Beruflich Dokumente
Kultur Dokumente
Measles (Rubeola) : Communicable Disease Management Protocol
Hochgeladen von
Marilyn GonzalesOriginaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Measles (Rubeola) : Communicable Disease Management Protocol
Hochgeladen von
Marilyn GonzalesCopyright:
Verfügbare Formate
1.
Case Definition
1.1 Confirmed Case
Consistent clinical illness
a
with laboratory
confirmation, in the absence of recent
immunization (one to 14 days prior) with measles-
containing vaccine. Laboratory confirmation
includes at least one of:
isolation of measles virus from an
appropriate clinical specimen
OR
detection of measles virus RNA
OR
seroconversion or a significant rise in
measles IgG titre between acute and
convalescent sera by any standard
serologic assay
OR
positive serologic test for measles specific
IgM antibody using a recommended
assay
b
OR
In the absence of laboratory confirmation, clinical
illness
a
in a person with an epidemiologic link to a
laboratory-confirmed case (1).
1.2 Probable Case
Clinical illness
a
in the absence of appropriate
laboratory tests and in the absence of an
epidemiologic link to a laboratory-confirmed case
in a person who has recently been to an area of
known measles activity.
Note: Surveillance for measles focuses on evident
disease rather than infection. Therefore, surveillance
definitions do not take into account asymptomatic
or subclinical infections that may be detectable by
laboratory methods (1).
2. Reporting Requirements
Laboratory:
All positive laboratory results should be
faxed (204-948-3044 secure fax) and
called into Manitoba Health, Public
Health Surveillance Unit at
204-788-6736 or 204-788-8666 (24
hours) for rapid notification.
Operators of Manitoba clinical laboratories
are required to submit to Cadham
Provincial Laboratory (CPL) the residual
serum, plasma or respiratory specimens or
respiratory viral isolate sub-cultures from
individuals who tested positive for measles
virus within seven days of report.
Health Care Professional:
The Communicable Disease Control
Investigation Form (available at:
www.gov.mb.ca/health/publichealth/cdc/protocol/form2.pdf)
should be faxed (204-948-3044 secure
fax) to Manitoba Health, Public Health
Surveillance Unit when a health
professional becomes aware that a person
meets or has recently met the probable or
confirmed case definition for measles.
a Clinical illness is characterized by all of the following:
fever 38.3C or greater;
cough, coryza (runny nose) or conjunctivitis; and
generalized maculopapular rash for at least three days.
Atypical cases in immunocompromised or partially
immune persons may lack hallmark symptoms.
b IgM serology may be a false positive. If the clinical
presentation is inconsistent with a diagnosis of measles
or in the absence of recent travel/exposure history, IgM
results must be confirmed by another listed
confirmatory method. Most acute measles cases develop
IgM three days or more after rash onset. Therefore, a
suspected measles case where serum collected 3 days
post rash onset initially tests IgM negative should have a
second serum collected > 3 days post rash onset for
retesting for IgM.
Communicable Disease Management Protocol
July 2010
1
Communicable Disease Control Branch
Communicable Disease Management Protocol Measles (Rubeola)
Measles (Rubeola)
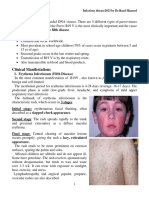
3. Clinical Presentation/Natural
History
The prodromal phase begins 8-12 days after
exposure in susceptible persons (2) and may
resemble a severe upper respiratory tract infection
(3). This phase is characterized by malaise, fever,
anorexia, conjunctivitis and respiratory symptoms
such as cough and coryza (3). Other symptoms
may include diarrhea, especially in infants, and
generalized lymphadenopathy (4). Older children
may complain of photophobia and occasionally of
arthralgia (5). Koplik spots may appear toward the
end of the prodrome, just before the appearance of
the rash (3). Koplik spots (white spots on a red base
on the inner lining of the mouth) usually occur on
the mucosa opposite the second molars and begin
to slough as the rash appears (3) but occasionally
occur on the conjunctiva, vaginal mucosa or the
gastrointestinal mucosa (6). The maculopapular
rash of measles is usually identified approximately
14 days after exposure (2). It begins on the face,
then progresses down the body to the extremities,
including the palms and soles (3, 4), and lasts
approximately five days (3). Patients tend to be
most ill on the first or second day of the rash (3).
The rash fades in the same sequence it appears,
from head to extremities (4). The characteristic rash
may not develop in immunocompromised patients
(7). Uncomplicated illness, from late prodrome to
resolution of the fever and rash, lasts seven to 10
days (3). The disease is usually more severe in
infants and adults than in children (10). Measles is
also often more severe in immunocompromised
individuals (4) and malnourished children,
including those with Vitamin A deficiency (4).
Uncomplicated recovery from measles is the norm
in resource-rich areas; but serious complications of
the respiratory tract (pneumonia) and central
nervous system (CNS) (acute encephalitis) may
occur (3). Other potential serious complications
include myocarditis, pericarditis and hepatitis.
Complications are more common among children
younger than five years of age and adults 20 years
of age and older (4). Measles virus may directly
cause croup, bronchiolitis and pneumonia (3).
Secondary viral (6) or bacterial invasion may also
occur, resulting in complications such as
pneumonia and otitis media (3). Measles associated
with Vitamin A deficiency is a common cause of
blindness in developing countries (8). Measles
occurring during pregnancy has been associated
with spontaneous abortion, premature delivery (3,
9) and low birth weight infants (9). Acute measles
encephalitis occurs in approximately one of every
1,000 reported cases and may result in permanent
neurologic damage (9). In Canada, death is
estimated to occur once in 3,000 cases of measles
(9).
A rare late complication of measles infection is
subacute sclerosing panencephalitis (SSPE), a
chronic and degenerative central nervous system
disease characterized by behavioural and intellectual
deterioration and seizures progressing to death (2).
