurNoch keine Bewertungen
- Transforming Office Depot: A Plan For Renewal and ReinvigorationDokument113 SeitenTransforming Office Depot: A Plan For Renewal and ReinvigorationAnonymous Feglbx5Noch keine Bewertungen
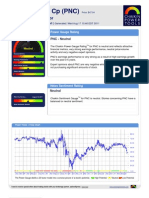
- Stock Research Report For PNC As of 8/17/11 - Chaikin Power ToolsDokument4 SeitenStock Research Report For PNC As of 8/17/11 - Chaikin Power ToolsChaikin Analytics, LLCNoch keine Bewertungen
- Man AHL Analysis CTA Intelligence - On Trend-Following CTAs and Quant Multi-Strategy Funds ENG 20140901Dokument1 SeiteMan AHL Analysis CTA Intelligence - On Trend-Following CTAs and Quant Multi-Strategy Funds ENG 20140901kevinNoch keine Bewertungen
- Stock Research Report For Amer Finl Group AFG As of 9/22/11 - Chaikin Power ToolsDokument4 SeitenStock Research Report For Amer Finl Group AFG As of 9/22/11 - Chaikin Power ToolsChaikin Analytics, LLCNoch keine Bewertungen
- Review of Google's 3Q07 Earnings Call: October 19, 2007Dokument7 SeitenReview of Google's 3Q07 Earnings Call: October 19, 2007Brian BolanNoch keine Bewertungen
- Financial Statement Analysis Project - Fall 2012-1Dokument9 SeitenFinancial Statement Analysis Project - Fall 2012-1rajesh934Noch keine Bewertungen
- Stock Research Report For UNH As of 7/27/11 - Chaikin Power ToolsDokument4 SeitenStock Research Report For UNH As of 7/27/11 - Chaikin Power ToolsChaikin Analytics, LLCNoch keine Bewertungen
- Gujarat Gas LTD: Put in Play by British Gas - ALERTDokument4 SeitenGujarat Gas LTD: Put in Play by British Gas - ALERTChaitanya JagarlapudiNoch keine Bewertungen
- Jackson Securities, LLC: A Review of Research Published in April and Updated Coverage ListDokument6 SeitenJackson Securities, LLC: A Review of Research Published in April and Updated Coverage ListBrian BolanNoch keine Bewertungen
- Agricultural Bank of ChinaDokument7 SeitenAgricultural Bank of ChinaJack YuanNoch keine Bewertungen
- CCL Products - Buy - 12th December 2022Dokument5 SeitenCCL Products - Buy - 12th December 2022Positive ThinkerNoch keine Bewertungen
- Pfizer Reports First-Quarter 2014 ResultsDokument26 SeitenPfizer Reports First-Quarter 2014 Resultspathanfor786Noch keine Bewertungen
- Corrected BrandDokument63 SeitenCorrected BrandRajesh KhannaNoch keine Bewertungen
- C.B. Worth - "Money in Motion"Dokument24 SeitenC.B. Worth - "Money in Motion"DealBookNoch keine Bewertungen
- CRISIL Research Ier Report Apollo Hosp Ent 2014 Q3FY14fcDokument10 SeitenCRISIL Research Ier Report Apollo Hosp Ent 2014 Q3FY14fcRakesh SrivastavaNoch keine Bewertungen
- 3Q16 Earnings PresentationDokument18 Seiten3Q16 Earnings PresentationmisterbeNoch keine Bewertungen
- Opportunities in Times of Adversity!: Event UpdateDokument3 SeitenOpportunities in Times of Adversity!: Event UpdateJanani SubramanianNoch keine Bewertungen
- Glaxosmithkline PLC: Research ReportDokument8 SeitenGlaxosmithkline PLC: Research Reportrumi2accaNoch keine Bewertungen
- Stock Research Report For AIG As of 8/17/11 - Chaikin Power ToolsDokument4 SeitenStock Research Report For AIG As of 8/17/11 - Chaikin Power ToolsChaikin Analytics, LLCNoch keine Bewertungen
- Data Driven 1 3321Dokument6 SeitenData Driven 1 3321api-532693362Noch keine Bewertungen
- Summary of Gregory R. Caruso's The Art of Business ValuationVon EverandSummary of Gregory R. Caruso's The Art of Business ValuationNoch keine Bewertungen
