Beruflich Dokumente
Kultur Dokumente
Calculating PVT Properties
Hochgeladen von
Anonymous yjF4yygpPbCopyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Calculating PVT Properties
Hochgeladen von
Anonymous yjF4yygpPbCopyright:
Verfügbare Formate
Calculating PVT properties
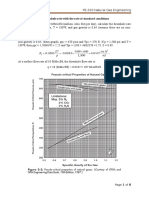
This is an example of calculating PVT properties. The specific correlations that should be used for a specific crude oil or reservoir may vary, as discussed in the referenced pages
focusing on specific properties.
Determine the PVT properties for a United States midcontinental crude oil and natural gas system with properties listed in Table 1. Table 2 lists the correlations to be used.
Measured data are provided for comparison with the calculated results. For correlations that rely on other correlations, these data illustrate the effects of error propagation in the
calculations.
(/File%3AVol1_Page_302_Image_0002.png)
Table 1
(/File%3AVol1_Page_303_Image_0002.png)
Table 2
Contents
1 Gravity and molecular weight
2 Bubblepoint pressure
3 Bubblepoint oil formation volume factor
4 Isothermal compressibility
5 Undersaturated oil formation volume factor
6 Oil density
7 Dead oil viscosity
8 Bubblepoint oil viscosity
9 Undersaturated oil viscosity
10 Gas/oil interfacial tension
11 Water/oil interfacial tension
12 Nomenclature
13 References
14 Noteworthy papers in OnePetro
15 External links
16 See also
Gravity and molecular weight
Determine the crude oil specific gravity (/Crude_oil_characterization),
(/File%3AVol1_page_0291_eq_002.png)....................(1)
and molecular weight,
(/File%3AVol1_page_0292_eq_001.png)....................(2)
Bubblepoint pressure
Use the Lasater
[1]
correlation to estimate bubblepoint pressure (/Oil_bubblepoint_pressure). Calculate the gas mole fraction in the oil,
(/File%3AVol1_page_0292_eq_002.png)....................(3)
and the Lasater bubblepoint pressure factor,
(/File%3AVol1_page_0292_eq_003.png)....................(4)
with Lasaters relationship between bubblepoint pressure factor and bubblepoint pressure,
(/File%3AVol1_page_0292_eq_004.png)....................(5)
For comparison, Standing
[2][3]
= 2,316 psia, Glas
[4]
= 2,725 psia, Al-Shammasi
[5]
= 2,421 psia, and Velardi
[6]
= 2,411 psia.
Modify the calculated bubblepoint pressure to account for the effects of nitrogen in the surface gas with Jacobsons equation.
(/File%3AVol1_page_0293_eq_001.png)....................(6)
Therefore, the bubblepoint pressure should be increased by 9.8% to 2,251 psia. The measured bubblepoint pressure was reported to be 2,479 psia.
Bubblepoint oil formation volume factor
Calculate the bubblepoint oil formation volume factor (/Oil_formation_volume_factor) (FVF) using the correlation from Al-Shammasi.
[5]
(/File%3AVol1_page_0293_eq_002.png)....................(7)
(/File%3AVol1_page_0293_eq_003.png)
For comparison (in bbl/STB), Standing
[2][3]
= 1.410, Glas
[4]
= 1.386, Al-Marhoun
[7]
= 1.364, Farshad
[8]
= 1.364, and Kartoatmodjo
[9][10][11]
= 1.358. The measured bubblepoint
oil FVF is 1.398 bbl/STB.
Isothermal compressibility
Calculate the isothermal compressibility of oil (/Isothermal_compressibility_of_oil) using the Farshad
[8]
correlation.
(/File%3AVol1_page_0294_eq_001.png)....................(8)
(/File%3AVol1_page_0294_eq_002.png)....................(9)
(/File%3AVol1_page_0294_eq_003.png)
The measured isothermal compressibility is 11.06 10
-6
psi
-1
.
