Beruflich Dokumente
Kultur Dokumente
Galactomanana Judith 2014 - GPC
Hochgeladen von
Rômulo Couto AlvesOriginaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Galactomanana Judith 2014 - GPC
Hochgeladen von
Rômulo Couto AlvesCopyright:
Verfügbare Formate
Characterisation of partially hydrolysed galactomannan from
Caesalpinia pulcherrima seeds as a potential dietary bre
Flvia C.A. Buriti
a, b
, Karina M.O. dos Santos
a,
*
, Vencios G. Sombra
c
, Jeanny S. Maciel
c
,
Daniele M.A. Teixeira S
d
, Hvila O. Salles
a
, Gilcenara Oliveira
e
, Regina C.M. de Paula
c
,
Judith P.A. Feitosa
c
, Ana C.O. Monteiro Moreira
f
, Renato A. Moreira
f
, Antonio S. Egito
a
a
Empresa Brasileira de Pesquisa Agropecuria, Embrapa Caprinos e Ovinos, P.O. Box 145, 62010-970 Sobral, Cear, Brazil
b
Universidade Estadual da Paraba, Departamento de Farmcia, Rua Juvncio Arruda, s/n, 58100-001 Campina Grande, Paraba, Brazil
c
Universidade Federal do Cear, Departamento de Qumica Orgnica e Inorgnica, P.O. Box 6021, 60455-760 Fortaleza, Cear, Brazil
d
Instituto Federal de Educao Cincia e Tecnologia do Cear, Campus de Sobral, Av. Dr. Guarani, 317, 62040-370 Sobral, Cear, Brazil
e
Universidade de Fortaleza, Centro de Cincias Tecnolgicas, Av. Washington soares, 1321, 60811-905 Fortaleza, Cear, Brazil
f
Universidade de Fortaleza, Centro de Cincias da Sade, Av. Washington Soares, 1321, 60811-905 Fortaleza, Cear, Brazil
a r t i c l e i n f o
Article history:
Received 3 April 2013
Accepted 16 July 2013
Keywords:
Caesalpinia pulcherrima
Dietary bre
Galactomannan
Guar substitute
Enzymatic hydrolysis
Absolute viscosity
a b s t r a c t
A galactomannan extracted from the endosperm of Caesalpinia pulcherrima seeds was submitted to
partial enzymatic hydrolysis to overcome the limitation of use of this polysaccharide as a dietary bre
ingredient, since the intact galactomannan may promote a considerable increase in the viscosity of food
products, even when added at low concentrations. The total dietary bre recovered from the partially
hydrolysed galactomannan (PHGM) was 78.27%. The molar mass of PHGM and the absolute viscosity of
aqueous solutions were reduced in relation to the intact galactomannan. Nevertheless, their structural
characteristics were very similar when evaluated by thermogravimetric analysis, infrared spectroscopy,
and also by
1
H and
13
C nuclear magnetic resonance. A lack of growth promotion in the food-grade strains
tested using PHGM during a preliminary in vitro fermentation assay suggests that the metabolism of
these microorganisms most likely would not affect the galactomannan content in products during their
production and storage. PHGM presented suitable properties to be added as an alternative dietary bre
source in a wide range of food products, particularly in beverages.
2013 Elsevier Ltd. All rights reserved.
1. Introduction
Galactomannans are used as a form of carbohydrate storage in
plants, consisting of a main chain of (1 / 4) linked b-D-man-
nopyranosyl residues with a single unit of a-D-galactopyranosyl
side-chain residues. The molecular weight, mannose/galactose ra-
tio (M/G) and galactose units distribution over the mannose back-
bone are properties that depend on the galactomannan source and
inuence the technological applications of the polysaccharides
(Jiang, Jian, Cristhian, Zang, & Sun, 2011; Tapie, Malhiac, Hucher, &
Grisel, 2008).
The major plant sources of galactomannans for commercial ap-
plications in food and non-food products are Cyamopsis tetragono-
loba (guar gum), Caesalpinia spinosa (tara gum) and Ceratonia siliqua
(locust bean gum) (Cerqueira et al., 2009, 2011a). In the food
industry, guar, tara and locust bean gums are primarily used as
thickening and stabilising agents (Oliveira et al., 2011; Reddy,
Mohan, Satla, & Gaikwad, 2011). Guar gum also has been used as a
dietary bre supplement (Ellis, Wang, Rayment, Ren, & Ross-
Murphy, 2001); however, it is considered a potential deterrent to
palatability since sensorial characteristics of guar-containing food
products tend to be poor due to high levels of viscosity (Cui, Ikeda, &
Eskin, 2007). A low viscosity ingredient was obtained by partial
hydrolysis of the guar gumto expand the applications of this dietary
bre source in food products, especially in beverages, yogurts, milk
drinks, whipped creams, soups and enteral solutions (Kapoor &
Juneja, 2009). Despite the worldwide use of guar gum in the food
industry, frequent supply shortages and the high cost of this
ingredient caused by variations in crop production have caused
manufacturers to seek alternative and sustainable sources of seed
gums (Byrne, 2012; Mathur, 2011; Mathur & Mathur, 2005).
Although information about potential guar gum substitutes is
found in the literature (Cerqueira et al., 2011a; Cunha, Vieira,
Arriaga, de Paula, & Feitosa, 2009; Hu, Kong, Yang, & Pan, 2011;
* Corresponding author. Tel.: 55 88 31127562; fax: 55 88 31127455.
E-mail address: karina.dos-santos@embrapa.br (K.M.O. dos Santos).
Contents lists available at ScienceDirect
Food Hydrocolloids
j ournal homepage: www. el sevi er. com/ l ocat e/ f oodhyd
0268-005X/$ e see front matter 2013 Elsevier Ltd. All rights reserved.
http://dx.doi.org/10.1016/j.foodhyd.2013.07.015
Food Hydrocolloids 35 (2014) 512e521
de Souza, Lucyszyn, Ferraz, & Sierakowsky, 2010), there is a lack of
studies dealing with the characterisation of alternative
galactomannan-based ingredients to partially hydrolysed guar gum.
Caesalpinia pulcherrima (EN: Dwarf Poinciana, Pride of
Barbados; PT: amboyant-mirim, amboyanzinho) is a plant from
the family Fabaceae e Leguminosae and is largely found in Brazil.
Its seeds accumulate considerable amounts of galactomannan with
potential applications in the food industry, as hydrocolloid, texture
modier and source of dietary bre. The possibility of growing
C. pulcherrima for commercial and industrial uses highlights op-
portunities for economic and social developments in the semi-arid
areas of the Caatinga, which is the main biome of the Brazilian
Northeastern region, because this plant is well-adapted to this
particular environment. The main characteristics of the gal-
actomannan extracted from C. pulcherrima seeds were described in
studies that focused on its potential applications in the industry
(Andrade, Azero, Luciano, & Gonalves, 1999; Azero & Andrade,
1999, 2002; Braga et al., 2011; Cerqueira et al., 2009, 2011b).
Nonetheless, similar to intact guar gum, the galactomannan of
C. pulcherrima has a high viscosity and gelling capacity at low
concentrations, which impede its application as a dietary bre
source in semi-solid and liquid products. Partial hydrolysis of this
polysaccharide would overcome these limitations and allow its
addition in a wide range of products at amounts high enough to be
used as a dietary bre ingredient.
The present study evaluated the production of partially hydro-
lysed galactomannan from C. pulcherrima using enzymatic hydro-
lysis. Structural and physicochemical characterisations of intact and
hydrolysed galactomannan were carried out. Due to the possibility
of fermentation of this carbohydrate by some food-grade cultures
commonly used in industry and by some harmful bacteria repre-
sentative of gut microbiota, in vitro fermentation of hydrolysed
galactomannan from C. pulcherrima was also investigated.