It occurs several to many years after an attack of
measles (3).
Modified forms of measles with generally mild
symptoms may occur in infants who still have
partial protection from maternal antibody and,
occasionally, in persons with only partial protection
from the vaccine (5).
Atypical measles may occur in persons who received
inactivated (killed) measles vaccine (KMV) and
are subsequently exposed to wild-type measles virus
(4). This is prevented by revaccinating with live
measles vaccine (4).
4. Etiology
Measles virus is an RNA virus with only one
serotype, classified as a member of the genus
Morbillivirus of the family Paramyxoviridae. There
are numerous distinct genotypes (3). Molecular
characterization of a specific virus genotype may
identify an outbreak strain when the epidemiologic
observations are consistent (5). The primary site of
infection is the respiratory epithelium of the
nasopharynx (4).
Communicable Disease Management Protocol
July 2010
2
Communicable Disease Management Protocol Measles (Rubeola)
5. Epidemiology
5.1 Reservoir and Source
Humans (10). No non-human reservoir or source
of infection has been identified (9). An
asymptomatic carrier state has not been
documented (4, 11).
5.2 Transmission
Measles is spread by airborne droplets (sneezing or
coughing) or direct (close personal) contact with
nasal or throat secretions of infected persons (12).
The virus may remain infective in droplet nuclei in
the air for several hours (3, 4), especially under
conditions of low relative humidity (3). It is spread
less commonly by articles freshly soiled with nose
and throat secretions (10, 12).
In Canada, sustained transmission has been mostly
eliminated with high vaccine coverage and the
current two dose vaccine schedule for specific
groups (9). However, secondary spread from
imported cases to the few remaining vulnerable
Canadians may occur (9).
Respiratory excretion of the attenuated measles
virus used in vaccines may occur after vaccination;
however, person-to-person transmission has not
been documented (5).
5.3 Occurrence
General: Measles is endemic worldwide (4, 5), with
approximately 20 million cases occurring annually
(13). In temperate climates, outbreaks generally
occur in late winter and early spring (5). In tropical
climates, transmission appears to increase after the
rainy season (5). Prior to the introduction of
universal vaccination, epidemics of measles lasting
three to four months occurred consistently every
two to five years (3). Outbreaks are attributed to
the accumulation of individuals susceptible to
measles virus, including both unvaccinated or
partially vaccinated persons and those who were
vaccinated but failed to seroconvert (5). Since the
initiation of universal measles vaccination with the
resulting decrease in measles virus circulation, the
average age at which infection occurs has increased
(5). Measles is still a common disease and an
important cause of death and disability in countries
with limited health infrastructure (11). Between
2000 and 2007, the number of reported cases of
measles worldwide declined by two-thirds (14).
However, there is substantial underreporting of
measles cases even in industrialized countries (14).
Global mortality from measles was reduced by 74%
during this period from an estimated 750,000 to
197,000 (14, 15). The largest regional percentage
reduction in estimated measles mortality during
2000-2007 occurred in the Eastern Mediterranean
and African regions (14).
Canada: Between 2001 and 2005, the number of
measles cases reported annually ranged from six
(2005) to 34 (2001), with a yearly average of 14
(9). All cases were imported or import-related (9).
Manitoba: The last large outbreak occurred in
1986 with greater than 3,000 cases. From 2000 to
2009 inclusive, three cases of measles were
reported, one in each of 2002, 2003 and 2004.
5.4 Incubation Period
The incubation period of measles following
exposure to the onset of the prodrome is 8-12 days
(2). Exposure to rash onset averages 14 days (range,
7-18 days) (4). Incubation may be slightly longer in
adults (3). Immune globulin given for passive
protection early in the incubation period may
extend the incubation period rather than prevent
disease (10).
5.5 Host Susceptibility and Resistance
All individuals who have not had infection or been
effectively immunized or those with profound
cellular immunosuppression are susceptible (10).
Immunity following natural infection is believed to
be lifelong, and vaccination with measles-
containing vaccine has been shown to be protective
for at least 20 years (5). In Canada, measles-
containing vaccine is only available in combination
with the rubella and mumps vaccine (MMR).
Communicable Disease Management Protocol
July 2010
3
Communicable Disease Management Protocol Measles (Rubeola)
Individuals are presumed to be immune to measles
if they were born before 1970, have a history of
laboratory-confirmed measles disease, documented
evidence of vaccination with two doses of measles-
containing vaccine after their first birthday, or have
laboratory evidence of immunity (9). Vaccination
protects against all wild-type genotypes (5). Infants
whose biological mothers have had disease are
generally protected until six to nine months of age
or longer by passively acquired maternal measles
antibody (10). Infants whose mothers have been
immunized have lower levels of passive antibody
and may have a shorter duration of protection (10).
5.6 Period of Communicability
Measles is one of the most highly communicable
diseases in humans (10). It is most infectious
during the late prodromal phase of the illness,
when cough and coryza are at their peak (3). The
virus can be spread for about four days before rash
onset (i.e., one to two days before fever onset) until
about four days after rash onset (4, 5, 10, 12).
Secondary attack rates among susceptible
household contacts have been reported to be
75%- 90% (5). Patients with SSPE are not
contagious (2).
6. Laboratory Diagnosis
All lab specimens should include date of onset of
both fever and rash.
Virus Detection:
Cadham Provincial Laboratory (CPL) virology
section (204-945-6123) should be consulted
prior to sending specimens for virus detection.
All specimens should be transported with a cold
pack (to maintain a temperature of
approximately 4C) to CPL as soon as possible.
When investigating a sporadic case, a
nasopharyngeal (refer to:
http://www.gov.mb.ca/health/publichealth/cpl/docs/nasopharyngeal_collection.pdf )
or throat swab should be collected for virus
isolation. Nasopharyngeal swabs are preferred.