Undersaturated oil formation volume factor
With the results from Lasaters
[1]
method for bubblepoint pressure, use Al-Shammasis
[5]
method for bubblepoint oil FVF, and Farshads
[8]
equation for isothermal compressibility,
the undersaturated oil FVF is given by
(/File%3AVol1_page_0294_eq_004.png)....................(10)
(/File%3AVol1_page_0294_eq_005.png)
which compares to a measured value of 1.367 bbl/STB. Because this calculation uses the results from multiple correlations, individual correlation error compounds and propagates
through to the final result. The calculated value is 1.367 bbl/STB with the actual bubblepoint value of 1.398 bbl/STB; therefore, the accuracy of the bubblepoint FVF is primarily
affected by the accuracy of the undersaturated FVF.
Oil density
Calculate the oil density (/Oil_density).
(/File%3AVol1_page_0295_eq_001.png)....................(11)
Dead oil viscosity
Calculate the dead oil viscosity (/Oil_viscosity) using the correlation from Glas.
[4]
(/File%3AVol1_page_0295_eq_002.png)....................(12)
For comparison, Fitzgerald
[12][13][14]
= 1.808 cp, and Bergman
[15][16]
= 2.851 cp. The measured dead oil viscosity is 1.67 cp.
Bubblepoint oil viscosity
Calculate the bubblepoint oil viscosity (/Oil_viscosity) using the method developed by Chew and Connally.
[17][18]
(/File%3AVol1_page_0295_eq_003.png)....................(13)
(/File%3AVol1_page_0296_eq_001.png)....................(14)
(/File%3AVol1_page_0296_eq_002.png)....................(15)
For comparison, Beggs and Robinson
[19]
= 0.515 cp. The measured viscosity at bubblepoint is 0.401 cp.
Undersaturated oil viscosity
Calculate the undersaturated oil viscosity by applying the Vazquez and Beggs
[20][21]
correlation.
(/File%3AVol1_page_0296_eq_003.png)....................(16)
(/File%3AVol1_page_0296_eq_004.png)
For comparison, Beal
[22]
= 0.730 cp and Kouzel
[23]
= 0.778 cp. The measured value is 0.475 cp. This example illustrates the steps necessary to calculate oil viscosity requiring
correlations for dead oil viscosity, bubblepoint viscosity, undersaturated viscosity, and bubblepoint pressure/solution GOR. Errors in individual correlations can compound and
propagate through to the resulting answer. For instance, if the measured bubblepoint viscosity is used in Eq. 16, the result is 0.52 cpmuch closer to the measured value. Therefore,
care should be exercised in the selection of accurate correlations for individual properties.
Gas/oil interfacial tension
Estimate the gas/oil surface tension (/Interfacial_tension) using the method developed by Abdul-Majeed.
[24]
Calculate the dead oil surface tension.
(/File%3AVol1_page_0297_eq_001.png)....................(17)
(/File%3AVol1_page_0297_eq_002.png)
Determine the live oil adjustment factor.
(/File%3AVol1_page_0297_eq_003.png)....................(18)
(/File%3AVol1_page_0297_eq_004.png)
Calculate the live gas/oil surface tension.
(/File%3AVol1_page_0297_eq_005.png)....................(19)
(/File%3AVol1_page_0298_eq_001.png)
For comparison, Baker and Swerdloff
[25][26]
= 4.73 dynes/cm.
Water/oil interfacial tension
Estimate the water/oil surface tension (/Interfacial_tension) using Firoozabadi and Ramey.
[27]
Calculate the pseudocritical temperature of the dead oil.
(/File%3AVol1_page_0298_eq_002.png)....................(20)
(/File%3AVol1_page_0298_eq_003.png)
Calculate the pseudocritical temperature of the gas.
(/File%3AVol1_page_0298_eq_004.png)....................(21)
(/File%3AVol1_page_0298_eq_005.png)
Calculate the pseudocritical temperature of the live gas/oil mixture.
(/File%3AVol1_page_0298_eq_006.png)....................(22)
Convert oil density units from lbm/ft
3
to g/cm
3
.
(/File%3AVol1_page_0300_eq_001.png)....................(23)
Calculate the surface tension between the oil and water phases.