2. Material and methods
2.1. Galactomannan extraction
The pods of C. pulcherrima were collected in the cities of For-
taleza, Quixeramobim and Sobral (Cear State, Brazil), between
September 2009 and August 2010. A voucher specimen of
C. pulcherrima seeds has been deposited at Herbarium Prisco
Bezerra e EAC (Federal University of Cear, Fortaleza, Brazil) under
the number 44718.
The polysaccharide extraction was based on the procedures
described by Cerqueira et al. (2009). Seeds were removed from the
pods, cleaned and placed in a blender to be mechanically broken.
Afterwards, the endospermwas manually separated from the germ
and the hull. The endosperm obtained was suspended in 92.8%
ethanol (w/w) in a proportion of 1:3 (seeds:ethanol) at 70
C for
15 min to inactivate the enzymes and eliminate the low-molecular-
weight compounds. Ethanol was removed and distilled water was
added in a 1:5 (endosperm:water) proportion and the suspension
was left overnight. The next day, the amount of water was increased
to an endosperm:water ratio of 1:10 and mixed in a blender for
5 min. Next, the viscous solution was ltered through a nylon net
and precipitated by adding 92.8% ethanol (w/w) at ratio of 1:2. The
precipitate was successively washed with acetone, dried with hot
air and milled. The sample obtained in this step was labelled GM.
2.2. Enzymatic hydrolysis of galactomannan
The GM powder was dissolved in distilled water (3 L per batch)
to a concentration of 15 g/L under constant stirring for 3 h at room
temperature using a mechanical mixer Fisatom (model 713, So
Paulo, Brazil) starting at 1000 rpm with subsequent increase up to
2000 rpm to form a uniform gel. For the enzymatic hydrolysis, a
commercial cellulase from Aspergillus niger (1.24 U/mg, Sigma,
Buchs, Switzerland) was dissolved in distilled water to achieve a
concentration of 6.4 U/mL. The enzyme dispersion was added to
polysaccharide suspension in a proportion of 12.8 U of cellulase to
1 g of galactomannan. The gel was stirred for 2 h at room tem-
perature with a successive decrease from 2000 rpm to 1000 rpm.
These conditions of hydrolysis were established in a preliminary
study by evaluating the relative viscosity and the dietary bre
content of suspensions containing GM throughout the time after
the cellulase addition (Buriti et al., 2011). The hydrolysed suspen-
sion was transferred to borosilicate asks with caps and immedi-
ately autoclaved at 121
C for 20 min. The suspension was cooled,
passed through a sieve (0.25 mmopening) and dried in a mini spray
drier Bchi (model B-290, Flawil, Switzerland) using an inlet tem-
perature of 160
C, an outlet temperature of 103e107
C, air owof
538 L/h and a pump speed of 233 mL/h. A total of 11 batches were
prepared and the sample obtained in this step was labelled PHGM.
The yield of PHGM in this step was 48.7 4.5 (%, w/w).
2.3. Galactomannan samples characterisation
2.3.1. Moisture content
The moisture content was determined for the GM and PHGM
samples by drying 1 g of the samples at 105
C to a constant weight.
2.3.2. Total dietary bre
The total dietary bre was determined for the GM and PHGM
samples using the AOAC 985.29 method (Prosky et al., 1985).
2.3.3. Thermogravimetric analysis
Thermogravimetric analysis (TGA) of the GM and PHGM sam-
ples was performed using a TA Instruments analyser (model Q50,
New Castle, DE, USA). Approximately 10 mg of the sample was
placed in an aluminium pan and heated over a temperature range
of 25e900
C at 10
C/min under a synthetic air atmosphere.
2.3.4. Gel permeation chromatography
The weight average molar mass (M
w
) and number average molar
mass (M
n
) of the GM and PHGM samples were determined by gel
permeation chromatography (GPC) with a Shimadzu instrument
(model LC-10AD, Kyoto, Japan) at room temperature using an
Ultrahydrogel linear column (7.8 300 mm, Waters, Milford, MA,
USA), a ow rate of 0.5 mL/min, a polysaccharide concentration of
1 mg/mL, water as the solvent and NaNO
3
0.1 M as the eluent. A
differential refractometer was used as the detector. The elution
volume was corrected using ethylene glycol as an internal marker
at 11.25 mL. The GPC was calibrated using pullulan samples (Sho-
dex, Showa Denko, Kawasaki, Japan) as standards.
2.3.5. Infrared spectral analysis
The Fourier transform infrared spectra (FT-IR) of the GM and
PHGM samples were recorded with a Shimadzu IR spectropho-
tometer (model 8300, Kyoto, Japan) in the range of 400e
4000 cm
1
. The samples were analysed as KBr pellet.
2.3.6. Nuclear magnetic resonance spectroscopy
The
1
H and
13
C nuclear magnetic resonance (NMR) spectra of
10 mg/mL solutions of the GM and PHGM samples dissolved in
D
2
O were recorded at 70
C on a Fourier transform Bruker
Avance spectrometer (model DRX 500, Rheinstetten, Germany)
with an inverse multinuclear gradient probe-head equipped
with z-shielded gradient coils and Silicon Graphics. Sodium 2,2-
dimethylsilapentane-5-sulphonate (DSS) was used as an internal
F.C.A. Buriti et al. / Food Hydrocolloids 35 (2014) 512e521 513
standard (0.00 ppm for
1
H). A distortionless enhancement by
polarisation transfer (DEPT 135) spectrum was recorded to deter-
mine the hydrogenation of each carbon. The acquisition and delay
times were 1.0 s. Correlation spectroscopy,
1
H,
13
C-HSQC were
performed using the parameters supplied in the Bruker manual.
2.3.7. Rheological measurements
The effect of shear rate on the viscosity of the GM and PHGM
aqueous solutions at concentrations of 0.3, 0.5 and 1% (w/v) was
evaluated using a TA Instrument Rheometer (model AR-550, New
Castle, DE, USA). Tests were performed at 25
C.
2.3.8. In vitro fermentation assay
The effect of PHGM on the growth of different bacteria
compared to the absence and presence of other carbohydrate
sources was evaluated in a preliminary assay using an in vitro
model adapted from Ryu, Kim, Park, Lee, and Lee (2007) with a
modied MRS mediumand phenol red as indicator. One food-grade
strain commonly used as starter culture in fermented dairy prod-
ucts, Streptococcus thermophilus TA-40 (Danisco, Sassenage,
France), ve benecial food-grade strains, Lactobacillus acidophilus
LA-5 (Chr. Hansen, Hrsholm, Denmark), L. acidophilus NCFM
(Danisco, Madison, WI, USA), Lactobacillus rhamnosus Lr-32 (Dan-
isco, Madison, WI, USA), Bidobacteriumanimalis subsp. lactis BB-12
(Chr. Hansen, Hrsholm, Denmark) and B. animalis subsp. lactis Bi-
07 (Danisco, Madison, WI, USA), as well as two harmful bacteria
representative of gut microbiota, Escherichia coli INCQS 00033
(Fundao Oswaldo Cruz, Rio de Janeiro, Brazil) and Clostridium
perfringens INCQS 00130 (Fundao Oswaldo Cruz, Rio de Janeiro,
Brazil), were tested. The broth medium consisted of peptone (10 g,
Himedia, Mumbai, India), meat extract (8 g, Himedia, Mumbai,
India), yeast extract (4 g, Acumedia, Lansing, MI, USA), Tween
80 (1 mL, Cromoline, Diadema, Brazil), ammonium acetate (2 g),
MgSO
4
$7H
2
O (0.18 g), MnSO
4
$H
2
O (0.05 g), Na
2
SO
4
(2 g), K
2
SO
4
(1.25 g), Na
2
CO
3
(0.2 g), CaCl
2
$2H
2
O (0.11 g), L()-cysteine
HCl (0.5 g), phenol red (0.18 g) and distilled water (1 L). Each pre-
cultured culture was diluted in peptone water and 0.1 mL aliquots
(6e7 log colony forming units [CFU]) were inoculated into 5 mL of
the broth media without or with 0.5% (w/v) PHGM and/or other
carbohydrates. The carbohydrates evaluated were inulin (Orafti
HP-Gel, Beneo, Oreye, Belgium), oligofructose (FOS, Orafti P95,
Beneo, Oreye, Belgium), lactose (Vetec, Rio de Janeiro, Brazil) and
glucose (Quimibras, Rio de Janeiro, Brazil). Cell density was deter-
mined by measuring the absorbance at 660 nm using a Shimadzu
spectrophotometer (model UV1200, Kyoto, Japan) before (0 h) and
after 6 h, 24 h and 48 h of anaerobic incubation (Anaerobic System
Anaerogen, Oxoid, Basingstoke, UK) at 37
C. The assays were
performed in triplicate and the absorbance results were converted
into microbial concentrations (log CFU/mL) using growth curves
from each microorganism. Statistical analysis was performed for
the in vitro fermentation assay data using SAS (Statistical Analysis
Systems) software version 9.2 (SAS Institute Inc., Cary, NC, USA).
