Beruflich Dokumente
Kultur Dokumente
Notes From Drug Metabolism
Hochgeladen von
Daniil LyalkoCopyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Notes From Drug Metabolism
Hochgeladen von
Daniil LyalkoCopyright:
Verfügbare Formate
Notes from Drug metabolism
1. Most of the chemicals we are exposed to on a day to day basis are not required for normal
biological function.
a. May even be harmful
2. Nature has evolved an array of Enzyme-catalyzed processes to rid our bodies of these
invading substances.
a. Substances that are foreign to the body are called xenobiotics
b. This study of the biological fate of xenobiotics is what we refer to as Drug Metabolism
3. Basics of drug metabolism
a. Polar substances can be excreted via the kidneys into the urine
b. Most Medicine
Very lipid-soluble
Reabsorbed from kidney tubule back into the blood stream
The whole point of drug metabolism is to have the compounds (medicine)
undergo metabolism, generating polar species which can avoid being
reabsorbed via the renal system and can be excreted via urine
c. Common but misleading to think drug metabolism is just a detoxifying or deactivating
process
Metabolites may actually be similar in pharmacological activity to the parent
1. Nortriptyline, the N-dimethylated metabolite of the antidepressant
amitriptyline
Metabolites may have a life-threatening toxicity
1. Painkiller paracetamol, gets metabolized to a highly reactive species
which damages the liver in overdose
4. The Chemistry of Drug Metabolism
a. The chemical pathways by which drugs are metabolized are extremely varied.
b. Divided into phase 1 and phase 2 reactions
Phase 1 Metabolism
1. Functionalization occurs where a polar grouping is added to or exposed
on the molecule
2. MOST IMPORTANT phase 1 pathway is Oxidation, although other
reactions notably reduction and hydrolysis do occur
3. Cytochrome P450 monooxygenases (CYPs) are the enzymes largly
responsible for drug oxidation
a. Initial reaction is mainly the insertion of a high-energy oxygen
atom into a single C-H or multiple C-C bond, is the same for all
substrates, the end result may be quite different
Phase 2 metabolism
1. These reactions are ones of conjugation in which a polar group possibly
formed during a phase 1 reaction, reacts with an endogenous molecule
(substance that originates from within an organism, tissue, or cell.) such
as glucuronic acid to form a water-soluble product suitable for excretion
2. In some cases, a phase 2 metabolite can be more pharmacologically
active than the parent drug
5. The biochemistry of drug metabolism
a. Sites of metabolism
Enzymes that metabolize drugs are present mainly in the liver although
significant metabolism may take place in the intestines, lungs, or brain as well as
other places.
When a drug is administered orally, the metabolism in the liver may be so
efficient that little or none of the drug enters the systemic blood circulation.
1. Termed the first pass effect
a. Drugs undergoing the first pass metabolism may need to be
administered via a non-oral route.
b. Phase 1 Enzymes
Enzyme Catalyzing phase 1 reactions include
1. CYPs, Flavin monooxygenases, aldehyde oxidases, and epoxide
hydrolases.
2. CYPs (cytochromep450) studied the most
a. Named for ability to absorb light at 450nm in the reduced form
and complexed to carbon monoxide
b. The endogenous (natural) role regulation of steroids,
elcosanoids, and other hormones by oxidative processes
c. Represents a super family of several hundred members
arranged in multigene gene families and subfamilies according
to their amino acid sequence
d. CYPs 1A, 2C, 2D, 2E, and 3A are primarily involved with drug
metabolism in humans
i. A given drug could be a substrate (a molecule upon
which an enzyme acts) for more than one CYP isoform
ii. A single form of CYP may be capable of metabolizing a
range of drugs
1. CYP2D6 metabolizes many widely used drugs
such as antidepressants, antischizophrenic
agents, cardiovascular drugs, pain killers, even
drugs of abuse such as ecstasy.
iii. Various contributions of the CYP isoforms are well
illustrated by Warfarin an anti-blood-coagulant
1. Administered as a racemic mixture (S)-Warfarin
and (R)-Warfarin.