- Citigroup Inc: Stock Report - May 7, 2016 - NYS Symbol: C - C Is in The S&P 500Dokument11 SeitenCitigroup Inc: Stock Report - May 7, 2016 - NYS Symbol: C - C Is in The S&P 500Luis Fernando EscobarNoch keine Bewertungen
- Stock Research Report For Yamana Gold Inc GAS As of 11/17/11 - Chaikin Power ToolsDokument4 SeitenStock Research Report For Yamana Gold Inc GAS As of 11/17/11 - Chaikin Power ToolsChaikin Analytics, LLCNoch keine Bewertungen
- Arquitos Capital Management - Q3 2014 Investor LetterDokument4 SeitenArquitos Capital Management - Q3 2014 Investor LetterCanadianValueNoch keine Bewertungen
- Stock Research Report For Manulife Finl MFC As of 9/22/11 - Chaikin Power ToolsDokument4 SeitenStock Research Report For Manulife Finl MFC As of 9/22/11 - Chaikin Power ToolsChaikin Analytics, LLCNoch keine Bewertungen
- Aria 2Dokument5 SeitenAria 2Susan LaskoNoch keine Bewertungen
- Premarket KnowledgeBrunch Microsec 30.11.16Dokument5 SeitenPremarket KnowledgeBrunch Microsec 30.11.16Rajasekhar Reddy AnekalluNoch keine Bewertungen
- Neural Fair Value 25 User GuideDokument2 SeitenNeural Fair Value 25 User GuideCosta MoullasNoch keine Bewertungen
- Warrant Report May 9thDokument3 SeitenWarrant Report May 9thMichael CiprianoNoch keine Bewertungen
- Is This The Market Correction We Were Waiting ForDokument5 SeitenIs This The Market Correction We Were Waiting ForPIYUSH GOPALNoch keine Bewertungen
- Icahn Enterprises - May 2015 Investor PresentationDokument41 SeitenIcahn Enterprises - May 2015 Investor PresentationCanadianValue100% (2)
- 2013 q2 Crescent Commentary Steve RomickDokument16 Seiten2013 q2 Crescent Commentary Steve RomickCanadianValueNoch keine Bewertungen
- KDP DPS Keurig DR Pepper Investor Day March 2018Dokument112 SeitenKDP DPS Keurig DR Pepper Investor Day March 2018Ala BasterNoch keine Bewertungen
- Report 3-Governance&Debtb PDFDokument31 SeitenReport 3-Governance&Debtb PDFIceberg Research100% (1)
- Man AHL Analysis CTA Intelligence - On The Dangers of Following The Consensus ENG 20141022Dokument1 SeiteMan AHL Analysis CTA Intelligence - On The Dangers of Following The Consensus ENG 20141022kevinNoch keine Bewertungen
- GOI Incorporates Bad Bank: INDIA - FINANCIALS - Event UpdateDokument4 SeitenGOI Incorporates Bad Bank: INDIA - FINANCIALS - Event UpdateGaganNoch keine Bewertungen
- Life Sciences Sales Incentive Compensation: Sales Incentive CompensationVon EverandLife Sciences Sales Incentive Compensation: Sales Incentive CompensationNoch keine Bewertungen
- RGA Investment Advisors Envestnet Slides PDFDokument20 SeitenRGA Investment Advisors Envestnet Slides PDFJason Gilbert100% (1)
- The Future of Your Wealth: How the World Is Changing and What You Need to Do about It: A Guide for High Net Worth Individuals and FamiliesVon EverandThe Future of Your Wealth: How the World Is Changing and What You Need to Do about It: A Guide for High Net Worth Individuals and FamiliesNoch keine Bewertungen
- Summary of Michael J. Mauboussin & Alfred Rappaport's Expectations InvestingVon EverandSummary of Michael J. Mauboussin & Alfred Rappaport's Expectations InvestingNoch keine Bewertungen
- Beams AdvAcc11 ChapterDokument21 SeitenBeams AdvAcc11 Chaptermd salehinNoch keine Bewertungen
- Comparative Investment ReportDokument8 SeitenComparative Investment ReportNelby Actub MacalaguingNoch keine Bewertungen