Repeated measures analysis of variance (RM-ANOVA) was used to
determine signicant differences among samples (P < 0.05), fol-
lowed by the Tukey post-hoc test to identify contrasts. Homoge-
neity of variance among samples was checked by the Cochran and
Bartlett tests (P < 0.05).
3. Results and discussion
3.1. Composition and structural characterisation of the intact and
hydrolysed galactomannans
Table 1 shows the main characteristics obtained for the intact
and hydrolysed galactomannans. The moisture content and the
residual mass were similar for the GM and PHGM samples. The
hydrolysis process reduced the dietary bre content in the PHGM
sample to 78.3% (w/w) compared to 87.5% (w/w) in the GM sample.
Nevertheless, after hydrolysis the total dietary bre recovered in
the PHGM sample was still considered high, as it was equivalent to
the dietary bre content of 76% (w/w) found in partially hydrolysed
guar gum Sunber
(Kapoor & Juneja, 2009). It is important to
emphasise that, although with this reduction in the dietary bre
content, partially hydrolysed galactomannans usually give lower
viscosity, and therefore they could be added in higher amounts in
food products for improvement of total dietary bre when
compared with the intact galactomannans (Yoon, Chu, & Juneja,
2008), as it will be discussed later.
The FT-IR spectra were very similar for the GM and PHGM
samples (Fig. 1), showing characteristic absorption bands of the
galactomannans as reported in the literature (Cerqueira et al.,
2011b; Figueir, Ges, Moreira, & Sombra, 2004; Hu et al., 2011;
Mudgil, Barak, & Khatkar, 2012b; Shobha, Vishu Kumar,
Tharanathan, Koka, & Gaonkar, 2005). Bands approximately
3400 cm
1
are attributed to OeH stretching vibration of the poly-
saccharides and the region approximately 2900 cm
1
is represen-
tative of eCeH stretching modes (Cerqueira et al., 2011b; Hu et al.,
2011; Shobha et al., 2005). The absorption of approximately
1645 cm
1
is due to the bound water (Hu et al., 2011; Shobha et al.,
2005). The region approximately 1380 cm
1
and 1430 cm
1
cor-
responds to eCH
2
deformation modes and the broad band between
800 cm
1
and 1200 cm
1
results from the highly coupled CeCeO,
CeOH and CeOeC stretching modes of the polymer backbone
(Mudgil et al., 2012b; Shobha et al., 2005). The peak at 1151 cm
1
is
attributed to bending vibrational modes of the CeO present in the
pyranose ring and the bands at 813 cm
1
and 872 cm
1
indicate the
Table 1
Main characteristics of intact and partially hydrolysed galactomannans from
C. pulcherrima (GM and PHGM, respectively).
GM PHGM
Moisture e drying at 105
C (%, w/w) 11.11 11.38
TGA residual mass (%, w/w) 0.3531 0.3184
Total dietary bre (%, w/w) 87.77 78.27
Mannose:galactose ratio 3.65:1 3.24:1
M
n
(g/mol) 2.48 10
6
9.89 10
4
M
w
(g/mol) 8.03 10
6
2.47 10
5
Polydispersity index (M
w
/M
n
) 3.23 2.50
Fig. 1. FT-IR spectra for intact and partially hydrolysed galactomannans from
C. pulcherrima (GM and PHGM, respectively): GM ( ) and PHGM ( ).
F.C.A. Buriti et al. / Food Hydrocolloids 35 (2014) 512e521 514
presence of a-linked D-galactopyranose units and b-linked D-man-
nopyranose units, respectively (Cerqueira et al., 2011b; Figueir
et al., 2004). In agreement with the results of the present study,
Mudgil et al. (2012b) did not nd structural modications in the FT-
IR spectra of the guar gum after its partial hydrolysis with a
cellulase from the A. niger, when compared to the intact guar gum.
The mannose/galactose (M/G) ratios obtained from the inte-
grated peak areas from the
1
H NMR spectrum for the anomeric
protons of mannose and galactose units at d 4.74 and d 5.02,
respectively (Fig. 2(a) and (b)), were 3.65:1 for the GM sample and
3.24:1 for the PHGM sample (Table 1). The reduced values of M/G
ratio for PHGM compared to the intact polysaccharide (GM) indi-
cate that galactose was not cleaved during the enzymatic hydro-
lysis. These results were expected, since the enzymatic hydrolysis
was performed with a cellulase from A. niger. In this case, the
cleavage of the linkages should occur only between the mannose
units. The value of M/G ratio for GMwas similar to the ratio of 3.6:1
determined by Braga et al. (2011), however smaller values were
reported by Andrade et al. (1999) and Cerqueira et al. (2009), 2.83:1
and 2.88:1 respectively. The resonances of protons and carbons in
the
1
H and
13
C NMR spectra for the GM and PHGM obtained from
C. pulcherrima were assigned based on the literature for gal-
actomannan (Bociek, Izzard, Morrison, & Weti, 1981; Chaubey &
Kapoor, 2001; Cunha et al., 2009) and the
1
H,
13
C-HSQC correla-
tion analysis (Fig. 2(c) and (d)). A summary of the assigned
1
H and
13
C NMR shifts is shown in Table 2. A similar
1
He
13
C-HSQC spec-
trum was obtained for the GM and the PHGM samples. Three sig-
nals were veried in the anomeric region of the
13
C NMR spectra (d
90e110) and were assigned as C-1 of a-D-galactose at d 99.46 for the
GM and PHGM samples, C-1 of b-D-mannose at d 100.75 and
d 100.76 for the GM and the PHGM, respectively, and C-1 of
substituted b-D-mannose at d 100.56 for the GM and PHGM sam-
ples. In the
1
He
13
C-HSQC spectrum from the GM sample, it was
observed that there were only two correlations from the non-
substituted units b-mannopyranosyl (d 4.72/100.7) and the a-gal-
actopyranosyl (d 5.01/99.5) (Fig. 2(c)). The three C-6 signals at
opposite amplitudes in the DEPT 135 spectrum (data not shown)
exhibited correlations with their protons at d 3.91/61.2, d 3.75/61.7,
and d 3.81/67.3, due to the H-6/C-6 correlations of the non-
substituted units b-mannopyranosyl, a-galactopyranosyl and the
two diastereotopic protons from the O-substituted b-mannopyr-
anosyl, respectively. The correlations from the PHGM sample were
similar (Fig. 2(d)).