Nasopharyngeal and throat swabs should be
collected within four days of rash onset. Since the
virus is cell associated, the technique should be
vigorous enough to capture some epithelial cells.
Swabs should be placed in a tube containing 2-3 ml
of viral transport medium (VTM). Also acceptable
but least preferred of the virological specimens is
urine, 50 mL of which may be collected within
seven days of rash onset.
Further strain characterization may be indicated for
epidemiological and public health control activities.
Serology:
Serological testing is preferred during established
outbreaks. Generally, IgM is used for diagnostic
testing and IgG for immune status testing. A
specimen for the detection of measles specific IgM
antibodies should ideally be taken within three to
seven days after rash onset, but may be taken up to
28 days after rash onset. Both false positive and
false negative measles IgM results can occur. If the
clinical presentation is inconsistent with a diagnosis
of measles or in the absence of recent
travel/exposure history, a positive IgM result must
be confirmed by one of the confirmatory methods
listed in section 1, Case Definition. If a specimen
taken 3 days after rash onset is negative for
measles IgM, a second specimen should be
obtained three days later. Consideration should also
be given to investigation for other exanthem
viruses, including parvovirus and rubella.
7. Key Information for Public Health
Response
Immunization history including date(s)
and type of vaccine if known.
Recent exposure/travel history of cases
(i.e., 7-18 days before rash onset).
Identification and appropriate follow-up
of susceptible contacts.
8. Control
8.1 Management of Cases
Measles is an uncommon infection in Manitoba
that may present with signs and symptoms
suggesting a wide differential diagnosis and has a
possibility for severe adverse outcomes if not
Communicable Disease Management Protocol
July 2010
4
Communicable Disease Management Protocol Measles (Rubeola)
managed appropriately. Consultation with an
expert in infectious diseases is recommended for the
management of individual cases of probable or
confirmed disease. Reported cases are referred to
the Regional Health Authority (RHA) of patient
residence or First Nations Inuit Health (if
applicable) for follow-up.
Airborne Precautions in addition to Routine
Practices should be followed when individuals with
probable measles present to a health care setting.
Refer to Infection Control Guidelines: Routine
Practices and Additional Precautions for Preventing
the Transmission of Infection in Health Care.
Canada Communicable Disease Report CCDR,
1999; 25S4: 1-142.
All cases should be advised to stay home (self-
isolate) from school, post-secondary educational
institutions, child care facilities, workplaces and
other group settings for four days after rash onset.
Cases should be advised to practice good hand
hygiene, avoid sharing drinking glasses or utensils
and cover coughs and sneezes with a tissue or
forearm.
Treatment:
Consultation with an expert in infectious
diseases.
Treatment is supportive (3).
Bacterial superinfection should be
promptly diagnosed and treated with
appropriate antimicrobials; prophylactic
antibiotics to prevent superinfection are of
unproven value and not recommended
(3).
The World Health Organization (WHO)
currently recommends Vitamin A for all
children with acute measles, regardless of
the country of residence. The following
doses, once daily for two days are
recommended (2):
200,000 IU for children 12 months of
age or older;
100,000 IU for infants six through 11
months of age; and
50,000 IU for infants younger than
six months of age.
Cases who are health care workers should be
advised to immediately notify Occupational Health
and/or Infection Prevention and Control for the
facility/regional program in which they work and in
consultation with them determine when it is
appropriate to return to work. A health care worker
(HCW) includes individuals who have the
potential to acquire or transmit infection during
the course of their work and includes nurses,
physicians, other hospital workers, students,
volunteers, home-care workers, emergency
responders, and support staff (16).
Public Health Measures:
Individuals with confirmed or probable
measles should be excluded from child
care facilities, schools, universities and
workplaces for four days following
appearance of the rash (7, 10).
Hospitalized Patients:
Only measles immune
c
personnel
should enter the room.
In addition to Routine Practices,
Airborne Precautions from onset of
catarrhal stage of the prodromal
period through fourth day of rash
should be used to reduce the exposure
of other patients. Refer to Infection
Control Guidelines: Routine Practices
and Additional Precautions for
Preventing the Transmission of Infection
in Health Care. Canada Communicable
Disease Report CCDR, 1999; 25S4: 1-
142.
c Individuals are presumed to be immune to measles if
they were born before 1970, have a history of
laboratory-confirmed measles disease, documented
evidence of vaccination with two doses of measles-
containing vaccine after their first birthday or have
laboratory evidence of immunity (9).
Communicable Disease Management Protocol
July 2010
5
Communicable Disease Management Protocol Measles (Rubeola)
8.2 Management of Contacts
Regional Public Health (of case area of residence)
or First Nations Inuit Health (FNIH) (if
applicable) will contact all reported cases to
establish a list of exposed persons and identify
susceptible contacts. Examples of exposure
situations where contacts should be identified
include home, child care facility, school, school bus,
workplace, physician office and hospital emergency
department. Airline passengers who have been
exposed to a confirmed measles case on the same
flight should be considered for notification of
exposure, assessment of immune status, and
recommendation for vaccination if appropriate (i.e.,
where vaccination is able to be completed within
72 hours of exposure).
Contacts should be counselled regarding the signs
and symptoms of measles and the need to report to
their health care provider should they occur.
Symptomatic contacts should be instructed to call
before presenting to a health care provider to
reduce the potential impact on susceptible
individuals. Contacts should be encouraged to
practice good hand hygiene, avoid sharing drinking
glasses or utensils and cover coughs and sneezes
with a tissue or forearm.
Contacts who are health care workers should be
advised to notify Occupational Health and/or
Infection Prevention and Control for the
facility/regional program in which they work and in
consultation with them determine when it is
appropriate to return to work.