(/File%3AVol1_page_0300_eq_002.png)....................(24)
(/File%3AVol1_page_0300_eq_003.png)
Nomenclature
B
g
=
gas FVF, ft
3
/scf
B
o
= oil FVF, bbl/STB
B
ob
= oil formation volume at bubblepoint pressure, bbl/STB
c
o
=
oil isothermal compressibility, Lt
2
/m, psi
-1
c
ob
=
oil isothermal compressibility at bubblepoint, Lt
2
/m, psi
-1
K
w
=
Watson characterization factor, R
1/3
M
g
= gas molecular weight, m, lbm/lbm mol
M
go
= gas/oil mixture molecular weight, m, lbm/lbm mol
M
o
= oil molecular weight, m, lbm/lbm mol
M
og
= oil-gas mixture molecular weight, m, lbm/lbm mol
p =
pressure, m/Lt
2
, psia
p
b
=
bubblepoint pressure, m/Lt
2
, psia
(/File%3AVol1_page_0304_inline_003.png) = bubblepoint pressure of oil with N
2
present in surface gas, m/Lt
2
, psia
p
bh
=
bubblepoint pressure of oil without nonhydrocarbons, m/Lt
2
, psia
p
f
= bubblepoint pressure factor, psia/R
p
r
= pressure ratio (fraction of bubblepoint pressure)
R
s
= solution GOR, scf/STB
T = temperature, T, F
T
abs
= temperature, T, R
T
b
= mean average boiling point temperature, T, R
T
cg
= gas pseudocritical temperature, T, R
T
cm
= mixture pseudocritical temperature, T, R
T
co
= oil pseudocritical temperature, T, R
T
r
= reduced temperature, T
T
sc
= temperature at standard conditions, T, F
V =
volume, L
3
V
o
=
volume of crude oil, L
3
W
g
= weight of dissolved gas, m
W
o
= weight of crude oil, m
x
g
= gas "component" mole fraction in oil
x
o
= oil "component" mole fraction in oil
y
g
= gas "component" mole fraction in gas
(/File%3AVol1_page_0305_inline_005.png) =
mole fraction N
2
in surface gas
yo = oil "component" mole fraction in gas
Z = gas compressibility factor
API
= oil API gravity
g
= gas specific gravity, air=1
gc = gas specific gravity adjusted for separator conditions, air=1
ghc
= gas specific gravity of hydrocarbon components in a gas mixture, air=1
gs
= separator gas specific gravity, air=1
o
= oil specific gravity
o
= oil viscosity, m/Lt, cp
ob
= bubblepoint oil viscosity, m/Lt, cp
od
= dead oil viscosity, m/Lt, cp
g
=
gas density, m/L
3
, lbm/ft
3
o
=
oil density, m/L
3
, lbm/ft
3
ob
=
bubblepoint oil density, m/L
3
, lbm/ft
3
w
=
water density, m/L
3
, g/cm
3
hw
=
hydrocarbon/water surface tension, m/t
2
, dynes/cm
go
=
gas/oil surface tension, m/t
2
, dynes/cm
od
=
dead oil surface tension, m/t
2
, dynes/cm
References
1.
1.0
1.1
Lasater, J.A. 1958. Bubble Point Pressure Correlations. J Pet Technol 10 (5): 6567. SPE-957-G. http://dx.doi.org/10.2118/957-G (http://dx.doi.org/10.2118/957-G).
2.
2.0
2.1
Standing, M.B. 1981. Volumetric and Phase Behavior of Oil Field Hydrocarbon Systems, ninth edition. Richardson, Texas: Society of Petroleum Engineers of AIME
3.
3.0
3.1
Standing, M.B. 1947. A Pressure-Volume-Temperature Correlation for Mixtures of California Oils and Gases. API Drilling and Production Practice (1947): 275-287.
4.
4.0
4.1
4.2
Glas, . 1980. Generalized Pressure-Volume-Temperature Correlations. J Pet Technol 32 (5): 785-795. SPE-8016-PA. http://dx.doi.org/10.2118/8016-PA
(http://dx.doi.org/10.2118/8016-PA)
5.