The split of C-4 in the spectra of the intact and partially
hydrolysed galactomannans was used to determine the probabili-
ties of a-D-galactose substitution on the (1 /4)-b-D-mannopyr-
anose based on the study of Dea and Morrison (1975). Three signals
were assigned in the
13
C NMR spectrum region of the C-4 in the
mannose residues for both the GM and PHGM samples (Fig. 3(a)
and (b)). The rst signal at d 77.5 is attributed to two continuous
Fig. 2. Anomeric region
1
H NMR ((a) and (b)) and
1
He
13
C-HSQC spectra ((c) and (d)) for intact and partially hydrolysed galactomannans from C. pulcherrima (GM and PHGM,
respectively): GM (a) and (c); PHGM (b) and (d).
F.C.A. Buriti et al. / Food Hydrocolloids 35 (2014) 512e521 515
substituted b-D-mannose units (denoted as I), the second at d 77.3 is
attributed to dyads in which only one of the two b-D-mannose
units is substituted (denoted as II) and the third at d 77.0 is due to
the unsubstituted b-D-mannose units that are adjacent to other
unsubstituted unit of the same monosaccharide (denoted as III)
(Cunha et al., 2009; de Souza et al., 2010). If the cleavage occurs in
the mannose units without substitution, the C-4 signal due to dyads
with unsubstituted b-D-mannose units (III) will decrease and,
consequently the ratio of dyads in which only one of the two b-D-
mannose units is substituted (II) or the ratio of two continuous
substituted b-D-mannose units (I) will increase. In GM and PHGM
spectra, the ratios of signal I/III and II/III were, respectively, 0.16 and
1.04 for GM and 0.23 and 1.20 for PHGM indicating that the
cleavage occurred in the unsubstituted b-D-mannose units. These
results indicate that the galactosyl groups in the backbone of PHGM
were not affected by the enzymatic hydrolysis and thermal treat-
ment. Similar results were veried in the commercial partially
hydrolysed guar gum (Yoon et al., 2008).
3.2. Gel permeation chromatography (GPC)
Fig. 4 shows the GPC chromatograms of intact and hydrolysed
galactomannan of C. pulcherrima. It was observed that the partial
hydrolysis occurred in a homogeneous form, as only one peak is
observed in Fig. 4 for PHGM. In the present study, the number
average molar mass (M
n
) and the weight average molar mass (M
w
)
obtained for the GM sample were 2.48 10
6
g/mol, and
8.03 10
6
g/mol, respectively (Table 1). The M
w
value was higher
than the intrinsic viscosity molar mass of 1.75 10
6
g/mol and
2.10 10
6
g/mol, as reported by Cerqueira et al. (2009) and Andrade
et al. (1999) for intact galactomannan from C. pulcherrima.
Furthermore, the M
n
and M
w
values for the PHGM sample,
9.89 10
4
g/mol and 2.47 10
5
g/mol, respectively, were 25e30-
fold lower when compared to the GM sample (Table 1). These
values were higher than those reported in the literature for the
Table 2
1
HNMR and
13
C NMR shifts for intact and partially hydrolysed galactomannans from
C. pulcherrima (GM and PHGM, respectively).
Position
13
C (d, ppm)
1
H (d, ppm)
GM PHGM GM PHGM
a-D-Galactopyranosyl
C-1/H-1 99.46 99.46 5.02 5.02
C-2/H-2 69.07 69.01 3.84 3.82
C-3/H-3 70.60 70.11 4.11 4.10
C-4/H-4 69.93 69.93 4.00 4.01
C-5/H-5 71.82 71.79 3.89 3.88
C-6/H-6 61.77 61.75 3.75 3.74
4-Linked-b-D-mannopyranosyl
C-1/H-1 100.75 100.76 4.73 4.72
C-2/H-2 70.60 70.62 4.11 4.11
C-3/H-3 72.07 72.08 3.79 3.79
C-4/H-4 77.30 77.10 3.85 3.82
C-5/H-5 75.70 75.69 3.55 3.55
C-6/H-6 61.19 61.22 3.89 3.89
4,6-Linked-b-D-mannopyranosyl
C-1/H-1 100.57 100.57 4.74 4.74
C-4/H-4
a
77.34 77.34 3.82 3.82
C-4/H-4
b
77.29 77.29
C-5/H-5 74.04 74.03 3.74 3.73
C-6/H-6 67.24 67.29 3.81 3.80
a
Samples from two continuous O-substituted b-D-mannopyranosyl units.
b
Samples from dyads in which only one of the two b-D-mannopyranosyl units is
substituted.
Fig. 3.
13
C NMR spectral region of C-4 (mannose) for intact and partially hydrolysed galactomannans from C. pulcherrima (GM and PHGM, respectively): GM (a) and PHGM (b).
F.C.A. Buriti et al. / Food Hydrocolloids 35 (2014) 512e521 516
partially hydrolysed guar gum Sunbre
, whose M
w
is 2.0 10
4
g/
mol and the range is 10
3
e10
5
g/mol (Ohashi et al., 2012; Okubo
et al., 1994; Yoon et al., 2008).
The polydispersity index of 3.23 for the GMsample (Table 1) was
higher than the values reported for the galactomannans extracted
from plant sources found in the Brazilian ora of 1.2 and 1.3 for
Mimosa scabrella (Vendruscolo et al., 2009), 1.55 for Caesalpinia
ferrea (de Souza et al., 2010), 2.06 for Dimorphandra gardneriana
(Cunha et al., 2009), and 2.84 for Parkinsonia aculeata (Garros-Rosa,
Reicher, Petkowicz, Sierakowski, & Moreira, 2006). The hydrolysis
process reduced the polydispersity index to 2.50 in the PHGM
sample (Table 1).
3.3. Thermogravimetric analysis
The thermogravimetric (TG) curves were quite similar for the
GM and PHGM samples (Fig. 5). Three mass-loss events were
observed for both samples. The rst event was at 58
C and at 59
C
for the GM and PHGM samples, respectively, which might be
associated with water evaporation. The following events are related
to the thermal composition of the samples and occurred at 302
C
and at 300
C for the GM and PHGM samples, respectively, and at
480
C for both the GM and the PHGM samples. After the moisture
loss, a constant weight was observed up to approximately 260
C.
The highest weight loss was approximately 80% (w/w) of the dry
weight and occurred between 290
C and 350
C. Cerqueira et al.
(2011b) veried the two events during the TG experiments with
galactomannans from C. pulcherrima, Gleditsia triacanthos and
Adenanthera pavonina. According to these authors, a weight loss of
approximately 45% (w/w) was observed in the second event for the
three polysaccharides studied. For the galactomannan from
C. pulcherrima, the peak of the derivate at the weight loss curve
(DTG) was 321.73
C, which is close to the temperature of the
second event for the GM and PHGM samples in the present study.
These results indicate that the PHGM sample would tolerate most
temperatures used in the thermal treatment of food products,
specically pasteurisation and sterilisation. However, the possible
interactions of PHGM with other food components during thermal
treatment may affect the products characteristics and should be
investigated further.