Definitions:
Contact: Someone who shared the same airspace
(no minimum length of time) during the infectious
period
d
.
Susceptible Contact: A contact (defined above)
born during or after 1970
e
and not meeting any of
the following criteria (9):
Documented evidence of vaccination with
two doses of measles-containing vaccine
after their first birthday;
Laboratory evidence of immunity; or
History of laboratory-confirmed measles
disease.
High Risk Susceptible Contact: A susceptible
contact (as defined above) meeting one or more of
the following criteria:
Immunocompromised;
Pregnant;
Infant < 12 months of age; or
Other valid contraindication to the
receipt of measles vaccine (e.g., allergy to
a vaccine component) (9).
8.21 Management of Susceptible Contacts
Susceptible contacts, as defined above, who are
12 months of age AND do not have
contraindications to measles-containing vaccine
(e.g., immunocompromised, pregnant, see High
Risk Susceptible Contact above) should be
immunized with MMR as soon as possible.
Immunization within 72 hours of exposure may
prevent disease. There are no known adverse effects
when vaccine is given to people incubating the
disease (9). A second dose of MMR vaccine given
at least 28 days after the first dose is indicated only
for susceptible contacts who received no doses of
measles-containing vaccine prior to the exposure.
Susceptible contacts who received one dose of
measles-containing vaccine prior to the exposure do
not require a second post-exposure dose of MMR
vaccine. Routine post-immunization serology is not
indicated (9).
d The infectious period is four days prior to and four days
after rash appears (4, 5, 10, 12).
e Depending on the number of cases, their age and
immunization histories, a decision may be made by the
Public Health and Primary Health Care Division or
Regional Health Authority, in consultation with an
Outbreak Response Team, to consider older persons,
without two doses of live vaccine, as susceptible.
Communicable Disease Management Protocol
July 2010
6
Communicable Disease Management Protocol Measles (Rubeola)
Immune globulin (Ig) should be considered for
susceptible contacts presenting more than 72 hours
but within six days of exposure (i.e., too late for
vaccine). The recommended dose for
immunocompetent individuals is 0.25 mL/kg body
weight up to a maximum of 15 mL (9). Refer to the
current Canadian Immunization Guide and the
product monograph for information on clinical use.
Refer to Follow-up After Ig Administration below.
8.22 Management of High Risk Susceptible
Contacts
Immune globulin (Ig) should be administered as
soon as possible to high risk susceptible contacts as
defined above (i.e., for whom measles-containing
vaccine is contraindicated), preferably within three
days of exposure but as long as six days after
exposure (9). The recommended dose for
immunocompetent individuals is 0.25 mL/kg body
weight up to a maximum of 15 mL (9). For
immunocompromised persons, 0.5 mL/kg is given,
up to a maximum of 15 mL (9). Refer to the current
Canadian Immunization Guide and the product
monograph for information on clinical use.
Follow-up After Ig Administration: If clinical
measles does not develop in a person administered
Ig, measles-containing vaccine should be given five
or six months later depending on the Ig dose used
(refer to current Canadian Immunization Guide),
provided the individual is greater than one year of
age and there are no contraindications to the
vaccine (e.g., pregnant, immunocompromised) (9).
A second dose of MMR vaccine given at least 28
days after the first dose is indicated only for
susceptible contacts who received no doses of
measles-containing vaccine prior to the exposure.
Susceptible contacts who received one dose of
measles-containing vaccine prior to the exposure do
not require a second post-exposure dose of MMR
vaccine. Routine post-immunization serology is not
indicated (9).
8.23 Children in Schools and Child Care
Facilities
Parents/guardians of susceptible
f
children attending
facilities where a case has occurred should be
informed that:
The measles virus is extremely contagious.
Susceptible
f
children who have not
received vaccine within 72 hours of first
exposure or Ig within six days of first
exposure are highly likely to develop
measles infection. Therefore, self-
exclusion for 14 days after disease onset in
the last known case in the facility is
recommended to prevent infection in
unimmunized children (2).
Note: Susceptible
f
individuals in workplaces and
adult educational facilities where a case has
occurred who have not received vaccine within 72
hours of first exposure or Ig within six days of first
exposure should be informed that self-exclusion for
14 days after disease onset in the last known case is
recommended (2, 17) to prevent infection.
Individuals attending such facilities who were born
before 1970 are presumed to be immune to
measles.
Refer also to section 8.3 Management of
Outbreaks below.
8.24 In Hospitals
Susceptible
f
contacts should be discharged if
possible before the fifth day after first exposure.
Otherwise, place susceptible contacts on Airborne
Precautions from day five after first exposure to day
21 after last exposure (11). Refer to Infection
Control Guidelines: Routine Practices and
Additional Precautions for Preventing the
Transmission of Infection in Health Care. Canada
Communicable Disease Report CCDR, 1999: 25S4:
1-142.
f An individual born during or after 1970 who has no
documented evidence of vaccination with two doses of
measles-containing vaccine after their first birthday, no
laboratory evidence of immunity and no history of
laboratory-confirmed measles disease. Depending on the
number of cases, their age and immunization histories, a
decision may be made by the Public Health and
Primary Health Care Division or Regional Health
Authority, in consultation with an Outbreak Response
Team, to consider older persons, without two doses of
live vaccine, as susceptible.
Communicable Disease Management Protocol
July 2010
7
Communicable Disease Management Protocol Measles (Rubeola)
8.3 Management of Outbreaks
Definition of Outbreak: Confirmed cases in excess
of what is expected in the jurisdiction over a given
time period.
With the current childhood two-dose schedule for
measles-containing vaccine, large outbreaks of
measles are not expected to occur (9). However,
imported cases will result in limited transmission of
measles, usually among unvaccinated children and
young adults who have not received two doses of
vaccine (9).