5.0
5.1
5.2
Al-Shammasi, A.A. 2001. A Review of Bubblepoint Pressure and Oil Formation Volume Factor Correlations. SPE Res Eval & Eng 4 (2): 146-160. SPE-71302-PA.
http://dx.doi.org/10.2118/71302-PA (http://dx.doi.org/10.2118/71302-PA)
6. Velarde, J., Blasingame, T.A., and McCain Jr., W.D. 1997. Correlation of Black Oil Properties At Pressures Below Bubble Point Pressure - A New Approach. Presented at
the Annual Technical Meeting of CIM, Calgary, Alberta, 811 June. PETSOC-97-93. http://dx.doi.org/10.2118/97-93 (http://dx.doi.org/10.2118/97-93)
7. Al-Marhoun, M.A. 1992. New Correlations For Formation Volume Factors Of Oil And Gas Mixtures. J Can Pet Technol 31 (3): 22. PETSOC-92-03-02.
http://dx.doi.org/10.2118/92-03-02 (http://dx.doi.org/10.2118/92-03-02)
8.
8.0
8.1
8.2
Frashad, F., LeBlanc, J.L., Garber, J.D. et al. 1996. Empirical PVT Correlations For Colombian Crude Oils. Presented at the SPE Latin American and Caribbean
Petroleum Engineering Conference, Port of Spain, Trinidad and Tobago, 2326 April. SPE-36105-MS. http://dx.doi.org/10.2118/36105-MS (http://dx.doi.org/10.2118/36105-
MS)
9. Kartoatmodjo, R.S.T. 1990. New Correlations for Estimating Hydrocarbon Liquid Properties. MS thesis, University of Tulsa, Tulsa, Oklahoma.
10. Kartoatmodjo, T.R.S. and Schmidt, Z. 1991. New Correlations for Crude Oil Physical Properties, Society of Petroleum Engineers, unsolicited paper 23556-MS.
11. Kartoatmodjo, T. and Z., S. 1994. Large Data Bank Improves Crude Physical Property Correlations. Oil Gas J. 92 (27): 5155.
12. Fitzgerald, D.J. 1994. A Predictive Method for Estimating the Viscosity of Undefined Hydrocarbon Liquid Mixtures. MS thesis, Pennsylvania State University, State College,
Pennsylvania.
13. Daubert, T.E. and Danner, R.P. 1997. API Technical Data BookPetroleum Refining, 6th edition, Chap. 11. Washington, DC: American Petroleum Institute (API).
14. Sutton, R.P. and Farshad, F. 1990. Evaluation of Empirically Derived PVT Properties for Gulf of Mexico Crude Oils. SPE Res Eng 5 (1): 79-86. SPE-13172-PA.
http://dx.doi.org/10.2118/13172-PA (http://dx.doi.org/10.2118/13172-PA)
15. Whitson, C.H. and Brul, M.R. 2000. Phase Behavior, No. 20, Chap. 3. Richardson, Texas: Henry L. Doherty Monograph Series, Society of Petroleum Engineers.
16. Bergman, D.F. 2004. Dont Forget Viscosity. Presented at the Petroleum Technology Transfer Council 2nd Annual Reservoir Engineering Symposium, Lafayette, Louisiana,
28 July.
17. Chew, J. and Connally, C.A. Jr. 1959. A Viscosity Correlation for Gas-Saturated Crude Oils. In Transactions of the American Institute of Mining, Metallurgical, and
Petroleum Engineers, Vol. 216, 23. Dallas, Texas: Society of Petroleum Engineers of AIME.
18. Aziz, K. and Govier, G.W. 1972. Pressure Drop in Wells Producing Oil and Gas. J Can Pet Technol 11 (3): 38. PETSOC-72-03-04. http://dx.doi.org/10.2118/72-03-04
(http://dx.doi.org/10.2118/72-03-04)
19. Beggs, H.D. and Robinson, J.R. 1975. Estimating the Viscosity of Crude Oil Systems. J Pet Technol 27 (9): 1140-1141. SPE-5434-PA. http://dx.doi.org/10.2118/5434-PA
(http://dx.doi.org/10.2118/5434-PA)
20. Vazquez, M.E. 1976. Correlations for Fluid Physical Property Prediction. MS thesis, University of Tulsa, Tulsa, Oklahoma.