2. (S)-Warfarin is metabolized more quickly via the
major pathway being 7-hydroxylation, CYP2C9
responsible for that.
3. (r)-warfarin is metabolized mainly via 6- and 8-
hydroxylation pathways catalyzed by CYPs 1A1
and 1A2.
iv. Less commonly, CYP and other enzymes act in reductive
roles, substrates include azo and nitro compounds, also
some epoxides
c. Phase 2 Enzymes
Mediated by a diverse group of transferase enzymes (general name for the class
of enzymes that enact the transfer of specific functional groups from one
molecule the donor to another called the acceptor)
1. Include, glucoronosyl transferases, sulfotransferases, and glutathione-S-
transferases
2. There is strong evidence that phase 2 enzymes, notably uridine
diphosphate glucuronosyl transferases (UDPGTs) occur in multiple
forms.
3. UDPGTs require uridine diphosphate glucuronic acid as a cofactor. (Non-
protein chemical compound that is required for the proteins biological
activity. Can be considered helper molecules that assist in biochemical
transformations.)
a. Gets derived enzymatically from glucose and is present / found
everywhere in body tissues
4. Responsible for the conjugation of a range of endogenous molecules
(originate from within an organism, tissue, or cell)
a. Glucuronidation is the most common phase 2 reaction, due to
abundance of UDPGA
i. The addition of glucuronic acid to a substrate (a
molecule upon which an enzyme acts)
ii. Mechanism
1.
This part is confusing what is the relevance? Talk to Dr. Fab tomorrow!
6. Clinical and Industrial Applications
a. Catalytic activities of the drug-metabolizing enzymes, especially CYPs, vary widely
between species and between individuals.
Because of this differing ability to eliminate drugs via metabolism, the
concentration of a drug in the blood and at the tissue where it acts may vary
greatly between individuals given the same dose
Many factors ranging from genetic makeup to lifestyle play a role
Das könnte Ihnen auch gefallen
- Routes of Drug Administration: Asst Professor Dept of Pharmacology Govt Medical College, AkolaDokument27 SeitenRoutes of Drug Administration: Asst Professor Dept of Pharmacology Govt Medical College, Akolaنور الهدىNoch keine Bewertungen
- Volpe's Understanding EvolutionDokument303 SeitenVolpe's Understanding EvolutionAnonymous kg7YBMFH100% (1)
- Blood: TransportationDokument29 SeitenBlood: TransportationShobhit GajbhiyeNoch keine Bewertungen
- Metabolic DisordersDokument80 SeitenMetabolic DisordersXeniyaFedoryakNoch keine Bewertungen
- Introduction of PharmacologyDokument33 SeitenIntroduction of PharmacologykayarohanamNoch keine Bewertungen
- Calcium Metabolism & Calcium Metabolism DisordersDokument45 SeitenCalcium Metabolism & Calcium Metabolism Disorderstrisya arthaputriNoch keine Bewertungen
- Hematinics BPTDokument17 SeitenHematinics BPTbpt2Noch keine Bewertungen
- Drug Metabolism FinalDokument78 SeitenDrug Metabolism FinalPrasanna DNoch keine Bewertungen
- 4-Pharmacokinetics IDokument88 Seiten4-Pharmacokinetics IMarc Imhotep Cray, M.D.Noch keine Bewertungen
- PharmacologyDokument57 SeitenPharmacologyarun231187Noch keine Bewertungen
- Biopharmaceutics Note 7th SemDokument38 SeitenBiopharmaceutics Note 7th Semmj.vinoth@gmail.comNoch keine Bewertungen