- Apple Inc StrategyDokument4 SeitenApple Inc Strategyvaibhav_gupta20110% (1)
- Oman - Corporate SummaryDokument12 SeitenOman - Corporate SummarymujeebmuscatNoch keine Bewertungen
- Dow Chemicals Bid Case Analysis PDFDokument3 SeitenDow Chemicals Bid Case Analysis PDFFerSorinNoch keine Bewertungen
- LIST OF YGC and AYALA COMPANIESDokument3 SeitenLIST OF YGC and AYALA COMPANIESJibber JabberNoch keine Bewertungen
- 51 Checklist Buy BackDokument3 Seiten51 Checklist Buy BackvrkesavanNoch keine Bewertungen
- Fundamental Accounting Ii Final ModuleDokument174 SeitenFundamental Accounting Ii Final ModuleFanuel Derese100% (2)
- Role of Central Bank in BangladeshDokument20 SeitenRole of Central Bank in BangladeshAsadul HoqueNoch keine Bewertungen
- Checkpoint Exam 2 (Study Sessions 10-15)Dokument11 SeitenCheckpoint Exam 2 (Study Sessions 10-15)Bảo TrâmNoch keine Bewertungen
- EOU FAQsDokument6 SeitenEOU FAQsSri KanthNoch keine Bewertungen
- Innovation All ChapterDokument94 SeitenInnovation All Chapterbereket amareNoch keine Bewertungen
- Estate Planning & Administration OutlineDokument9 SeitenEstate Planning & Administration OutlineSean Robertson100% (1)
- Client / Brand Analysis: 1.1. About GraingerDokument11 SeitenClient / Brand Analysis: 1.1. About GraingerMiguel Angel RuizNoch keine Bewertungen
- FIN211 Financial Management Lecture Notes Text Reference: Chapter 1 Topic: Role of Financial ManagementDokument8 SeitenFIN211 Financial Management Lecture Notes Text Reference: Chapter 1 Topic: Role of Financial ManagementAnonymous y3E7iaNoch keine Bewertungen
- Micro Tutorial 8 - 2017Dokument2 SeitenMicro Tutorial 8 - 2017AndyNoch keine Bewertungen
- 08641IBM Institute For Business ValueDokument10 Seiten08641IBM Institute For Business ValueanashussainNoch keine Bewertungen
- Risk Quantification - ERMDokument20 SeitenRisk Quantification - ERMSeth ValdezNoch keine Bewertungen
- Superannuation Choice FormDokument6 SeitenSuperannuation Choice FormMollyNoch keine Bewertungen
- MCQs On 301 SM PDFDokument3 SeitenMCQs On 301 SM PDFNeelNoch keine Bewertungen
- Punjab Spatial Planning StrategyDokument12 SeitenPunjab Spatial Planning Strategybaloch47Noch keine Bewertungen
- Developing Marketing Strategies and PlansDokument43 SeitenDeveloping Marketing Strategies and PlansThaer Abu Odeh100% (1)
- Quality Management and Practises in Automobile SectorDokument57 SeitenQuality Management and Practises in Automobile Sectorshivi73100% (16)
- BAHRE 311 - Week 1 2 - ULO BDokument6 SeitenBAHRE 311 - Week 1 2 - ULO BMarlyn TugapNoch keine Bewertungen
- Find Solutions For Your Homework: Books Study Writing Flashcards Math Solver InternshipsDokument8 SeitenFind Solutions For Your Homework: Books Study Writing Flashcards Math Solver InternshipsAbd ur Rehman BodlaNoch keine Bewertungen
- Aluminum Building and Construction Usage Study: April 2021Dokument25 SeitenAluminum Building and Construction Usage Study: April 2021Anver AkperovNoch keine Bewertungen
- BusFin PT 5Dokument5 SeitenBusFin PT 5Nadjmeah AbdillahNoch keine Bewertungen
- Kritika AbstractDokument3 SeitenKritika AbstractKritika SinghNoch keine Bewertungen
- Corporate Governance AssignmentDokument12 SeitenCorporate Governance AssignmentMayank Rajpoot0% (1)
- Erd 4 F 003Dokument3 SeitenErd 4 F 003GCLT Logistics and Transport and Trucking ServicesNoch keine Bewertungen