Beruflich Dokumente
Kultur Dokumente
Volume of Compressed Gas in A Cylinder
Hochgeladen von
Waleed EmaraOriginaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Volume of Compressed Gas in A Cylinder
Hochgeladen von
Waleed EmaraCopyright:
Verfügbare Formate
Volume of Compressed Gas in a Cylinder
To find the volume of gas available from a compressed gas cylinder, we apply the Ideal Gas Law (PV =
nT!" In a high#pressure cylinder, the volume will be affected by the content$s compressibility factor %
(PV = %nT!" &or e'ample, an (L cylinder of pure helium may contain )*+ cu" ft" of gas while the same
cylinder of pure air may contain )++ cu" ft" under the same conditions" &or these practical calculations,
however, we assume ideal gas behavior for simplicity"
The Ideal Gas Law PV = nT
,here-
P is pressure
V is volume
n is the number of moles
is the gas constant
T is the absolute temperature
,hen the temperature is .ept constant, we can derive the e/uation-
P()! ' V()! = P(0! ' V(0!
,here-
P()! is the pressure of the compressed gas in the cylinder (psi!
V()! is the internal volume of the cylinder, often referred to as water volume (liter!1
P(0! is the atmospheric pressure () atm # )+"2 psi!
V(0! is the volume of gas at pressure P (0! (liter!
&or e'ample, an (L si3ed cylinder is filled with nitrogen at 0444 psi" ,hat is the gas volume of nitrogen
from the cylinder5
P()! is 0444 psi
V()! is the internal volume of (L cylinder 06"7 liter1
P(0! is )+"2 psi
V(0! is the un.nown volume of gas
8olving the e/uation above for V(0! gives-
V(0! = 9p()! ' V()!:;P(0! = (0444 psi ' 06"7 liters!;)+"2 psi = +4)* liters
(appro'imately )+4 cu" ft"!
1The water volume of the high#pressure cylinders can be found on this
Conversion of PPM (mole basis) to Grams per Liter
for Gas Mixtures
<'ample = Given a )44 ppm (mole basis! gas mi'ture of >ompound ( in nitrogen, how
much of >ompound ( will be contained in a ) liter volume5
?ey (ssumptions-
)" Temperature of sample is assumed to be at 0)")@> (24@&!"
0" Pressure of sample is assumed to be ) atm Aust before inAection" (t around atmospheric
pressure, gases behave in close to ideal manner"
>alculation-
Bsing the Ideal Gas Law (PV = nT! for a temperature of 06+")@? (0)")@>!, a pressure of ) atm, and
the gas constant of 4"4C0) liter ' atm;mole ' degree ?, we find that ) mole of ideal gas occupies
0+")7 liters"
Dne liter of gas will then contain ();0+")7 ! moles" 8ince the concentration of >ompound ( is )44 ppm,
the total number of moles of >ompound ( in ) liter is-
(total E moles per liter! ' (concentration of >ompound (!"
The concentration of )44 ppm (parts per million! is unit#less, and e/uals )44 mole#parts per ),444,444
total moles = 4"444)44 in decimal formF thus the amount of moles of >ompound ( in one liter of mi'ture
is-
();0+")7! moles per liter ' 4"444)44 =
4"44444+ moles of >ompound ( per liter
In order to find the weight of >ompound (, we need to .now its molecular weight" &or e'ample, if
>ompound ( is hydrogen sulfide, with a molecular weight of *+"4C gram;mole, we obtain the following
concentration-
4"44444+ moles per liter ' *+"4C gram per mole =
4"444)+4 gram per liter or 4")+4 milligram per liter
General &ormula for >onversion of ppm (mole! to grams per liter for Gas Gi'tures
(for temperature 0)")@> (24@&! and pressure ) atm!
>onversion of grams per liter to ppm (mole basis! for Gas Gi'ture-
(for temperature 0)")@> (24@&! and pressure ) atm!