Before any intervention is initiated,
probable measles cases should be
promptly confirmed by culture or
serology (9).
Public notification should occur: the level
of notification will be at the discretion of
regional Public Health and/or the Office
of Disaster Management.
Contacts
g
of confirmed cases should be
informed that measles virus is circulating
and that immunization should be
updated if necessary (9). Measles-
containing vaccine may be given as early
as six months of age in outbreak
situations, with an additional two doses
given after the childs first birthday
according to the recommended
immunization schedule below under
section 8.4 (2, 9).
Management of outbreaks may require
mass immunization campaigns for
selected age ranges. This will be
determined by the Public Health and
Primary Health Care Division or
Regional Health Authority, in
consultation with an Outbreak Response
Team.
Ig is not recommended as a general
strategy to control measles outbreaks;
however, it should be administered to
high risk susceptible contacts as described
above under Management of Contacts
(9).
Active surveillance measures should
remain in place until four weeks after the
last case occurs (17).
8.4 Preventive Measures
Prompt identification and management of
cases and contacts.
Immunization:
Children: Routine two-dose
immunization of all children, unless
contraindicated (9). Infants should
receive a first dose combined with
mumps and rubella vaccines (MMR) on
or shortly after their first birthday; the
second dose should be given at least 28
days after the first dose and after 15
months of age, but before school entry
(9). Refer to the current Manitoba
Recommended Immunization Schedules for
Infants, Children and Adults available at:
http://www.gov.mb.ca/health/publichealth/cdc/fs/irg.pdf.
Vaccination of children as early as six
months of age may be recommended
during outbreaks or international
travel to an area where measles is
endemic with an additional two doses
of MMR given after the child's first
birthday (2, 9).
Adults: Immunization of adults born
during or after 1970 who have not
received a measles-containing vaccine or
had natural measles infection, unless
contraindicated (9). A second dose of
MMR should be offered only to adults
born during or after 1970 who are at
greater risk of exposure specifically:
Travellers to measles endemic areas;
Health care workers;
Military recruits; and
Students at post-secondary
institutions.
g For practical purposes, all students attending the same
school or facility should be considered contacts (9).
Communicable Disease Management Protocol
July 2010
8
Communicable Disease Management Protocol Measles (Rubeola)
Please refer to the current Canadian Immunization
Guide as well as the manufacturers package insert
instructions for clinical use information on measles-
containing vaccine.
Note: Not all recommended vaccines and
immune globulins are provided by Manitoba
Health (e.g., second MMR dose for travel). Refer
to Manitoba Healths eligibility criteria for high
risk individuals available at:
http://www.gov.mb.ca/health/publichealth/cdc/vaccineeligibility.html.
9. Additional Resources
Manitoba Immunization Schedules
available at:
http://www.gov.mb.ca/health/publichealth/cdc/schedule.html
Canadian Immunization Guide
References
1. Public Health Agency of Canada. Case
Definitions for Communicable Diseases under
National Surveillance. Canada Communicable
Disease Report CCDR, 2009; 35S2: 71-72.
2. American Academy of Pediatrics. Measles. In:
Pickering LK ed. Redbook 2009 Report of the
Committee on Infectious Diseases 28th ed. Elk
Grove Village, IL: American Academy of
Pediatrics, 2009; 444-455.
3. Gershon Anne A. Measles Virus (Rubeola). In:
Mandell GL, Bennell JE, Dolin R eds.
Principles and Practice of Infectious Diseases 6th
ed. Elsevier, Philadelphia, 2007; 2031-2038.
4. Centers for Disease Control and Prevention.
Chapter Measles. Epidemiology and
Prevention of Vaccine-Preventable Diseases, The
Pink Book: Updated 11th Edition 2009.
5. Pan American Health Organization. Measles
Elimination Field Guide Second Edition 2005.
6. Steichan, Oliver and Dautheville Sandrine.
Koplik spots in early measles. Canadian
Medical Association Journal 2009; 180(5): 583.
7. Manitoba Health Communicable Disease
Control Branch. Measles Communicable
Disease Management Protocol, November 2001.
8. Perry, Robert T and Halsey, Neal A. The
Clinical Significance of Measles: A Review.
Journal of Infectious Diseases 2004; 189 (Suppl
1 ): S4-16.
9. National Advisory Committee on
Immunization. Canadian Immunization Guide
7th ed. Public Health Agency of Canada, 2006;
228-236.
10. Heymann David L. Measles. In: Control of
Communicable Diseases Manual 19th ed,
American Public Health Association,
Washington, 2008; 402-408.
11. World Health Organization. Measles vaccines:
WHO position paper. Weekly Epidemiological
Record 2009; No. 35, 84: 349-360.
12. Public Health Agency of Canada. Vaccine-
Preventable Diseases Measles. Updated:
February 19, 2008.
13. Centers for Disease Control and Prevention.
Measles United States, January 1-April 25,
2008. Morb Mort Wkly Rep MMWR 2008;
57(18): 494-498.
14. World Health Organization. Progress in global
measles control and mortality reduction, 2000-
2007. Weekly Epidemiological Record 2008; No.
49, 441-448.
15. Joint News Release WHO/UNICEF/American
Red Cross/CDC/UN Foundation 4 December
2008. Global Measles Deaths Drop by 74%:
The Eastern Mediterranean region achieves
measles goal three years early.
16. Public Health Agency of Canada. Guidelines
for the Prevention and Control of Mumps
Outbreaks in Canada. Canada Communicable
Disease Report CCDR, 2010; 36S1: 1-46.
17. Health Canada. Guidelines for Control of
Measles Outbreaks in Canada (Revised 1995).
Canada Communicable Disease Report, 1995;
21: 189-195.