21. Vazquez, M. and Beggs, H.D. 1980. Correlations for Fluid Physical Property Prediction. J Pet Technol 32 (6): 968-970. SPE-6719-PA. http://dx.doi.org/10.2118/6719-PA
(http://dx.doi.org/10.2118/6719-PA)
22. Beal, C. 1970. The Viscosity of Air, Water, Natural Gas, Crude Oil and Its Associated Gases at Oil Field Temperatures and Pressures, No. 3, 114127. Richardson, Texas:
Reprint Series (Oil and Gas Property Evaluation and Reserve Estimates), SPE.
23. Kouzel, B. 1965. How Pressure Affects Liquid Viscosity. Hydrocarb. Process. (March 1965): 120.
24. Abdul-Majeed, G.H. and Abu Al-Soof, N.B. 2000. Estimation of gasoil surface tension. J. Pet. Sci. Eng. 27 (34): 197-200. http://dx.doi.org/10.1016/S0920-
4105(00)00058-9 (http://dx.doi.org/10.1016/S0920-4105(00)00058-9)
25. Baker, O. and Swerdloff, W. 1955. Calculation of Surface Tension 3Calculating parachor Values. Oil Gas J. (5 December 1955): 141.
26. Baker, O. and Swerdloff, W. 1956. Calculation of Surface Tension 6Finding Surface Tension of Hydrocarbon Liquids. Oil Gas J. (2 January 1956): 125.
27. Firoozabadi, A. and Ramey Jr., H.J. 1988. Surface Tension of Water-Hydrocarbon Systems at Reservoir Conditions. J Can Pet Technol 27 (MayJune): 4148.
Noteworthy papers in OnePetro
Use this section to list papers in OnePetro that a reader who wants to learn more should definitely read
External links
Use this section to provide links to relevant material on websites other than PetroWiki and OnePetro
See also
Oil fluid properties (/Oil_fluid_properties)
PEH:Oil System Correlations (/PEH%3AOil_System_Correlations)
(https://www.onepetro.org/search?q=Calculating PVT properties) (http://scholar.google.ca/scholar?q=Calculating PVT properties)
(http://www.worldcat.org/search?q=Calculating PVT properties) (http://wiki.seg.org/index.php?
title=Special%3ASearch&redirs=1&fulltext=Search&ns0=1&ns4=1&ns500=1&redirs=1&title=Special%3ASearch&advanced=1&fulltext=Advanced+search&search=Calculating
PVT properties) (http://wiki.aapg.org/index.php?
title=Special%3ASearch&profile=advanced&fulltext=Search&ns0=1&ns4=1&ns102=1&ns104=1&ns106=1&ns108=1&ns420=1&ns828=1&redirs=1&profile=advanced&search=Calculating
PVT properties)
Das könnte Ihnen auch gefallen
- Oil Correlations FEKETEDokument21 SeitenOil Correlations FEKETEOscarCaballeroNoch keine Bewertungen
- PVT ModellingDokument6 SeitenPVT ModellingFan JackNoch keine Bewertungen
- Effect of On Equation-of-State Predictions: C Prop ErtiesDokument12 SeitenEffect of On Equation-of-State Predictions: C Prop ErtiesAllanNoch keine Bewertungen
- Abdullwahid Ahmed EXPDokument14 SeitenAbdullwahid Ahmed EXPAbdullwahid AhmedNoch keine Bewertungen
- 634183496451357500Dokument24 Seiten634183496451357500Tiar_Rahman_9553Noch keine Bewertungen
- MMP Prediction Using Equation of StateDokument12 SeitenMMP Prediction Using Equation of StateArie IrNoch keine Bewertungen
- Ec. de Martin PDFDokument17 SeitenEc. de Martin PDFMaggyBalcazarNoch keine Bewertungen
- Beggs and Brill Correlation for Liquid Holdup and Friction FactorDokument6 SeitenBeggs and Brill Correlation for Liquid Holdup and Friction FactorAli Al AkbarNoch keine Bewertungen
- Chapter 3 - Single Phase Fluid FlowDokument128 SeitenChapter 3 - Single Phase Fluid FlowZulfikri ZulkifliNoch keine Bewertungen
- The Five Reservoir FluidsDokument18 SeitenThe Five Reservoir FluidsFanny BalamNoch keine Bewertungen
