Beruflich Dokumente
Kultur Dokumente
U1 5thermochemistryexitticket
Hochgeladen von
api-2590404080 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
49 Ansichten1 SeiteOriginaltitel
u1 5thermochemistryexitticket
Copyright
© © All Rights Reserved
Verfügbare Formate
DOCX, PDF, TXT oder online auf Scribd lesen
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
© All Rights Reserved
Verfügbare Formate
Als DOCX, PDF, TXT herunterladen oder online auf Scribd lesen
0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
49 Ansichten1 SeiteU1 5thermochemistryexitticket
Hochgeladen von
api-259040408Copyright:
© All Rights Reserved
Verfügbare Formate
Als DOCX, PDF, TXT herunterladen oder online auf Scribd lesen
Sie sind auf Seite 1von 1
Name: ____________________________________
1.5 Thermochemistry Exit Ticket
1. A student mixes two liquid chemicals together and
notices that some vapor is produced. What type of
process is this?
a. Endothermic
b. Exothermic
c. Equilibrium
d. Equinox
2. Which of the following statements describes an
exothermic reaction, but not an endothermic reaction?
a. Energy is destroyed during the reaction
b. Energy is used to form chemical bonds
c. Energy is used to break chemical bonds
d. Energy is released as heat during the reaction
3. Which of the following correctly classifies the phase
change of a liquid to a solid and provides the correct
reasoning?
a. Endothermic because the liquid releases energy
to change into a solid.
b. Endothermic because the liquid absorbs energy
to change into a solid.
c. Exothermic because the liquid releases energy to
change into a solid.
d. Exothermic because the liquid absorbs energy to
change into a solid.
4. How much joules of energy are needed to heat 19
grams of water for a total of 25C?
a. 1,985.5 J
b. 475 J
c. 113.6 J
d. 5.5 J
5. If the heat of fusion for Copper is 205 kg/kJ, how much
energy would be required to melt a 500 kg sample?
a. 0.410 kJ
b. 0.41 kJ
c. 0.4 kJ
d. 0.41 J
Name: ____________________________________
1.5 Thermochemistry Exit Ticket
1. A student mixes two liquid chemicals together and
notices that some vapor is produced. What type of
process is this?
e. Endothermic
f. Exothermic
g. Equilibrium
h. Equinox
2. Which of the following statements describes an
exothermic reaction, but not an endothermic reaction?
e. Energy is destroyed during the reaction
f. Energy is used to form chemical bonds
g. Energy is used to break chemical bonds
h. Energy is released as heat during the reaction
3. Which of the following correctly classifies the phase
change of a liquid to a solid and provides the correct
reasoning?
e. Endothermic because the liquid releases energy
to change into a solid.
f. Endothermic because the liquid absorbs energy
to change into a solid.
g. Exothermic because the liquid releases energy to
change into a solid.
h. Exothermic because the liquid absorbs energy to
change into a solid.
4. How much joules of energy are needed to heat 19
grams of water for a total of 25C?
e. 1,985.5 J
f. 475 J
g. 113.6 J
h. 5.5 J
5. If the heat of fusion for Copper is 205 kg/kJ, how much
energy would be required to melt a 500 kg sample?
e. 0.410 kJ
f. 0.41 kJ
g. 0.4 kJ
h. 0.41 J
Das könnte Ihnen auch gefallen
- Capstone Community Service ProjectDokument4 SeitenCapstone Community Service Projectapi-259040408Noch keine Bewertungen
- 2 3 Atomic ModelsDokument19 Seiten2 3 Atomic Modelsapi-259040408Noch keine Bewertungen
- 2 3 Exit TicketDokument1 Seite2 3 Exit Ticketapi-259040408Noch keine Bewertungen
- 4 Community Service Commitment FormDokument1 Seite4 Community Service Commitment Formapi-259040408Noch keine Bewertungen
- 3 Proposal FormDokument1 Seite3 Proposal Formapi-259040408Noch keine Bewertungen
- 2 2 Exit TicketDokument1 Seite2 2 Exit Ticketapi-259040408Noch keine Bewertungen
- 2 2 The Atom and Periodic TableDokument29 Seiten2 2 The Atom and Periodic Tableapi-259040408Noch keine Bewertungen
- U1 5thermochemistryexitticketDokument1 SeiteU1 5thermochemistryexitticketapi-259040408Noch keine Bewertungen
- 2 1 Exit TicketDokument1 Seite2 1 Exit Ticketapi-259040408Noch keine Bewertungen
- 2 1 Elements Compounds and MixturesDokument23 Seiten2 1 Elements Compounds and Mixturesapi-259040408Noch keine Bewertungen