- BiotransformationDokument33 SeitenBiotransformationAlphaRaj Mekapogu67% (3)
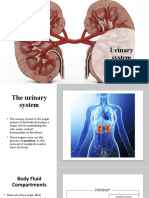
- Urinary SystemDokument16 SeitenUrinary SystemDragan KankarasNoch keine Bewertungen
- Pituitary Gland: The Master GlandDokument15 SeitenPituitary Gland: The Master GlandMohammed ShahanewzNoch keine Bewertungen
- Fundamentals of PharmacyDokument66 SeitenFundamentals of PharmacyLHYRA KATHLEEN LOPEZ100% (1)
- 1introDokument158 Seiten1introDea MaharanisNoch keine Bewertungen
- Adrenergic and Anti-Adrenergic Drugs: Manju PrathapDokument43 SeitenAdrenergic and Anti-Adrenergic Drugs: Manju PrathapManju PrathapNoch keine Bewertungen
- Intro PK PD Genomics - PharmaDokument110 SeitenIntro PK PD Genomics - PharmaKenneth NuñezNoch keine Bewertungen
- Principles of Pharmacology Chapter 1Dokument37 SeitenPrinciples of Pharmacology Chapter 1Muhammad ZakriaNoch keine Bewertungen
- Manual PDFDokument60 SeitenManual PDFMiriam BteichNoch keine Bewertungen
- Experimental and Quasi-Experimental Designs For Generalized Causal Inference PDFDokument81 SeitenExperimental and Quasi-Experimental Designs For Generalized Causal Inference PDFGonçalo SampaioNoch keine Bewertungen
- Route of Administration of Biotech ProductsDokument31 SeitenRoute of Administration of Biotech Productsheaven.protik100% (3)
- BiotransformationDokument18 SeitenBiotransformationRajesh PatelNoch keine Bewertungen
- PCR Primer Design PDFDokument62 SeitenPCR Primer Design PDFnesariaNoch keine Bewertungen
- Quarter 4 - Module 4: Cancer vs. CarcinogenDokument22 SeitenQuarter 4 - Module 4: Cancer vs. Carcinogenwetlog lolololimNoch keine Bewertungen
- Anti-Thyroid Drugs and Thyroid HormoneDokument7 SeitenAnti-Thyroid Drugs and Thyroid Hormonemicheal1960100% (1)
- General PharmacologyDokument20 SeitenGeneral PharmacologyDrMohan GuptaNoch keine Bewertungen
- Pharmacology of Ergot AlkaloidsDokument143 SeitenPharmacology of Ergot AlkaloidsParveen Hullon100% (1)
- Bioavailability and Bioequivalence: By: Kris May Lyn A. RamosDokument77 SeitenBioavailability and Bioequivalence: By: Kris May Lyn A. RamosValar Morghulis100% (1)
- Antioxidant and Anti-Inflammatory Assays Confirm Bioactive Compounds in Ajwa Date FruitDokument7 SeitenAntioxidant and Anti-Inflammatory Assays Confirm Bioactive Compounds in Ajwa Date Fruitrizla67100% (1)
- Anterior Pituitary HormonesDokument46 SeitenAnterior Pituitary Hormonespramod bhaleraoNoch keine Bewertungen
- Cell Communication and Signal TransductionDokument34 SeitenCell Communication and Signal TransductionArina SafiraNoch keine Bewertungen
- Types of Tablets and Their CharecteristicsDokument49 SeitenTypes of Tablets and Their CharecteristicsRohit IrkalNoch keine Bewertungen
- 2 Cell Injury, Adaptation, and Cell DeathDokument6 Seiten2 Cell Injury, Adaptation, and Cell DeathcedonuliNoch keine Bewertungen
- Biochemistry JD 2Dokument6 SeitenBiochemistry JD 2failinNoch keine Bewertungen
- Adrenergic AgonistsDokument40 SeitenAdrenergic AgonistsBenedict Brashi100% (1)
- Types of FermentersDokument3 SeitenTypes of FermentersHoney krishnaNoch keine Bewertungen
- Metabolism of XenobioticsDokument30 SeitenMetabolism of XenobioticsCzarina RiveraNoch keine Bewertungen