3.4. Rheological properties
The effects of the shear rate on absolute viscosity in the aqueous
solutions of the GM and PHGM samples at 0.3%, 0.5%, and 1% (w/v)
are shown in Fig. 6. At all concentrations studied, the GM and
PHGM solutions presented a pseudo-plastic behaviour at a low
shear rate. The absolute viscosity of the GM solution at 1% (w/v)
achieved 802 mPa s at a shear rate of 100 s
1
. The viscosity would
increase greatly if a liquid food product such as juices, fermented
milks or milk beverages was added with the GM at this proportion
or higher, since products considered to be source of dietary bre
usually contain at least 1.5 g bre per 100 kcal (Codex Alimentarius,
2009). According to Rezaei, Khomeiri, Kashaninejad, and Aalami
(2011), the addition of only 0.3% (w/w) of intact guar gum in
frozen yogurt caused the viscosity to increase to 3305 mPa s, more
than twice the value obtained in control samples without guar gum
(1522 mPa s). However, in the present study the absolute viscosity
of the different aqueous solutions in the PHGM sample was 10e
100-fold lower than those obtained for the GM sample at the same
concentrations and shear rate. At a shear rate of 400 s
1
, the GM
and PHGM samples at a concentration of 1% (w/v) showed absolute
viscosities of 286 mPa s and 6 mPa s, respectively. Likewise, Mudgil,
Fig. 5. Weight loss curves for intact and partially hydrolysed galactomannans from
C. pulcherrima (GM and PHGM, respectively) at a heating rate of 10
C min
1
under
synthetic air: GM ( ) and PHGM ( ).
Fig. 6. Effect of shear rate on viscosity of intact and partially hydrolysed gal-
actomannans from C. pulcherrima (GM and PHGM, respectively) at concentration (w/v)
of 0.3% (6), 0.5% (B) and 1% (,). Closed symbols represent GM and open symbols
represent PHGM.
Fig. 4. GPC curves for intact and partially hydrolysed galactomannans from
C. pulcherrima (GM and PHGM, respectively) at concentration of 1 mg/mL: GM ( )
and PHGM ( ).
F.C.A. Buriti et al. / Food Hydrocolloids 35 (2014) 512e521 517
Barak, and Khatkar (2012a) observed that the aqueous solutions of
the intact guar gum (889,742 g/mol) and the partially hydrolysed
guar gum (7936 g/mol) at 1% (w/v) showed absolute viscosity of
approximately 100 mPa s and 4 mPa s, respectively, at a shear rate
of 400 s
1
. Taking into account the use of partially hydrolysed
galactomannans in liquid food products as a dietary bre source,
Brennan and Tudorica (2008) obtained skimmed yogurts added of
2% and 6% (w/w) partially hydrolysed guar gum (PHGG) with
apparent viscosities of approximately 2500 mPa s and 3500 mPa s,
respectively, at a shear rate of 10 s
1
, compared to approximately
1500 mPa s in skimmed yogurt without addition of PHGG. Ac-
cording to the results of absolute viscosity obtained in the present
study and their comparison with the data reported in the literature,
the PHGM sample might be considered suitable as an alternative
ingredient to PHGG to be applied in liquid food products such as
yogurts and milk fermented beverages to improve their dietary
bre content without excessive increase in their viscosity.
3.5. In vitro fermentation assay
The microorganisms evaluated in this study (S. thermophilus
TA-40, L. acidophilus e LA-5 and NCFM strains, L. rhamnosus Lr-32,
B. animalis subsp. lactis e BB-12 and Bi-07 strains, E. coli INCQS
00033, and C. perfringens INCQS 00130) were not able to metabolise
the PHGM in the modied MRS medium at 37
C, under anaerobic
conditions for 48 h (Figs. 7 and 8) because no signicant increase in
bacterial growth compared to the control samples (with no car-
bohydrate addition) was observed (P > 0.05). The growth of
L. acidophilus NCFM, E. coli, and C. perfringens in the presence of all
carbohydrates evaluated (PHGM, inulin, FOS, lactose and glucose),
during the different sampling periods (0, 6, 24 and 48 h), did not
differ signicantly from the control (P > 0.05). A signicant growth
of the B. animalis (BB-12 and Bi-07 strains) and the L. rhamnosus
species, compared to the control was only observed in the presence
of lactose and glucose (P < 0.05). However, there was no signicant
Fig. 7. Populations of S. thermophilus TA-40 (a), L. acidophilus LA-5 (b), L. acidophilus NCFM (c), and L. rhamnosus Lr-32 (d) in the control medium (without carbohydrates) and in the
media with partially hydrolysed galactomannan of C. pulcherrima (PHGM), inulin, oligofructose (FOS), lactose or glucose before ( ) and after 6 h ( ), 24 h ( ) and 48 h ( ) of
incubation under anaerobic conditions at 37
C.
A,B,C
Capital letters denote signicant differences between the growth of a same microorganism in the different media in a same
sampling period.
a,b
Lowercase letters denote signicant differences between the growth of a same microorganism in a same media in the different sampling periods.
F.C.A. Buriti et al. / Food Hydrocolloids 35 (2014) 512e521 518
difference between the populations of B. animalis in the media
containing inulin, FOS, lactose and glucose after 24 h and 48 h of
assay (P >0.05). Media containing FOS, lactose and glucose resulted
in a signicant growth of both S. thermophilus and L. acidophilus LA-
5 when compared to the control media after 48 h of assay.
Considering the use of the PHGM in food products containing the
strains of Streptococcus, Lactobacillus and Bidobacterium tested in
the in vitro fermentation assay of the present study, our preliminary
results indicate that the metabolism of these bacteria most likely
would not affect the dietary bre content of this ingredient during
the fermentation and shelf life of products, particularly in foods
stored under refrigerated conditions in which the bacterial meta-
bolism is decreased. Moreover, according with these results, the
strains tested would not be able to degrade directly gal-
actomannans with M
w
higher than 10
5
g/mol in the gut.
In agreement with the results of the present study using PHGM,
Okubo et al. (1994) did not observe growth of L. acidophilus (IF-164,
ATCC-4356 and Om strains), Lactobacillus casei (ATCC-7469 and
IFO-3425), C. perfringens (C-01 and ATCC-13124 strains), and E. coli
(O-601 and M602 strains) in Peptone Yeast Extract Fildes Solution
(PYF) broth containing 0.5% (w/v) of the commercial PHGG
Sunbre
when incubated anaerobically at 37
C for 48 h. Addi-
tionally, 16 Bidobacterium strains (belonging to the species Bi-
dobacterium bidum, Bidobacterium infantis, Bidobacterium breve,
Bidobacterium adolescentis and Bidobacterium longum) were not
able to grow in the presence of the PHGG using the same in vitro
conditions. For this in vitro experiment with the PHGG, the authors
reported a moderate growth for the Bacteroides ovatus, Clostridium
coccoides, Clostridium butyricum and Peptostreptococcus productus
species. However, the authors achieved a signicant increase in the
population of Bidobacteriumspp. in faeces of healthy humans who
consumed beverages containing 7% (w/v) PHGG (100 mL, three
times a day, totalling 21 g PHGG per day) over a period of 14 days.
The authors reported that the population of Lactobacillus spp. in
faeces also increased, although not to the same extent as veried
for Bidobacterium spp. Furthermore, the numbers of Clostridium
Fig. 8. Populations of B. animalis BB-12 (a), B. animalis Bi-07 (b), E. coli INCQS 00033 (c), and C. perfringens INCQS 00130 (d) in the control medium (without carbohydrates) and in
the media with partially hydrolysed galactomannan of C. pulcherrima (PHGM), inulin, oligofructose (FOS), lactose or glucose before ( ) and after 6 h ( ), 24 h ( ) and 48 h ( ) of
incubation under anaerobic conditions at 37
C.
A,B,C
Capital letters denote signicant differences between the growth of a same microorganism in the different media in a same
sampling period.
a,b
Lowercase letters denote signicant differences between the growth of a same microorganism in a same media in the different sampling periods.
F.C.A. Buriti et al. / Food Hydrocolloids 35 (2014) 512e521 519
spp., C. perfringens, Enterobacteriaceae and Streptococcaceae in
faeces, showed a tendency to decrease.