Communicable Disease Management Protocol
July 2010
9
Communicable Disease Management Protocol Measles (Rubeola)
Das könnte Ihnen auch gefallen
- Full Notes RadiographyDokument120 SeitenFull Notes RadiographyNishimwe Lambert100% (1)
- Jon Zonderman, Laurel, M.D. Shader Birth Control Pills Drugs The Straight FactsDokument96 SeitenJon Zonderman, Laurel, M.D. Shader Birth Control Pills Drugs The Straight FactsPaola Felippi CoelhoNoch keine Bewertungen
- Pod Uveitis Book RevDokument252 SeitenPod Uveitis Book Revelika dwi100% (1)
- MeaslesDokument16 SeitenMeaslesChristian Lloyd Nablea PlazaNoch keine Bewertungen
- MeaslesDokument20 SeitenMeaslessuryanto_malvinNoch keine Bewertungen
- Seminar On MeaslesDokument24 SeitenSeminar On MeaslesMARK ELUOKONoch keine Bewertungen
- Respiratory Infections - 30.10.2023Dokument61 SeitenRespiratory Infections - 30.10.2023johnnycash5404Noch keine Bewertungen
- Measles: 1. Disease ReportingDokument18 SeitenMeasles: 1. Disease ReportingMemo AliNoch keine Bewertungen
- E MumpsDokument3 SeitenE Mumpsolivia1026Noch keine Bewertungen
- Measles (Rubeola) Rubeola Red Spot: Dr. Eman Khammas Alsadi Community Medicine LecturerDokument34 SeitenMeasles (Rubeola) Rubeola Red Spot: Dr. Eman Khammas Alsadi Community Medicine Lecturereman khammasNoch keine Bewertungen
- Diseases PIDSRDokument25 SeitenDiseases PIDSRaringkinking100% (1)
- Measles Aug 2014Dokument23 SeitenMeasles Aug 2014Shahab UddinNoch keine Bewertungen
- Jurnallll 3Dokument15 SeitenJurnallll 3RiestaKierantiNoch keine Bewertungen
- Acep Fact Sheet: Measles (Rubeola) Brief DescriptionDokument2 SeitenAcep Fact Sheet: Measles (Rubeola) Brief DescriptionrobinsonknightsNoch keine Bewertungen
- Care of Clients With Problems in Inflammatory and Immunology Integumentary SystemDokument24 SeitenCare of Clients With Problems in Inflammatory and Immunology Integumentary Systemgrazelantonette.calubNoch keine Bewertungen
- COVID-19: UM Icrob Iolog yDokument50 SeitenCOVID-19: UM Icrob Iolog yJana AbdulrahimNoch keine Bewertungen
- Measles: Clinical Manifestations, Diagnosis, Treatment, and PreventionDokument15 SeitenMeasles: Clinical Manifestations, Diagnosis, Treatment, and PreventionWidya Ayu SetyaningrumNoch keine Bewertungen
- Dr. Rehab Aljerbi Assistant Lecturer-Department of Family and Community Medicine Tripoli UniversityDokument31 SeitenDr. Rehab Aljerbi Assistant Lecturer-Department of Family and Community Medicine Tripoli UniversityHannan AliNoch keine Bewertungen
- Tyhpoid FeverDokument6 SeitenTyhpoid FeverMade Oka Heryana100% (1)
- MeasDokument22 SeitenMeasSriLestariFajerinNoch keine Bewertungen
- The Reemergence of MeaslesDokument8 SeitenThe Reemergence of MeaslesAyuNoch keine Bewertungen
- Measles: Practice EssentialsDokument7 SeitenMeasles: Practice EssentialsMuhammad ANoch keine Bewertungen
- CDs L2 4th 2021 2022Dokument46 SeitenCDs L2 4th 2021 2022محمد رافد لطيفNoch keine Bewertungen
- MeaslesDokument4 SeitenMeaslespauline erika buenaventuraNoch keine Bewertungen
- ND Dengue FeverDokument6 SeitenND Dengue FeverAlia SalviraNoch keine Bewertungen
- Measles: Investigative GuidelinesDokument17 SeitenMeasles: Investigative GuidelinesDennis DennisNoch keine Bewertungen
- PAEDS 4 - 18.10.19 Viral InfectionDokument15 SeitenPAEDS 4 - 18.10.19 Viral Infectionlotp12Noch keine Bewertungen
- Smallpox Binder Chapter.2008.FINAL Id314Dokument14 SeitenSmallpox Binder Chapter.2008.FINAL Id314subhadipbhunia.studentNoch keine Bewertungen
- Varicella Zooster VirusDokument9 SeitenVaricella Zooster VirusChikita Artia SariNoch keine Bewertungen
- COVID-19 First Wave HitDokument5 SeitenCOVID-19 First Wave HitSarthak VNoch keine Bewertungen
- InfectiousDokument4 SeitenInfectiouszainabd1964Noch keine Bewertungen
- Chapter 14: Rubella: I. Disease DescriptionDokument11 SeitenChapter 14: Rubella: I. Disease DescriptionDhonna PurpleNoch keine Bewertungen
- Viral Exanthema: Dharmendra MandalDokument57 SeitenViral Exanthema: Dharmendra MandalMaunank TandelNoch keine Bewertungen
- REFERAT - Meassles - FirdaDokument19 SeitenREFERAT - Meassles - FirdaEriza LuthfansyahNoch keine Bewertungen
- Measles: EtiologyDokument14 SeitenMeasles: EtiologyHan SANoch keine Bewertungen
- 1-Measles (Rubeola) PDFDokument37 Seiten1-Measles (Rubeola) PDFمصطفى رسول هاديNoch keine Bewertungen
- Respiratory Infections: - Small Pox - Chicken Pox - TuberculosisDokument45 SeitenRespiratory Infections: - Small Pox - Chicken Pox - TuberculosisNamrata SharmaNoch keine Bewertungen