The concentration e'pressed in decimal form is unit#less" To find the concentration of >ompound ( in
ppm, multiply the answer from e/uation above with ),444,444" &or e'ample, a concentration of
>ompound ( of 4"444)44 (decimal form from e/uation above! would be )44 ppm, while a concentration
of 4"4)4444 would be )4,444 ppm or )H"
Das könnte Ihnen auch gefallen
- International Thermodynamic Tables of the Fluid State: Propylene (Propene)Von EverandInternational Thermodynamic Tables of the Fluid State: Propylene (Propene)Noch keine Bewertungen
- Nitrogen Cylinder Volume CalculationDokument1 SeiteNitrogen Cylinder Volume CalculationRamesh Ganesan GNoch keine Bewertungen
- Methane Number CalculationDokument7 SeitenMethane Number CalculationMile ZoricNoch keine Bewertungen
- International Thermodynamic Tables of the Fluid State, Argon, 1971: Division of Physical Chemistry, Commission on Thermodynamics and Thermochemistry, Thermodynamic Tables ProjectVon EverandInternational Thermodynamic Tables of the Fluid State, Argon, 1971: Division of Physical Chemistry, Commission on Thermodynamics and Thermochemistry, Thermodynamic Tables ProjectNoch keine Bewertungen
- Accurately Calculate Nitrogen RequirementDokument6 SeitenAccurately Calculate Nitrogen RequirementRachel BaileyNoch keine Bewertungen
- ZZ 1207573397 IsoFraction LNG Sampling SystemR2Dokument3 SeitenZZ 1207573397 IsoFraction LNG Sampling SystemR2kaysb786133Noch keine Bewertungen
- ASTM TablesDokument460 SeitenASTM TablesAbhishek PandeyNoch keine Bewertungen
- Costald 06-82Dokument6 SeitenCostald 06-82boyd.george@bp.com100% (1)
- Wobbe IndexDokument2 SeitenWobbe IndexLi Fang HuangNoch keine Bewertungen
- Heating Value Estimation For Natural Gas ApplicationsDokument4 SeitenHeating Value Estimation For Natural Gas Applicationspavanchem61Noch keine Bewertungen
- CO2 Capture Using Novel Biomass Derived Carbon DissertationDokument4 SeitenCO2 Capture Using Novel Biomass Derived Carbon Dissertationali AbbasNoch keine Bewertungen
- Mole Fraction Volume FractionDokument9 SeitenMole Fraction Volume FractionameyckulkarniNoch keine Bewertungen
- GPA Table of Physical PropertiesDokument15 SeitenGPA Table of Physical PropertiesMiissaEll Garciia100% (1)
- Tabla EstadisticaDokument1 SeiteTabla EstadisticaMichell RiveraNoch keine Bewertungen
- COSTALD Correlation For Saturated Liquid DensitiesDokument3 SeitenCOSTALD Correlation For Saturated Liquid DensitiesPrasad patgaonkarNoch keine Bewertungen
- Aspentech Course Catalog Fy18Dokument27 SeitenAspentech Course Catalog Fy18Waseem AkramNoch keine Bewertungen
- VLE CalculationDokument8 SeitenVLE CalculationDa SaintNoch keine Bewertungen
- GAS QUALITY Methane Number Calculation 2012-04-04Dokument4 SeitenGAS QUALITY Methane Number Calculation 2012-04-04Gabriel BerrioNoch keine Bewertungen
- R CalculationDokument17 SeitenR CalculationAee TrDNoch keine Bewertungen
- Ethylene Gas Equation StateDokument7 SeitenEthylene Gas Equation StateIñaki EseberriNoch keine Bewertungen
- GPA Standard 2174-93Dokument15 SeitenGPA Standard 2174-93marcosNoch keine Bewertungen
- 10.1 Sampling Part1Dokument52 Seiten10.1 Sampling Part1kanakarao1Noch keine Bewertungen