Changes in the bacterial and fatty acid composition in the
presence of PHGG were also demonstrated in studies involving
in vitro fermentation of this carbohydrate with faecal inoculum
(Ohashi et al., 2012; Stewart & Slavin, 2006). However, with respect
to the lack of ability of pure cultures of bidobacteria to ferment the
partially hydrolysed galactomannan, as veried in the present
study, Ohashi et al. (2012) suggested that PHGG would not directly
stimulate these microorganisms in the same manner as well
established bidogenic carbohydrates, such as inulin-type fructans.
The increased number of bidobacteria in faeces after the intake of
PHGG is likely due to their ability to ferment the oligosaccharides
produced after the degradation of the partially hydrolysed gal-
actomannan by other bacteria in the large intestine. According to
the authors, the Roseburia/Eubacterium rectale group also plays an
important role in the fermentation of the PHGG through the pro-
duction of the butyric acid. There is some possibility that the PHGM
obtained in the present study might have the same fermentation
pattern as the PHGG in the gut since the M
w
of PHGM(2.47 10
5
g/
mol) is in the range of molar mass values for which guar gum
fermentation was demonstrated in the cited studies: 2.0 10
4
g/
mol (Okubo et al., 1994) and from1.5 10
4
g/mol to 1.1 10
6
g/mol
(Stewart & Slavin, 2006).
4. Conclusions
The molar mass of the partially hydrolysed galactomannan of
C. pulcherrima and the absolute viscosity of aqueous solutions were
reduced when compared to the intact galactomannan. However,
the structural characteristics of the polysaccharide evaluated by
thermogravimetric analysis, infrared spectroscopy, and also
1
H and
13
C nuclear magnetic resonance were unaffected after the hydro-
lysis process. These properties are comparable to the characteristics
described in the literature for the galactomannan obtained fromthe
partial hydrolysis of the guar gum. The partially hydrolysed gal-
actomannan of C. pulcherrima has not promoted the growth of the
bacterial strains evaluated in the preliminary in vitro fermentation
assay; these results indicate that this carbohydrate might be added
as a galactomannan ingredient to increase the dietary bre content
of products prepared with the food-grade strains tested, since it
would not be degraded by these microorganisms during processing
and storage. The partially hydrolysed galactomannan obtained in
this study appears to be a potential alternative to the partially
hydrolysed guar gum, enlarging the availability of novel food in-
gredients, since food manufacturers are increasingly looking for
guar gum substitutes with ready accessibility. Based on the prop-
erties achieved in this study, it would be advisable to use the
partially hydrolysed galactomannan of the C. pulcherrima as a di-
etary bre source, especially in liquid food products such as yogurts
and fermented milk beverages.
Acknowledgements
This study was sponsored by the Conselho Nacional de
Desenvolvimento Cientco e Tecnolgico (CNPq), Fundao
Cearense de Apoio ao Desenvolvimento Cientco e Tecnolgico
(FUNCAP) and Empresa Brasileira de Pesquisa Agropecuria
(EMBRAPA). F.C.A. Buriti gratefully acknowledges the fellowship
and the nancial support from CNPq (Procs. 68.0013/2008-3 and
35.0161/2009-7) and FUNCAP (DCR 010/09). K.M.O. dos Santos
gratefully acknowledges the nancial support from EMBRAPA
(Macroprograma-3 No. 03.09.06.026.00.00). The authors wish to
thank Prof. Pedro Matias de Vasconcelos for supplying the
C. pulcherrima seeds and Dr. Sidina Cordeiro de Freitas, as well as
Embrapa Agroindstria de Alimentos for the dietary bre anal-
ysis, Centro Nordestino de Aplicao e Uso de Ressonncia Mag-
ntica Nuclear (CENAUREMN) for the NMR spectra, and Beneo e
Orafti, Clariant S.A., Danisco Brasil Ltda. and the Instituto Nacional
de Qualidade em Sade e Fundao Oswaldo Cruz (INCQS e
FIOCRUZ) for providing part of the materials used in this study.
We also wish to thank Joo Batista Paula Ibiapina, Jorge Silvestre,
Jos Tabosa dos Santos, and Mrcio Freire Ponciano for their
technical assistance.
References
Andrade, C. T., Azero, E. G., Luciano, L., & Gonalves, M. P. (1999). Solution of the
galactomannans extracted from the seeds of Caesalpinia pulcherrima and Cassia
javanica: comparison with locust bean gum. International Journal of Biological
Macromolecules, 26, 181e185.
Azero, E. G., & Andrade, C. T. (1999). Extraction and characterization of the gal-
actomannan from the seeds of Caesalpinia pulcherrima. Polmeros, 9, 54e59.
Azero, E. G., & Andrade, C. T. (2002). Testing procedures for galactomannan puri-
cation. Polymer Testing, 21, 551e556.
Bociek, S. M., Izzard, M. J., Morrison, A., & Weti, D. (1981).
13
C-NMR spectra of (1-6)-
a-D-galactosyl-(1-4)-b-D-mannans. Carbohydrate Research, 93, 279e283.
Braga, R. C., Teixeira-S, D. M. A., Ribeiro, A. F., Miranda, R. L., de Almeida, L. M.,
Horta, A. C. G., et al. (2011). Evaluation of Caesalpinia pulcherrima endospermic
gum as afnity matrices for galactose-binding lectins interaction. Brazilian Ar-
chives of Biology and Technology, 54, 283e292.
Brennan, C. S., & Tudorica, C. M. (2008). Carbohydrate-based fat replacers in the
modication of the rheological, textural and sensory quality of yoghurt:
comparative study of the utilisation of barley beta-glucan, guar gum and inulin.
International Journal of Food Science and Technology, 43, 824e833.
Buriti, F. C. A., dos Santos, K. M. O., Oliveira, G., Pereira, T., Moreira, R. A., & Egito, A. S.
(2011). Hidrlise enzimtica parcial de Caesalpinia pulcherrima (ambo-
lyanzinho) para sua aplicao como fonte de bra alimentar em alimentos
Congresso Nacional da SBAN, Fortaleza, CE, Brazil, 20e23 June 2011. Abstract
PS-21-039. Nutrire: Journal of the Brazilian Society of Food and Nutrition,
36(Suppl. 1), 69.
Byrne, J. (23 Jan. 2012). Tight supply and high prices in guar gum look set to continue.
Food Navigator.com. http://www.foodnavigator.com/content/view/print/60
5752.
Cerqueira, M. A., Bourbon, A. I., Pinheiro, A. C., Martins, J. T., Souza, B. W. S.,
Teixeira, J. A., et al. (2011a). Galactomannans use in the development of edible
lms/coating for food applications. Trends in Food Science and Technology, 22,
662e671.
Cerqueira, M. A., Pinheiro, A. C., Souza, B. W. S., Lima, A. M. P., Ribeiro, C., Miranda, C.,
et al. (2009). Extraction, purication and characterization of galactomannans
from non-traditional sources. Carbohydrate Polymers, 75, 408e414.
Cerqueira, M. A., Souza, B. W. S., Simes, J., Teixeira, J. A., Domingues, M. R. M.,
Coimbra, M. A., et al. (2011b). Structural and thermal characterization of
galactomannans from non-conventional sources. Carbohydrate Polymers, 83,
179e185.
Chaubey, M., & Kapoor, V. P. (2001). Structure of a galactomannan from the seeds of
Cassia angustifolia Vahl. Carbohydrate Research, 332, 439e444.
Codex Alimentarius. (2009). Thirty second session, Rome, Italy. Report of the 30th
session of the Codex Committee on nutrition and foods for special dietary uses,
Cape Town, South Africa. ALINORM 09/32/26 http://www.codexalimentarius.
net/download/report/710/al32_26e.pdf.