- Viral Infection: NAME-Rahul Pawar (Roll No.17) Sanket Joshi (Roll No.25)Dokument25 SeitenViral Infection: NAME-Rahul Pawar (Roll No.17) Sanket Joshi (Roll No.25)aditya_aditya_mayekar199Noch keine Bewertungen
- Poliomyelitis CDCDokument12 SeitenPoliomyelitis CDCAsiah JelitaNoch keine Bewertungen
- IOG - National Clinical Guidelines - Monkeypox Guidelines - 2022Dokument9 SeitenIOG - National Clinical Guidelines - Monkeypox Guidelines - 2022Maryam YasminNoch keine Bewertungen
- Rubella (Acquired and Congenital) : 1. Disease ReportingDokument15 SeitenRubella (Acquired and Congenital) : 1. Disease Reportingshreyaz90Noch keine Bewertungen
- Campak 11 PDFDokument12 SeitenCampak 11 PDFSa 'ng WijayaNoch keine Bewertungen
- Background: Otitis MediaDokument3 SeitenBackground: Otitis MediaHugo SalazarNoch keine Bewertungen
- Shig Ellos IsDokument8 SeitenShig Ellos IsnabilahNoch keine Bewertungen
- 420 063 Guideline MeaslesDokument25 Seiten420 063 Guideline MeaslesJerome ProfetanaNoch keine Bewertungen
- Swine Flu: Diseasealso Called Swine Influenza, Influenza A (H1N1), Hog Flu, or Pig FluDokument9 SeitenSwine Flu: Diseasealso Called Swine Influenza, Influenza A (H1N1), Hog Flu, or Pig FluSarvesh JaiswalNoch keine Bewertungen
- Virus Paramyxovirus: MorbillivirusDokument9 SeitenVirus Paramyxovirus: MorbillivirusRhoda Mae Dano JandayanNoch keine Bewertungen
- Chickenpox and SmallpoxDokument28 SeitenChickenpox and SmallpoxFourth YearNoch keine Bewertungen
- J School Nurs 2012 - p9-12Dokument4 SeitenJ School Nurs 2012 - p9-12Astri Faluna SheylavontiaNoch keine Bewertungen
- Covid 19: Dennis N. Muñoz, RN, RM, LPTDokument103 SeitenCovid 19: Dennis N. Muñoz, RN, RM, LPTDennis Nabor Muñoz, RN,RMNoch keine Bewertungen
- Respiratory 2 Diphtheria and Whooping CoughDokument30 SeitenRespiratory 2 Diphtheria and Whooping Cougheman khammasNoch keine Bewertungen
- Epidemiological Summary - Monkeypox - July 12 2023 - 230712 - 171702Dokument7 SeitenEpidemiological Summary - Monkeypox - July 12 2023 - 230712 - 171702Emmanuel RedmanNoch keine Bewertungen
- Coronavirus Dise/ase 2019 (COVID-19) Is ADokument40 SeitenCoronavirus Dise/ase 2019 (COVID-19) Is Asahil Narang call of dutyNoch keine Bewertungen
- MeaslesDokument4 SeitenMeaslesMohammad Yordan GandaraNoch keine Bewertungen
- 308-Article Text-623-1-10-20161213Dokument3 Seiten308-Article Text-623-1-10-20161213fadiah maharaniNoch keine Bewertungen
- Lect 4 Viral InfectionDokument44 SeitenLect 4 Viral InfectionAMIT GUPTANoch keine Bewertungen
- Varicella/Herpes Zoster: Communicable Disease Management ProtocolDokument0 SeitenVaricella/Herpes Zoster: Communicable Disease Management ProtocolJohn StenlyNoch keine Bewertungen
- HerpesDokument7 SeitenHerpesandrei efremovNoch keine Bewertungen
- Chapter 9: MeaslesDokument16 SeitenChapter 9: MeaslesTrixie Marie Sabile AbdullaNoch keine Bewertungen
- Yellow Fever: Key FactsDokument4 SeitenYellow Fever: Key FactsJuliapertiwi264Noch keine Bewertungen
- Guidelines For Management of Monkeypox DiseaseDokument23 SeitenGuidelines For Management of Monkeypox DiseaseMinerva Medical Treatment Pvt LtdNoch keine Bewertungen
- MeaslesDokument5 SeitenMeaslesVioline John ShannieNoch keine Bewertungen
- FDI Dental Ethics ManualDokument136 SeitenFDI Dental Ethics Manualdocx1975100% (2)
- BJR 84 1121 PDFDokument4 SeitenBJR 84 1121 PDFMarilyn GonzalesNoch keine Bewertungen
- ILEP2015 ReferenceDokument3 SeitenILEP2015 ReferenceraafatbadawyNoch keine Bewertungen
- Journal of Endodontics 2013 Ball - Henrik - Skjerven - 11!06!2013Dokument10 SeitenJournal of Endodontics 2013 Ball - Henrik - Skjerven - 11!06!2013Marilyn GonzalesNoch keine Bewertungen
- Measles and Rubella: Learning A Lesson The Hard WayDokument2 SeitenMeasles and Rubella: Learning A Lesson The Hard WayMarilyn GonzalesNoch keine Bewertungen
- A Review of The Clinical Significance of The Occlusal Plane Variation and Head PostureDokument74 SeitenA Review of The Clinical Significance of The Occlusal Plane Variation and Head PostureFaheemuddin MuhammadNoch keine Bewertungen
- Radiation Doses of Indirect and Direct Digital Cephalometric RadiographyDokument4 SeitenRadiation Doses of Indirect and Direct Digital Cephalometric RadiographyMarilyn GonzalesNoch keine Bewertungen