- U1 5thermochemistryDokument22 SeitenU1 5thermochemistryapi-259040408Noch keine Bewertungen
- U1 5thermochemistryDokument22 SeitenU1 5thermochemistryapi-259040408Noch keine Bewertungen
- Unit 2 ScheduleDokument1 SeiteUnit 2 Scheduleapi-259040408Noch keine Bewertungen
- U1 4phasechangeexitticketDokument1 SeiteU1 4phasechangeexitticketapi-259040408Noch keine Bewertungen
- U1 4phasechangeDokument25 SeitenU1 4phasechangeapi-259040408Noch keine Bewertungen
- 1 3 StatesofmatterDokument20 Seiten1 3 Statesofmatterapi-259040408Noch keine Bewertungen
- U1 4phasechangeDokument25 SeitenU1 4phasechangeapi-259040408Noch keine Bewertungen
- Unit 7 Gas Laws VocabDokument2 SeitenUnit 7 Gas Laws Vocabapi-259040408Noch keine Bewertungen
- U1 4phasechangeexitticketDokument1 SeiteU1 4phasechangeexitticketapi-259040408Noch keine Bewertungen
- Unit 8 Reactions VocabDokument4 SeitenUnit 8 Reactions Vocabapi-259040408Noch keine Bewertungen
- Unit 9 Solutions VocabDokument3 SeitenUnit 9 Solutions Vocabapi-259040408Noch keine Bewertungen
- U1 3slgexitticketDokument1 SeiteU1 3slgexitticketapi-259040408Noch keine Bewertungen
- Id Badge ProjectDokument1 SeiteId Badge Projectapi-259040408Noch keine Bewertungen
- Capstone Syllabus 2014-2015Dokument1 SeiteCapstone Syllabus 2014-2015api-259040408Noch keine Bewertungen
- Chemistry Syllabus 2014-2015 HonorsDokument2 SeitenChemistry Syllabus 2014-2015 Honorsapi-259040408Noch keine Bewertungen
- Chemistry Syllabus 2014-2015Dokument2 SeitenChemistry Syllabus 2014-2015api-259040408Noch keine Bewertungen
- 1 Student Parent Commitment FormDokument1 Seite1 Student Parent Commitment Formapi-259040408Noch keine Bewertungen
- Capstone Community Service ProjectDokument4 SeitenCapstone Community Service Projectapi-259040408Noch keine Bewertungen
- About Me Content RubricDokument1 SeiteAbout Me Content Rubricapi-259040408Noch keine Bewertungen
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceVon EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceBewertung: 4 von 5 Sternen4/5 (895)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeVon EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeBewertung: 4 von 5 Sternen4/5 (5794)
- The Yellow House: A Memoir (2019 National Book Award Winner)Von EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Bewertung: 4 von 5 Sternen4/5 (98)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureVon EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureBewertung: 4.5 von 5 Sternen4.5/5 (474)
- Shoe Dog: A Memoir by the Creator of NikeVon EverandShoe Dog: A Memoir by the Creator of NikeBewertung: 4.5 von 5 Sternen4.5/5 (537)
- The Little Book of Hygge: Danish Secrets to Happy LivingVon EverandThe Little Book of Hygge: Danish Secrets to Happy LivingBewertung: 3.5 von 5 Sternen3.5/5 (399)
- On Fire: The (Burning) Case for a Green New DealVon EverandOn Fire: The (Burning) Case for a Green New DealBewertung: 4 von 5 Sternen4/5 (73)
- Never Split the Difference: Negotiating As If Your Life Depended On ItVon EverandNever Split the Difference: Negotiating As If Your Life Depended On ItBewertung: 4.5 von 5 Sternen4.5/5 (838)
- Grit: The Power of Passion and PerseveranceVon EverandGrit: The Power of Passion and PerseveranceBewertung: 4 von 5 Sternen4/5 (588)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryVon EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryBewertung: 3.5 von 5 Sternen3.5/5 (231)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaVon EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaBewertung: 4.5 von 5 Sternen4.5/5 (266)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersVon EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersBewertung: 4.5 von 5 Sternen4.5/5 (344)
- The Emperor of All Maladies: A Biography of CancerVon EverandThe Emperor of All Maladies: A Biography of CancerBewertung: 4.5 von 5 Sternen4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnVon EverandTeam of Rivals: The Political Genius of Abraham LincolnBewertung: 4.5 von 5 Sternen4.5/5 (234)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreVon EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreBewertung: 4 von 5 Sternen4/5 (1090)