- SGS-OI-Safe Talk-Heat Stress and Ramadan-A4-EN-004Dokument4 SeitenSGS-OI-Safe Talk-Heat Stress and Ramadan-A4-EN-004Faraz AliNoch keine Bewertungen
- ch8 PDFDokument144 Seitench8 PDFJuan ZamoraNoch keine Bewertungen
- Gas Chromatography Analysis Speeds LPG Distillation: Analytical InstrumentationDokument2 SeitenGas Chromatography Analysis Speeds LPG Distillation: Analytical InstrumentationCarolina OñateNoch keine Bewertungen
- Reading A MicrometerDokument1 SeiteReading A Micrometermatrix93Noch keine Bewertungen
- 4 SOP-Tank Farm Final Sent To ZonesDokument37 Seiten4 SOP-Tank Farm Final Sent To ZonesVijayNoch keine Bewertungen
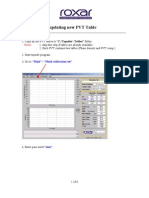
- Procedure For Down Loading PVT TableDokument6 SeitenProcedure For Down Loading PVT TablejnizamarNoch keine Bewertungen
- LNG PhysicsDokument25 SeitenLNG PhysicsRodrigo AbdoNoch keine Bewertungen
- CNG Specs - Iso 15403Dokument20 SeitenCNG Specs - Iso 15403Shasahank JoshiNoch keine Bewertungen
- EEMUADokument7 SeitenEEMUAzabirNoch keine Bewertungen
- Mis Production ReportDokument56 SeitenMis Production ReportKasturi YadavNoch keine Bewertungen
- Vertical Coalescer Separators For API-1581 Category C Type SDokument2 SeitenVertical Coalescer Separators For API-1581 Category C Type SGaluh AjengNoch keine Bewertungen
- Gas Flowmeter Calibration1957045249Dokument49 SeitenGas Flowmeter Calibration1957045249Mohamed NaserNoch keine Bewertungen
- Gas Hydrate Tutorial PDFDokument134 SeitenGas Hydrate Tutorial PDFGinoNoch keine Bewertungen
- Minimum Maximum LPG Energy Content (Btu/f)Dokument5 SeitenMinimum Maximum LPG Energy Content (Btu/f)Mirza Aatir SalmanNoch keine Bewertungen
- Production of OlefinsDokument39 SeitenProduction of OlefinsshubhamNoch keine Bewertungen
- Nm3 To M3Dokument4 SeitenNm3 To M3Aniket royNoch keine Bewertungen
- Reciprocating Pumps - NDPDDokument2 SeitenReciprocating Pumps - NDPDDhanny MiharjaNoch keine Bewertungen
- ViscosityDokument4 SeitenViscosityJan SetiawanNoch keine Bewertungen
- Ullage Report For DeliveryDokument72 SeitenUllage Report For DeliveryAbu Syeed Md. Aurangzeb Al MasumNoch keine Bewertungen
- Crude Oil Conversion TableDokument61 SeitenCrude Oil Conversion Tablerufffennew2Noch keine Bewertungen
- ch3 PDFDokument96 Seitench3 PDFJuan Zamora100% (1)
- Changing The Tool: Other Tools Can Be Downloaded From The GHG Protocol WebsiteDokument20 SeitenChanging The Tool: Other Tools Can Be Downloaded From The GHG Protocol WebsiteDonn CorreaNoch keine Bewertungen
- 5 - LNG Calculations WarsawDokument72 Seiten5 - LNG Calculations WarsawBrijesh Ojha100% (1)
- Tabel ASTM 53 PDFDokument32 SeitenTabel ASTM 53 PDFArunkumar ChandaranNoch keine Bewertungen
- Gas Properties: Molecular WeightDokument2 SeitenGas Properties: Molecular WeightDamar WibisonoNoch keine Bewertungen
- Agenda: - Coriolis Flow Meter Theory of Operation - Bunkering - Marine Fuel Management - Viscosity - QuestionsDokument17 SeitenAgenda: - Coriolis Flow Meter Theory of Operation - Bunkering - Marine Fuel Management - Viscosity - QuestionsMahaManthraNoch keine Bewertungen
- Thermo Scientific Orion LaboratoryDokument100 SeitenThermo Scientific Orion Laboratoryibnu samsiNoch keine Bewertungen