- Linear Mandibular Measurements: Comparison Between Orthopantomograms and Lateral CephalogramsDokument7 SeitenLinear Mandibular Measurements: Comparison Between Orthopantomograms and Lateral CephalogramsMarilyn GonzalesNoch keine Bewertungen
- KuDokument20 SeitenKuMarilyn GonzalesNoch keine Bewertungen
- Accuracy of Check BiteDokument9 SeitenAccuracy of Check BiteMuhammad Shakeel KhawajaNoch keine Bewertungen
- 02 D003 18267Dokument8 Seiten02 D003 18267Marilyn GonzalesNoch keine Bewertungen
- 84Dokument13 Seiten84Marilyn GonzalesNoch keine Bewertungen
- CephalometricsDokument112 SeitenCephalometricsatxnaeemNoch keine Bewertungen
- Soal Bahasa Inggris Kelompok 8 DoneDokument5 SeitenSoal Bahasa Inggris Kelompok 8 Doneputri dwi lailikaNoch keine Bewertungen
- Ethical Allocation of Future COVID-19 Vaccines: Rohit Gupta, Stephanie R MorainDokument5 SeitenEthical Allocation of Future COVID-19 Vaccines: Rohit Gupta, Stephanie R MorainNugraha SyahbaniNoch keine Bewertungen
- Nursing Care Plan Breast CancerDokument1 SeiteNursing Care Plan Breast CancerAhmed SalahNoch keine Bewertungen
- Plan Design Review GuideDokument37 SeitenPlan Design Review GuideclrhoadesNoch keine Bewertungen
- Pediatric Nursing Flashcards2 - QuizletDokument50 SeitenPediatric Nursing Flashcards2 - QuizletNursyNurseNoch keine Bewertungen
- Jessica Horsley ResumeDokument2 SeitenJessica Horsley Resumeapi-238896609Noch keine Bewertungen
- Attenuation ProjectDokument6 SeitenAttenuation Projectapi-237552707Noch keine Bewertungen
- HemolysisDokument22 SeitenHemolysisMohamoud MohamedNoch keine Bewertungen
- BurnsDokument27 SeitenBurnsThao TranNoch keine Bewertungen
- Zyrtec: Tablet Core: Tablet CoatingDokument5 SeitenZyrtec: Tablet Core: Tablet CoatingDwi WirastomoNoch keine Bewertungen
- Automated DatabasesDokument14 SeitenAutomated DatabasesAnilkumar Sagi100% (4)
- Syndactyly: Luke Geoghegan, Billie Georgina Knowles and Dariush NikkhahDokument2 SeitenSyndactyly: Luke Geoghegan, Billie Georgina Knowles and Dariush NikkhahMiguel JohnsonNoch keine Bewertungen
- SESSION 13 Abnormal PsychologyDokument47 SeitenSESSION 13 Abnormal PsychologyKainaat YaseenNoch keine Bewertungen
- MTY1215 Hematology 2 (Lec) HandoutsDokument7 SeitenMTY1215 Hematology 2 (Lec) HandoutsDarren Gabriel NicolasNoch keine Bewertungen
- For PrintDokument17 SeitenFor Printexample mailNoch keine Bewertungen
- Physical FormDokument4 SeitenPhysical Formapi-352931934Noch keine Bewertungen
- Nursing Care For HipopituitarismeDokument10 SeitenNursing Care For Hipopituitarismevita marta100% (1)
- Mariana Katkout, Health Psychology 7501PSYSCI (AP1) A Defense of The Biopsychosocial Model vs. The Biomedical ModelDokument13 SeitenMariana Katkout, Health Psychology 7501PSYSCI (AP1) A Defense of The Biopsychosocial Model vs. The Biomedical ModelMariana KatkoutNoch keine Bewertungen
- Protocolo Cochrane REHABILITACIÓN COGNITIVA en Demencia (2019)Dokument17 SeitenProtocolo Cochrane REHABILITACIÓN COGNITIVA en Demencia (2019)Sara Daoudi FuentesNoch keine Bewertungen
- Asiri Health Intermediate Care Centre: For Covid 19 Patients Siddhalepa Anarva Hotel A 4 Star, 100 Room FacilityDokument3 SeitenAsiri Health Intermediate Care Centre: For Covid 19 Patients Siddhalepa Anarva Hotel A 4 Star, 100 Room FacilityThilanka NiroshanaNoch keine Bewertungen
- Hospital Direct Roller Slide SheetDokument2 SeitenHospital Direct Roller Slide SheetdantefuerteNoch keine Bewertungen
- 5.3 - Structured Communication Using The SBAR ToolDokument15 Seiten5.3 - Structured Communication Using The SBAR ToolJehad AlturkNoch keine Bewertungen
- Pelvic Inflammatory Disease - Clinical Manifestations and DiagnosisDokument13 SeitenPelvic Inflammatory Disease - Clinical Manifestations and DiagnosisJavier Manuel Escobedo CalderónNoch keine Bewertungen
- Audits & Inspections CRO Perspective: DR - Prashant BodheDokument74 SeitenAudits & Inspections CRO Perspective: DR - Prashant BodheJayanta DasguptaNoch keine Bewertungen
- Acupressure Points For Knee PainDokument7 SeitenAcupressure Points For Knee Painلوليتا وردةNoch keine Bewertungen
- Recommendations For Physiotherapy Intervention After Stroke 5712Dokument9 SeitenRecommendations For Physiotherapy Intervention After Stroke 5712Anonymous Ezsgg0VSENoch keine Bewertungen
- FDA Marijuana Negative Monograph RejectionDokument7 SeitenFDA Marijuana Negative Monograph RejectionMarijuana MomentNoch keine Bewertungen
- Maternal Serum Alpha-Fetoprotein (MSAFP)Dokument2 SeitenMaternal Serum Alpha-Fetoprotein (MSAFP)Shaells JoshiNoch keine Bewertungen