Cui, S. W., Ikeda, S., & Eskin, M. N. A. (2007). Seed polysaccharide gums. In
C. G. Biliaderis, & M. S. Izydorczyk (Eds.), Functional food carbohydrates (pp.
127e165). Boca Raton: CRC.
Cunha, P. L. R., Vieira, I. G. P., Arriaga, A. M. C., de Paula, R. C. M., & Feitosa, J. P. A.
(2009). Isolation and characterization of galactomannan from Dimorphandra
gardneriana Tul. seeds as a potential guar gum substitute. Food Hydrocolloids,
23, 880e885.
de Souza, C. F., Lucyszyn, N., Ferraz, F. A, & Sierakowski, M. R. (2010). Caesalpinia
ferrea var. ferrea seeds as a new source of partially substituted galactomannan.
Carbohydrate Polymers, 82, 641e647.
Dea, I. C. M., & Morrison, A. (1975). Chemistry and interactions on seeds
galactomannans. Advances inCarbohydrate ChemistryandBiochemistry, 31, 241e312.
Ellis, P. R., Wang, Q., Rayment, P., Ren, Y., & Ross-Murphy, S. B. (2001). Guar gum:
agricultural and botanical aspects, physicochemical and nutritional properties,
and its use in the development of functional foods. In S. S. Cho, & M. L. Dreher
(Eds.), Handbook of dietary bre (pp. 613e657). New York: Marcel Dekker.
Figueir, S. D., Ges, J. C., Moreira, R. A., & Sombra, A. S. B. (2004). On the physico-
chemical and dielectric properties of glutaraldehyde crosslinked galactomannan-
collagen lms. Carbohydrate Polymers, 56, 313e320.
Garros-Rosa, I., Reicher, F., Petkowicz, C. L. O., Sierakowski, M. R., & Moreira, R. A.
(2006). Characterization of the galactomannans from Parkinsonia aculeata seeds
and their application on afnity chromatography. Polmeros, 16, 99e103.
Hu, C., Kong, Q., Yang, D., & Pan, Y. (2011). Isolation and structural characterization
of a novel galactomannan from Eremurus anisopterus (Ker. et Kir) Regel roots.
Carbohydrate Polymers, 84, 402e406.
F.C.A. Buriti et al. / Food Hydrocolloids 35 (2014) 512e521 520
Jiang, J., Jian, H., Cristhian, C., Zhang, W., & Sun, R. (2011). Structural and thermal
characterization of galactomannans from genus Gleditsia seeds as potential food
gum substitutes. Journal of the Science of Food and Agriculture, 91, 732e737.
Kapoor, M. P., & Juneja, L. R. (2009). Partially hydrolyzed guar gum dietary bre. In
S. S. Cho, & P. Samuel (Eds.), Fibre ingredients: Food applications and health
benets (pp. 79e120). Boca Raton: CRC.
Mathur, N. K. (2011). Fenugreek gum: the new food galactomannan. In Industrial
galactomannan polysaccharides (pp. 115e128). Boca Raton: CRC.
Mathur, V., & Mathur, N. K. (2005). Fenugreek and other lesser known legume
galactomannan-polysaccharides: scope for developments. Journal of Scientic
and Industrial Research, 64, 475e481.
Mudgil, D., Barak, S., & Khatkar, B. S. (2012a). Effect of enzymatic polymerization on
physicochemical and rheological properties of guar gum. Carbohydrate Poly-
mers, 90, 224e228.
Mudgil, D., Barak, S., & Khatkar, B. S. (2012b). X-ray diffraction, IR spectroscopy and
thermal characterization of partially hydrolyzed guar gum. International Journal
of Biological Macromolecules, 50, 1035e1039.
Ohashi, Y., Harada, K., Tokunaga, M., Ishihara, N., Okubo, T., Ogasawara, Y., et al.
(2012). Faecal fermentation of partially hydrolyzed guar gum. Journal of Func-
tional Foods, 4, 398e402.
Okubo, T., Ishihara, N., Takahashi, H., Fujisawa, T., Kim, M., Yamamoto, T., et al.
(1994). Effects of partially hydrolyzed guar gum intake on human intestinal
microora and its metabolism. Bioscience Biotechnology and Biochemistry, 58,
1364e1369.
Oliveira, N. M., Dourado, F. Q., Peres, A. M., Silva, M. V., Maia, J. M., & Teixeira, J. A.
(2011). Effect of guar gum on the physicochemical, thermal, rheological and
textural properties of green Edam cheese. Food and Bioprocess Technology, 4,
1414e1421.
Prosky, L., Asp, N. G., Furda, I., DeVries, J. W., Schweizer, T. F., & Harland, B. F.
(1985). Determination of total dietary bre in foods and food products:
collaborative study. Journal of the Association of Ofcial Analytical Chemists, 68,
677e679.
Reddy, K., Mohan, G. K., Satla, S., & Gaiwad, S. (2011). Natural polysaccharides:
versatile excipients for controlled drug delivery systems. Asian Journal of
Pharmaceutical Sciences, 6, 275e286.
Rezaei, R., Khomeiri, M., Kashaninejad, M., & Aalami, M. (2011). Effects of guar
gum and arabic gum on the physicochemical, sensory and ow behaviour
characteristics of frozen yoghurt. International Journal of Dairy Technology, 64,
563e568.
Ryu, S. I., Kim, B. G., Park, M. S., Lee, Y. B., & Lee, S. B. (2007). Evaluation of enhanced
hygroscopicity, bidogenicity, and anticariogenicity of enzymatically synthe-
sized b-galactosyl-trehalose oligosaccharides. Journal of Agricultural and Food
Chemistry, 55, 4184e4188.
Shobha, M. S., Vishu Kumar, A. B., Tharanathan, R. N., Koka, R., & Gaonkar, A. K.
(2005). Modication of guar galactomannan with the aid of Aspergillus niger
pectinase. Carbohydrate Polymers, 62, 267e273.
Stewart, M. L., & Slavin, J. L. (2006). Molecular weight of guar gum affects short-
chain fatty acid prole in model intestinal fermentation. Molecular Nutrition
and Food Research, 50, 971e976.
Tapie, N., Malhiac, C., Hucher, N., & Grisel, M. (2008). Determination of galactose
and mannose residues in natural galactomannans using a fast and efcient
high-performance liquid chromatography/UV detection. Journal of Chromatog-
raphy A, 1181, 45e50.
Vendruscolo, C. W., Ferrero, C., Pineda, E. A. G., Silveira, J. L. M., Freitas, R. A.,
Jimnez-Castellanos, M. R., et al. (2009). Physicochemical and mechanical
characterization of galactomannan from Mimosa scabrella: effect of drying
method. Carbohydrate Polymers, 76, 86e93.
Yoon, S. J., Chu, D. C., & Juneja, L. R. (2008). Chemical and physical properties, safety
and application of partially hydrolyzed guar gum as dietary bre. Journal of
Clinical Biochemistry and Nutrition, 42, 1e7.