- Training OfficersDokument92 SeitenTraining OfficersPriyank Sutariya100% (1)
- Compressibility For Non Ideal GasesDokument3 SeitenCompressibility For Non Ideal Gasescymy100% (1)
- Split Range Control Loop - EnggcyclopediaDokument6 SeitenSplit Range Control Loop - EnggcyclopediasgrsthNoch keine Bewertungen
- hb156 2012final PDFDokument80 Seitenhb156 2012final PDFJesika Andilia Setya WardaniNoch keine Bewertungen
- HFO Specific HeatDokument3 SeitenHFO Specific HeatMohammed Shafi AhmedNoch keine Bewertungen
- Calculation of Z Factors For Natural Gases Using Equations of State P.M. Dranchuk J.H. Abou-KassemDokument4 SeitenCalculation of Z Factors For Natural Gases Using Equations of State P.M. Dranchuk J.H. Abou-KassemAnonymous cCmpclQF6oNoch keine Bewertungen
- Predicting Dewpoints of Acid GasesDokument6 SeitenPredicting Dewpoints of Acid GasesShreyasGadkariNoch keine Bewertungen
- Diethylene Glycol Cargo Handling SheetDokument7 SeitenDiethylene Glycol Cargo Handling SheettoanvmpetrologxNoch keine Bewertungen
- AP Chemistry Chapter 10Dokument87 SeitenAP Chemistry Chapter 10Debalina DassNoch keine Bewertungen
- Ion Exchange Demineralizers: Big Problems, Small SolutionsDokument10 SeitenIon Exchange Demineralizers: Big Problems, Small SolutionsWaleed EmaraNoch keine Bewertungen
- Chemical Cleaning in Water SystemDokument29 SeitenChemical Cleaning in Water SystemAndriono Ndi HernandyNoch keine Bewertungen
- Polymers For Water Clarification - Treating Water and WastewaterDokument3 SeitenPolymers For Water Clarification - Treating Water and WastewaterWaleed EmaraNoch keine Bewertungen
- Assessment of Coagulants For Water TreatmentDokument2 SeitenAssessment of Coagulants For Water TreatmentWaleed EmaraNoch keine Bewertungen
- AVT-All Volatile TreatmentDokument31 SeitenAVT-All Volatile TreatmentRamachandran MarappanNoch keine Bewertungen
- Chemical CleaningDokument32 SeitenChemical Cleaningkae kae100% (2)
- A Power An Overview of Current FeedwaterDokument4 SeitenA Power An Overview of Current FeedwaterWaleed EmaraNoch keine Bewertungen
- AVT-All Volatile TreatmentDokument31 SeitenAVT-All Volatile TreatmentRamachandran MarappanNoch keine Bewertungen
- Calculate PPM To mg/m3 and PPM To mg/Nm3Dokument1 SeiteCalculate PPM To mg/m3 and PPM To mg/Nm3Waleed EmaraNoch keine Bewertungen
- How The Oxide Layer Deposit Is Formed in Various Heat Transfer Regions? - IRC Engineering Services (India) Pvt. Ltd.Dokument4 SeitenHow The Oxide Layer Deposit Is Formed in Various Heat Transfer Regions? - IRC Engineering Services (India) Pvt. Ltd.Waleed EmaraNoch keine Bewertungen
- Appendix III: Equivalent Weight of Substances Required in Volumetric Analysis - Engineering360Dokument3 SeitenAppendix III: Equivalent Weight of Substances Required in Volumetric Analysis - Engineering360Waleed EmaraNoch keine Bewertungen
- Calculate PPM To MGDokument1 SeiteCalculate PPM To MGWaleed EmaraNoch keine Bewertungen
- Insulating VarnishDokument5 SeitenInsulating VarnishWaleed EmaraNoch keine Bewertungen
- Collecting and Analyzing DepositsDokument4 SeitenCollecting and Analyzing DepositsWaleed EmaraNoch keine Bewertungen