- Physiology, Lecture 5, Blood (Lecture Notes)Dokument10 SeitenPhysiology, Lecture 5, Blood (Lecture Notes)Ali Al-Qudsi100% (1)
- ExcretionDokument35 SeitenExcretionHely PatelNoch keine Bewertungen
- Narcotic Analgesics - Notes - SY B. PharmaDokument12 SeitenNarcotic Analgesics - Notes - SY B. PharmaSneha charakNoch keine Bewertungen
- Drugs Affecting The Respiratory SystemDokument89 SeitenDrugs Affecting The Respiratory SystemkayarohanamNoch keine Bewertungen
- Excretion of DrugsDokument17 SeitenExcretion of DrugsdahiphalehNoch keine Bewertungen
- Drug AbsorptionDokument26 SeitenDrug Absorptionaman vermaNoch keine Bewertungen
- Unit I General PharmacologyDokument16 SeitenUnit I General PharmacologycuolyNoch keine Bewertungen
- Urine Formation: Reabsorption and Secretion, and Water ConservationDokument5 SeitenUrine Formation: Reabsorption and Secretion, and Water ConservationAshraf Moby100% (1)
- 1.1 Basic Principles of Cell InjuryDokument41 Seiten1.1 Basic Principles of Cell InjuryMUNEZERO Evase0% (1)
- Pharma 1.2 - Pharmacokinetics (Wini Ong) PDFDokument11 SeitenPharma 1.2 - Pharmacokinetics (Wini Ong) PDFVon Javier GamateroNoch keine Bewertungen
- Pharmacy Links 2Dokument2 SeitenPharmacy Links 2Bosch Pharmaceuticals50% (2)
- Cagayan State University College of Medicine and Surgery SY: 2009-2010Dokument41 SeitenCagayan State University College of Medicine and Surgery SY: 2009-2010ahmad usmanNoch keine Bewertungen
- Factors Influencing Drug Absorption Though Git PDFDokument59 SeitenFactors Influencing Drug Absorption Though Git PDFRamakant JoshiNoch keine Bewertungen
- UNIT-I Solubility of DrugsDokument23 SeitenUNIT-I Solubility of DrugsPranjul Shrivastava100% (1)
- Classification and Nomenclature of EnzymesDokument12 SeitenClassification and Nomenclature of EnzymesmcgilicuttyNoch keine Bewertungen
- Morphine: Somniferum, Dried and Powdered - Opium PowderDokument4 SeitenMorphine: Somniferum, Dried and Powdered - Opium PowderpharmbharathiNoch keine Bewertungen
- Unit 6 Targeted Drug DeliveryDokument42 SeitenUnit 6 Targeted Drug Deliveryabdullah2020100% (1)
- Neuro Muscular Junction NNDokument68 SeitenNeuro Muscular Junction NNAnak AyamNoch keine Bewertungen
- Pharmacology Notes CH 1 IntroductionDokument4 SeitenPharmacology Notes CH 1 Introductionridley45Noch keine Bewertungen
- Meg Acet in AnorexiaDokument45 SeitenMeg Acet in AnorexiaRaina MohakNoch keine Bewertungen
- General Prescribing Guidelines of Pedriatic PatientsDokument11 SeitenGeneral Prescribing Guidelines of Pedriatic PatientsGloomi100% (1)
- Ethiopia National Drug FormularyDokument572 SeitenEthiopia National Drug FormularyportosinNoch keine Bewertungen
- Catalyst O True Catalyst: ExampleDokument9 SeitenCatalyst O True Catalyst: ExampleAshley SaronNoch keine Bewertungen
- The Structure, Function and Organisation of The Human BodyDokument28 SeitenThe Structure, Function and Organisation of The Human BodyGemma WhitehouseNoch keine Bewertungen
- Prodrug Design PDFDokument29 SeitenProdrug Design PDFmehulpatel100% (1)
- Filogenia de Briofitos SensulatoDokument1 SeiteFilogenia de Briofitos SensulatoYuyitoS2714Noch keine Bewertungen