- The Unwinding: An Inner History of the New AmericaVon EverandThe Unwinding: An Inner History of the New AmericaBewertung: 4 von 5 Sternen4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyVon EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyBewertung: 3.5 von 5 Sternen3.5/5 (2259)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Von EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Bewertung: 4.5 von 5 Sternen4.5/5 (120)
- Her Body and Other Parties: StoriesVon EverandHer Body and Other Parties: StoriesBewertung: 4 von 5 Sternen4/5 (821)
- Technical Data Sheet: Silcoset 151 1 Part Adhesive SealantDokument2 SeitenTechnical Data Sheet: Silcoset 151 1 Part Adhesive SealantArun VaideeswaranNoch keine Bewertungen
- Crack-Tip Field: ES 247 Fracture Mechanics Zhigang SuoDokument12 SeitenCrack-Tip Field: ES 247 Fracture Mechanics Zhigang SuoRamesh SantanaNoch keine Bewertungen
- MODULE - 5 Metallurgy BPUT NotesDokument7 SeitenMODULE - 5 Metallurgy BPUT NotesShibashish RathNoch keine Bewertungen
- DANA-Technical Note (TN)Dokument8 SeitenDANA-Technical Note (TN)Samsul Imran BahromNoch keine Bewertungen
- P O L Y P R O P Y L E N E: Reliance Industries LimitedDokument1 SeiteP O L Y P R O P Y L E N E: Reliance Industries LimitedalbertoNoch keine Bewertungen
- Quotation Waterproofing For Basement Waterproofing - VECDokument4 SeitenQuotation Waterproofing For Basement Waterproofing - VECGaurav SinghNoch keine Bewertungen
- Chemistry Report (Size of Reactant)Dokument3 SeitenChemistry Report (Size of Reactant)Ain NajwaNoch keine Bewertungen
- ReferencesDokument42 SeitenReferencesArunKumar0203Noch keine Bewertungen
- Lab Manual For Soil TestingDokument58 SeitenLab Manual For Soil TestingSanjay MuthekarNoch keine Bewertungen
- Paper Chromatography HandoutDokument21 SeitenPaper Chromatography Handoutalem010Noch keine Bewertungen
- Position of Welds ComparisonDokument3 SeitenPosition of Welds ComparisonYuvaraj SathishNoch keine Bewertungen
- Valve Seat Seal Selection GuideDokument2 SeitenValve Seat Seal Selection Guideecovarrubias1Noch keine Bewertungen
- Maciej Zarow Metal Post and Core How To Improve Aesthetics Via WWW Styleitaliano OrgDokument27 SeitenMaciej Zarow Metal Post and Core How To Improve Aesthetics Via WWW Styleitaliano OrgghfhfdghNoch keine Bewertungen
- Dry Friction of Pure Aluminum Against Steel - N. RusinDokument8 SeitenDry Friction of Pure Aluminum Against Steel - N. RusinMiguel Dguez GurríaNoch keine Bewertungen
- ABREX NSSMC Abrasion Resistance Plate Catalogue PDFDokument8 SeitenABREX NSSMC Abrasion Resistance Plate Catalogue PDFomni_partsNoch keine Bewertungen
- Design of Basement WallDokument4 SeitenDesign of Basement WallMakAsifNoch keine Bewertungen
- BS en 13445-3 (2009)Dokument17 SeitenBS en 13445-3 (2009)Pedro_csNoch keine Bewertungen
- Retaining Wall Based On ACI 318-02Dokument6 SeitenRetaining Wall Based On ACI 318-02ابو عمر الأسمريNoch keine Bewertungen
- CE C30/ME C85 - Introduction To Solid MechanicsDokument2 SeitenCE C30/ME C85 - Introduction To Solid Mechanicsmindpower_146Noch keine Bewertungen
- RCC SyllabusDokument2 SeitenRCC SyllabusprashmceNoch keine Bewertungen
- (2003) US6603036 Process For The Manufacture of 2-Ethylhexyl AcrylateDokument6 Seiten(2003) US6603036 Process For The Manufacture of 2-Ethylhexyl Acrylateremi1988Noch keine Bewertungen
- Chart For Factor B of CSDokument8 SeitenChart For Factor B of CSnguyenvanphu1977Noch keine Bewertungen
- Effect of Self Weight On A Cantilever BeamDokument5 SeitenEffect of Self Weight On A Cantilever BeamBilal JavedNoch keine Bewertungen
- INSTAGDokument3 SeitenINSTAGJeeva Z FedricoNoch keine Bewertungen
- Stress-Strain Curve 3. Short Term Mechanical PropertiesDokument183 SeitenStress-Strain Curve 3. Short Term Mechanical PropertiesManprita BasumataryNoch keine Bewertungen
- Asia polymer-EV302Dokument1 SeiteAsia polymer-EV302ShahryarNoch keine Bewertungen
- Derakane Chem Guide 2010 AugDokument74 SeitenDerakane Chem Guide 2010 AugOliverNoch keine Bewertungen
- Peca Form TDS Feb 12Dokument4 SeitenPeca Form TDS Feb 12Øwięs MØhãmmedNoch keine Bewertungen
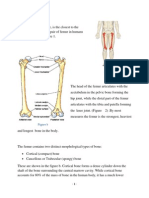
- Eiffel Tower and FemurDokument5 SeitenEiffel Tower and FemurAnand TRNoch keine Bewertungen