- Flow-Accelerated Corrosion - A Critical Issue Revisited - Power EngineeringDokument1 SeiteFlow-Accelerated Corrosion - A Critical Issue Revisited - Power EngineeringWaleed EmaraNoch keine Bewertungen
- Horizontal Cylindrical Tank Volume and Level CalculatorDokument5 SeitenHorizontal Cylindrical Tank Volume and Level CalculatorWaleed EmaraNoch keine Bewertungen
- Exp14d S2015 BDokument16 SeitenExp14d S2015 BWaleed EmaraNoch keine Bewertungen
- Performance of UV-Vis SpectrophotometersDokument6 SeitenPerformance of UV-Vis SpectrophotometersHuong ZamNoch keine Bewertungen
- Sulphite Test Procedure Using BuretteDokument1 SeiteSulphite Test Procedure Using BuretteWaleed EmaraNoch keine Bewertungen
- The Common Ion EffectDokument6 SeitenThe Common Ion EffectWaleed EmaraNoch keine Bewertungen
- Fast Cycling and Rapid Start-Up USDokument8 SeitenFast Cycling and Rapid Start-Up USanirudhalcNoch keine Bewertungen
- Henry's Law - NeutriumDokument8 SeitenHenry's Law - NeutriumWaleed EmaraNoch keine Bewertungen
- Combined Cycle JournalDokument9 SeitenCombined Cycle JournalWaleed EmaraNoch keine Bewertungen
- 43 How To Measure Total Iron PDFDokument24 Seiten43 How To Measure Total Iron PDFWaleed EmaraNoch keine Bewertungen
- Layup Practices For Fossil Plants 1Dokument6 SeitenLayup Practices For Fossil Plants 1Waleed EmaraNoch keine Bewertungen
- VTT R 03234 14 PDFDokument42 SeitenVTT R 03234 14 PDFWaleed EmaraNoch keine Bewertungen
- Chemistry SampleSolvedArihant Chap 1 4 PDFDokument62 SeitenChemistry SampleSolvedArihant Chap 1 4 PDFWaleed EmaraNoch keine Bewertungen
- Website Preservation PDFDokument8 SeitenWebsite Preservation PDFWaleed EmaraNoch keine Bewertungen
- Layup Practices For Fossil Plants 1Dokument6 SeitenLayup Practices For Fossil Plants 1Waleed EmaraNoch keine Bewertungen
- Chemistry Practicals First YearsDokument65 SeitenChemistry Practicals First YearsWaleed EmaraNoch keine Bewertungen
- An Introduction To FISIKA STATISTIK WORDDokument396 SeitenAn Introduction To FISIKA STATISTIK WORDAnonymous ZtYb6iFNoch keine Bewertungen
- Elementary Mechanics and Thermodynamics SOLUTIONS MANUAL - J. Norbury PDFDokument112 SeitenElementary Mechanics and Thermodynamics SOLUTIONS MANUAL - J. Norbury PDFsuryaaNoch keine Bewertungen
- Homework 2 Solutions CHEMISTRYDokument5 SeitenHomework 2 Solutions CHEMISTRYshaframenNoch keine Bewertungen
- 1st Year Chemistry Sc1 ChemistryDokument896 Seiten1st Year Chemistry Sc1 Chemistrybiranchi satapathyNoch keine Bewertungen
- AP Chemistry Chapter 10Dokument87 SeitenAP Chemistry Chapter 10Debalina DassNoch keine Bewertungen
- 11 Physics Notes ch13 PDFDokument2 Seiten11 Physics Notes ch13 PDFTalha MukhtarNoch keine Bewertungen
- Review Physics 2: Date: Xx/Xx/20XxDokument21 SeitenReview Physics 2: Date: Xx/Xx/20XxDương Hà Trúc TâmNoch keine Bewertungen
- Chapter 5Dokument95 SeitenChapter 5Joel OuanoNoch keine Bewertungen
- Gaseous State: Khoe Tjok TjinDokument16 SeitenGaseous State: Khoe Tjok TjinLuna eukharisNoch keine Bewertungen
- Agus Haryanto Agreng Dept. 06 MARET 2008Dokument41 SeitenAgus Haryanto Agreng Dept. 06 MARET 2008Cola JamNoch keine Bewertungen