- QualitativetestforspirinDokument7 SeitenQualitativetestforspirinYen BumNoch keine Bewertungen
- PlasmodiumDokument10 SeitenPlasmodiumBadar MinhasNoch keine Bewertungen
- Module 5 Anatomy I Splanchnology 1Dokument17 SeitenModule 5 Anatomy I Splanchnology 1neannaNoch keine Bewertungen
- Postdoc Cover Letter SampleDokument7 SeitenPostdoc Cover Letter Samplevofysyv1z1v3100% (1)
- Encyclopedia ES HealthDokument9 SeitenEncyclopedia ES HealthGantsooj BNoch keine Bewertungen
- Origin of Eukaryotic Cell PDFDokument8 SeitenOrigin of Eukaryotic Cell PDFaco_golarNoch keine Bewertungen
- Estudio Etnobotanico MonzanbiqueDokument11 SeitenEstudio Etnobotanico MonzanbiqueJavier JalilieNoch keine Bewertungen
- T Cell Development Thymic Education of T CellDokument34 SeitenT Cell Development Thymic Education of T Cellapi-273068056Noch keine Bewertungen
- Slotwise Details July-November 2021Dokument138 SeitenSlotwise Details July-November 2021Ch PrasadNoch keine Bewertungen
- Stingless Bee Tetragonula Laeviceps and T Aff BiroDokument9 SeitenStingless Bee Tetragonula Laeviceps and T Aff Birojays itemNoch keine Bewertungen
- Chapter4 Opt3Dokument30 SeitenChapter4 Opt3Cza VerwinNoch keine Bewertungen
- Heritability of Angular Leaf Spot Resistance in Populations of Common Bean Developed Using g5686, Mexico 54, Amendoim and Bat 332, As Donor ParentsDokument5 SeitenHeritability of Angular Leaf Spot Resistance in Populations of Common Bean Developed Using g5686, Mexico 54, Amendoim and Bat 332, As Donor ParentsijsidonlineinfoNoch keine Bewertungen
- Cell Note - BDB FiitjeeDokument5 SeitenCell Note - BDB FiitjeeSankar MandalNoch keine Bewertungen
- Growth Kinetics 2014Dokument33 SeitenGrowth Kinetics 2014Ivan Aulia BigbandNoch keine Bewertungen
- 1.wall of ThoraxDokument14 Seiten1.wall of ThoraxChandru ANoch keine Bewertungen
- Akram La Kilo Artikel Studi Potensi Pirazolin Tersubstitusi 1 N Dari Tiosemikarbazon Sebagai Agen Antiamuba Melalui Uji in SilicoDokument55 SeitenAkram La Kilo Artikel Studi Potensi Pirazolin Tersubstitusi 1 N Dari Tiosemikarbazon Sebagai Agen Antiamuba Melalui Uji in SilicoRetno SulistyaningrumNoch keine Bewertungen
- Krishna2019 ReferenceWorkEntry CiliaDokument5 SeitenKrishna2019 ReferenceWorkEntry CiliaWinada ReyhanNoch keine Bewertungen
- Two Week Learning Plan ScienceDokument4 SeitenTwo Week Learning Plan ScienceMaria Ramona FelixNoch keine Bewertungen
- Daily Lesson Plan: 7e's ApproachDokument6 SeitenDaily Lesson Plan: 7e's ApproachSonny MagdadaroNoch keine Bewertungen
- B.P.T ProjectDokument101 SeitenB.P.T ProjectVinay ChawlaNoch keine Bewertungen
- Pronunciation Stress Language ExchangesDokument5 SeitenPronunciation Stress Language ExchangesHàNhậtNguyễnNoch keine Bewertungen
- Substance Id enDokument118 SeitenSubstance Id ensmaps001Noch keine Bewertungen
- Biology The Essentials 2nd Edition by Marielle Hoefnagels ISBN Solution ManualDokument10 SeitenBiology The Essentials 2nd Edition by Marielle Hoefnagels ISBN Solution Manualmichael100% (25)
- Lazcano y Pereto. 2021. BioSystemsDokument6 SeitenLazcano y Pereto. 2021. BioSystemsRenato AmosNoch keine Bewertungen
- Fenofaze SuncokretaDokument9 SeitenFenofaze SuncokretaNastic PredragNoch keine Bewertungen