F.C.A. Buriti et al. / Food Hydrocolloids 35 (2014) 512e521 521
Das könnte Ihnen auch gefallen
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryVon EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryBewertung: 3.5 von 5 Sternen3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Von EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Bewertung: 4.5 von 5 Sternen4.5/5 (121)
- Grit: The Power of Passion and PerseveranceVon EverandGrit: The Power of Passion and PerseveranceBewertung: 4 von 5 Sternen4/5 (588)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaVon EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaBewertung: 4.5 von 5 Sternen4.5/5 (266)
- The Little Book of Hygge: Danish Secrets to Happy LivingVon EverandThe Little Book of Hygge: Danish Secrets to Happy LivingBewertung: 3.5 von 5 Sternen3.5/5 (399)
- Never Split the Difference: Negotiating As If Your Life Depended On ItVon EverandNever Split the Difference: Negotiating As If Your Life Depended On ItBewertung: 4.5 von 5 Sternen4.5/5 (838)
- Shoe Dog: A Memoir by the Creator of NikeVon EverandShoe Dog: A Memoir by the Creator of NikeBewertung: 4.5 von 5 Sternen4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerVon EverandThe Emperor of All Maladies: A Biography of CancerBewertung: 4.5 von 5 Sternen4.5/5 (271)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeVon EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeBewertung: 4 von 5 Sternen4/5 (5794)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyVon EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyBewertung: 3.5 von 5 Sternen3.5/5 (2259)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersVon EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersBewertung: 4.5 von 5 Sternen4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnVon EverandTeam of Rivals: The Political Genius of Abraham LincolnBewertung: 4.5 von 5 Sternen4.5/5 (234)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreVon EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreBewertung: 4 von 5 Sternen4/5 (1090)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceVon EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceBewertung: 4 von 5 Sternen4/5 (895)
- Her Body and Other Parties: StoriesVon EverandHer Body and Other Parties: StoriesBewertung: 4 von 5 Sternen4/5 (821)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureVon EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureBewertung: 4.5 von 5 Sternen4.5/5 (474)
- The Unwinding: An Inner History of the New AmericaVon EverandThe Unwinding: An Inner History of the New AmericaBewertung: 4 von 5 Sternen4/5 (45)
- Di Scovery and Meani NG: e Dead Sea Scrol L SDokument31 SeitenDi Scovery and Meani NG: e Dead Sea Scrol L SAnonymous vtKuitL100% (1)
- The Yellow House: A Memoir (2019 National Book Award Winner)Von EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Bewertung: 4 von 5 Sternen4/5 (98)
- On Fire: The (Burning) Case for a Green New DealVon EverandOn Fire: The (Burning) Case for a Green New DealBewertung: 4 von 5 Sternen4/5 (73)
- Chocolate Recipes 2Dokument81 SeitenChocolate Recipes 2kathrynbax100% (2)
- 8 Food IELTS Speaking Topic PDFDokument18 Seiten8 Food IELTS Speaking Topic PDFMary Joy BejeranoNoch keine Bewertungen
- MDSZ1 45Dokument607 SeitenMDSZ1 45Gerriza Nicole DacuycuyNoch keine Bewertungen
- OJT-EurotelDokument50 SeitenOJT-EurotelMichelle Calleja PaditNoch keine Bewertungen
- WWW - Srmuniv.ac - in Downloads Chapter-Ix Microbiological AssayDokument28 SeitenWWW - Srmuniv.ac - in Downloads Chapter-Ix Microbiological AssayAlexiel NguyenNoch keine Bewertungen
- Food Production NotesDokument66 SeitenFood Production NotesSunil BholaNoch keine Bewertungen
- Customer Service Handbook English PDFDokument22 SeitenCustomer Service Handbook English PDFsasikumar_s7898100% (1)
- Miniemulsion Polymerization of Styrene Using A PHDokument8 SeitenMiniemulsion Polymerization of Styrene Using A PHRômulo Couto AlvesNoch keine Bewertungen
- Jo 00354 A 013Dokument4 SeitenJo 00354 A 013Rômulo Couto AlvesNoch keine Bewertungen
- Israel: An Archaeological JourneyDokument71 SeitenIsrael: An Archaeological JourneyBen Stimpson100% (1)
- Aula 4 ADokument38 SeitenAula 4 ARômulo Couto AlvesNoch keine Bewertungen
- New Interchange 1-StudentBook by JBillyDokument148 SeitenNew Interchange 1-StudentBook by JBillyRômulo Couto AlvesNoch keine Bewertungen
- Genre Teks Tingkat SMPDokument15 SeitenGenre Teks Tingkat SMPHadiNoch keine Bewertungen
- Tandag National Science High School Humanities and Social SciencesDokument28 SeitenTandag National Science High School Humanities and Social SciencesArjean Shane CalihatNoch keine Bewertungen
- The Birch TreeDokument2 SeitenThe Birch TreeRichardClarksonNoch keine Bewertungen
- Kamote Tops (Ipomoeia Batatas Linn) Decoction Into JuiceDokument5 SeitenKamote Tops (Ipomoeia Batatas Linn) Decoction Into JuiceTata KurtoNoch keine Bewertungen
- PIDA - Inception ReportDokument270 SeitenPIDA - Inception ReportTrevor T ParazivaNoch keine Bewertungen
- Life at The Top Chapter 291Dokument18 SeitenLife at The Top Chapter 291Bryan Jude LegayadaNoch keine Bewertungen
- A Little Girl in Old Quebec by Douglas, Amanda Minnie, 1831-1916Dokument143 SeitenA Little Girl in Old Quebec by Douglas, Amanda Minnie, 1831-1916Gutenberg.org100% (1)
- Modul 2 Add Maths 2016 (JPPP)Dokument18 SeitenModul 2 Add Maths 2016 (JPPP)Tang Sok MinNoch keine Bewertungen
- TS Apun Ingles 3 BajaDokument407 SeitenTS Apun Ingles 3 BajaMayte MéndezNoch keine Bewertungen
- Takshashila International School, Kosamba: Food Menu For The Month of April 2022-23Dokument2 SeitenTakshashila International School, Kosamba: Food Menu For The Month of April 2022-23Yell YashNoch keine Bewertungen
- Ukk Kelas Xi EnglishDokument7 SeitenUkk Kelas Xi Englishalif fajriNoch keine Bewertungen
- 2017 - YEAR5 - BI - Paper1 - 1st PRODokument13 Seiten2017 - YEAR5 - BI - Paper1 - 1st PRORani ArumugamNoch keine Bewertungen
- Thinking About Complementary and Alternative Med PPL With CancerDokument13 SeitenThinking About Complementary and Alternative Med PPL With CancerTaranisaNoch keine Bewertungen
- 4H Handbook 2012Dokument42 Seiten4H Handbook 2012StJamesHigh0% (1)
- Cause and EffectDokument9 SeitenCause and Effectrani rahmaniNoch keine Bewertungen
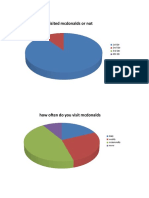
- Analysis Mcdonalds SurveyDokument8 SeitenAnalysis Mcdonalds SurveySanshu KhuranaNoch keine Bewertungen
- Menu BurgerDokument1 SeiteMenu Burgerahdiyatul fazlieNoch keine Bewertungen
- Active To Passive TransformationsDokument5 SeitenActive To Passive TransformationsDavid Burgos E Irene del PozoNoch keine Bewertungen
- School MealsDokument21 SeitenSchool MealsJFT482Noch keine Bewertungen
- In Vitro Propagation of Malaysian Cassava (Manihot Esculenta Crantz) Variety Through Low Cost Tissue Culture MediaDokument4 SeitenIn Vitro Propagation of Malaysian Cassava (Manihot Esculenta Crantz) Variety Through Low Cost Tissue Culture MediaIJEAB JournalNoch keine Bewertungen
- Oktoberfest: Ingeniería en MinasDokument10 SeitenOktoberfest: Ingeniería en MinasErik SotoNoch keine Bewertungen
- 1937 Dresden Sweet Potato FestivalDokument26 Seiten1937 Dresden Sweet Potato FestivalMary Pepin MoranNoch keine Bewertungen
- Unit 6 - Luyện chuyên sâu Ngữ pháp và Bài tập tiếng Anh 7 (HS)Dokument20 SeitenUnit 6 - Luyện chuyên sâu Ngữ pháp và Bài tập tiếng Anh 7 (HS)Thu TrầnNoch keine Bewertungen