- States of Matter - Mind Maps - Yakeen 2.0 2024 (Alpha)Dokument1 SeiteStates of Matter - Mind Maps - Yakeen 2.0 2024 (Alpha)Ananya SharmaNoch keine Bewertungen
- B.Sc. IIDokument60 SeitenB.Sc. IISahil JainNoch keine Bewertungen
- Crash XII Test # 01Dokument6 SeitenCrash XII Test # 01Kamran AliNoch keine Bewertungen
- Matter EnergyDokument10 SeitenMatter EnergyCyhsi BarrogaNoch keine Bewertungen
- Marcet BoilerDokument10 SeitenMarcet BoilerMD Atiqur Rahman Faisal100% (14)
- ENGG1500 Study Guide S1 2018 PDFDokument137 SeitenENGG1500 Study Guide S1 2018 PDFKatty TsaiNoch keine Bewertungen
- B.Sc. II Semester Physics:: Paper II Thermal Physics:: Imp QuestionsDokument2 SeitenB.Sc. II Semester Physics:: Paper II Thermal Physics:: Imp QuestionsReddyvari Venugopal100% (1)
- Advanced Level Physics Teaching SchemesDokument29 SeitenAdvanced Level Physics Teaching SchemesBeaugar MaxwellNoch keine Bewertungen
- Fluidization of Bulk SolidsDokument77 SeitenFluidization of Bulk SolidsManfredHeyde100% (1)
- ME 201 OutlineDokument2 SeitenME 201 Outlinemz_haqNoch keine Bewertungen
- Assignment 1 Soln PDFDokument13 SeitenAssignment 1 Soln PDFJawahar Khetan100% (1)
- 11th Chemistry - 1st Revision Test 2022 - Model Question Paper - Kanchipuram District - English Medium PDF DownloadDokument2 Seiten11th Chemistry - 1st Revision Test 2022 - Model Question Paper - Kanchipuram District - English Medium PDF Downloadshreenidhi umashankarNoch keine Bewertungen
- 9702 w02 QP 4Dokument16 Seiten9702 w02 QP 4rida yasinNoch keine Bewertungen
- F.Y.B.sc.-ChemistryDokument15 SeitenF.Y.B.sc.-ChemistryRakesh JamesNoch keine Bewertungen
- ANSYS CFX-Solver Theory GuideDokument372 SeitenANSYS CFX-Solver Theory GuideBhaskar NandiNoch keine Bewertungen
- 3 Properties of Ideal GasesDokument52 Seiten3 Properties of Ideal GasesBERN CERTEZANoch keine Bewertungen
- GisselaBonilla ADokument13 SeitenGisselaBonilla AGissela BTNoch keine Bewertungen
- Set 8Dokument2 SeitenSet 8venkatNoch keine Bewertungen
- (George D. Saravacos) Transport Properties of Food PDFDokument435 Seiten(George D. Saravacos) Transport Properties of Food PDFmanuparisottoNoch keine Bewertungen
- NBPhO 2021 With Solutions and Grading v4Dokument8 SeitenNBPhO 2021 With Solutions and Grading v4Diana PonceNoch keine Bewertungen
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincVon EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincBewertung: 3.5 von 5 Sternen3.5/5 (137)
- Chemistry for Breakfast: The Amazing Science of Everyday LifeVon EverandChemistry for Breakfast: The Amazing Science of Everyday LifeBewertung: 4.5 von 5 Sternen4.5/5 (90)
- Formulating, Packaging, and Marketing of Natural Cosmetic ProductsVon EverandFormulating, Packaging, and Marketing of Natural Cosmetic ProductsNoch keine Bewertungen
- AP® Chemistry Crash Course, For the 2020 Exam, Book + Online: Get a Higher Score in Less TimeVon EverandAP® Chemistry Crash Course, For the 2020 Exam, Book + Online: Get a Higher Score in Less TimeBewertung: 5 von 5 Sternen5/5 (1)
- The Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactVon EverandThe Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactBewertung: 5 von 5 Sternen5/5 (5)
- Is That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeVon EverandIs That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeBewertung: 5 von 5 Sternen5/5 (4)
- The Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsVon EverandThe Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsBewertung: 4 von 5 Sternen4/5 (146)
- Sodium Bicarbonate: Nature's Unique First Aid RemedyVon EverandSodium Bicarbonate: Nature's Unique First Aid RemedyBewertung: 5 von 5 Sternen5/5 (21)
- Guidelines for Chemical Process Quantitative Risk AnalysisVon EverandGuidelines for Chemical Process Quantitative Risk AnalysisBewertung: 5 von 5 Sternen5/5 (1)
- Handbook of Formulating Dermal Applications: A Definitive Practical GuideVon EverandHandbook of Formulating Dermal Applications: A Definitive Practical GuideNoch keine Bewertungen
- Organic Chemistry for Schools: Advanced Level and Senior High SchoolVon EverandOrganic Chemistry for Schools: Advanced Level and Senior High SchoolNoch keine Bewertungen
- Process Plant Equipment: Operation, Control, and ReliabilityVon EverandProcess Plant Equipment: Operation, Control, and ReliabilityBewertung: 5 von 5 Sternen5/5 (1)
- Taste: Surprising Stories and Science About Why Food Tastes GoodVon EverandTaste: Surprising Stories and Science About Why Food Tastes GoodBewertung: 3 von 5 Sternen3/5 (20)
- The Periodic Table: A Very Short IntroductionVon EverandThe Periodic Table: A Very Short IntroductionBewertung: 4.5 von 5 Sternen4.5/5 (3)
- Monkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeVon EverandMonkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeBewertung: 4 von 5 Sternen4/5 (1)
- AP Chemistry Flashcards, Fourth Edition: Up-to-Date Review and PracticeVon EverandAP Chemistry Flashcards, Fourth Edition: Up-to-Date Review and PracticeNoch keine Bewertungen
- The Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsVon EverandThe Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsBewertung: 5 von 5 Sternen5/5 (3)
- Tribology: Friction and Wear of Engineering MaterialsVon EverandTribology: Friction and Wear of Engineering MaterialsBewertung: 5 von 5 Sternen5/5 (1)
- The Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableVon EverandThe Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableBewertung: 3.5 von 5 Sternen3.5/5 (22)
- Functional Safety from Scratch: A Practical Guide to Process Industry ApplicationsVon EverandFunctional Safety from Scratch: A Practical Guide to Process Industry ApplicationsNoch keine Bewertungen
- Bioplastics: A Home Inventors HandbookVon EverandBioplastics: A Home Inventors HandbookBewertung: 4 von 5 Sternen4/5 (2)
- Ingredients: A Visual Exploration of 75 Additives & 25 Food ProductsVon EverandIngredients: A Visual Exploration of 75 Additives & 25 Food ProductsBewertung: 4 von 5 Sternen4/5 (1)
- Nuclear Energy in the 21st Century: World Nuclear University PressVon EverandNuclear Energy in the 21st Century: World Nuclear University PressBewertung: 4.5 von 5 Sternen4.5/5 (3)
- Chemistry for Breakfast: The Amazing Science of Everyday LifeVon EverandChemistry for Breakfast: The Amazing Science of Everyday LifeBewertung: 4.5 von 5 Sternen4.5/5 (14)