- Cce Test - 1677610418Dokument13 SeitenCce Test - 1677610418yosifNoch keine Bewertungen
- A Simple Method For Constructing Phase EnvelopesDokument9 SeitenA Simple Method For Constructing Phase Envelopesjlg314Noch keine Bewertungen
- Regional Government of KurdistanDokument16 SeitenRegional Government of KurdistanMohammed MohammedNoch keine Bewertungen
- SPE-175877-MS EOS Tuning - Comparison Between Several Valid Approaches and New RecommendationsDokument17 SeitenSPE-175877-MS EOS Tuning - Comparison Between Several Valid Approaches and New RecommendationsCamilo Benítez100% (1)
- Differential Liberation Experiment Results in TablesDokument3 SeitenDifferential Liberation Experiment Results in TablessaeedNoch keine Bewertungen
- 5-Reservoir Fluid Property Correlations State of The ArtDokument9 Seiten5-Reservoir Fluid Property Correlations State of The ArtAnonymous Vbv8SHv0bNoch keine Bewertungen
- Predicting The Hydrate FormationDokument10 SeitenPredicting The Hydrate FormationmviteazuNoch keine Bewertungen
- Two-Phase Flow Pressure DropDokument4 SeitenTwo-Phase Flow Pressure DropStevenMvuyana100% (1)
- Journal of Petroleum Science and Engineering: Ehsan Heidaryan, Jamshid Moghadasi, Masoud RahimiDokument6 SeitenJournal of Petroleum Science and Engineering: Ehsan Heidaryan, Jamshid Moghadasi, Masoud RahimipeNoch keine Bewertungen
- Hydrate Phase Diagrams For Methane + Ethane + Propane MixturesDokument13 SeitenHydrate Phase Diagrams For Methane + Ethane + Propane MixturesHuancong HuangNoch keine Bewertungen
- PVT ExperimentsDokument45 SeitenPVT Experimentsndlr81Noch keine Bewertungen
- PNG 520 - Course On Phase RelationsDokument172 SeitenPNG 520 - Course On Phase RelationsDenstar Ricardo SilalahiNoch keine Bewertungen
- ch3 PDFDokument96 Seitench3 PDFJuan Zamora100% (1)
- Enthalpy PDFDokument119 SeitenEnthalpy PDFEstuardo Javier Gan RodríguezNoch keine Bewertungen
- SC RE Chap12-Vapour Liquid EquilibriumDokument30 SeitenSC RE Chap12-Vapour Liquid EquilibriumweldsvNoch keine Bewertungen
- Spe 165382 MS PDokument19 SeitenSpe 165382 MS PTatianyNoch keine Bewertungen
- Example 3 Relating Downhole Rate With The Rate at Standard ConditionsDokument8 SeitenExample 3 Relating Downhole Rate With The Rate at Standard ConditionsMaisam AbbasNoch keine Bewertungen
- Empirical Correlations To Predict Gas - CondensateDokument9 SeitenEmpirical Correlations To Predict Gas - CondensateAysel NaibovaNoch keine Bewertungen
- Calculation of Phase Envelopes and Critical Points For Multicomponent MixturesDokument10 SeitenCalculation of Phase Envelopes and Critical Points For Multicomponent Mixturesflavio_cordero_1Noch keine Bewertungen
- Differential Liberation Test PDFDokument19 SeitenDifferential Liberation Test PDFSimone SanNoch keine Bewertungen
- Example #7-Pumping Oil Well (Example7.csv)Dokument5 SeitenExample #7-Pumping Oil Well (Example7.csv)Anonymous OtSaas5VNoch keine Bewertungen
- PB Lecture Notes 2017Dokument59 SeitenPB Lecture Notes 2017ganeshNoch keine Bewertungen
- Phase Oil Equilibria of Oil-Water-Brine - Yaun Kun LiDokument11 SeitenPhase Oil Equilibria of Oil-Water-Brine - Yaun Kun LiRonald NgueleNoch keine Bewertungen
- P-T Diagram For A Single CompoundDokument48 SeitenP-T Diagram For A Single CompoundilkerkozturkNoch keine Bewertungen
- PNGE 332 Lecture NotesDokument86 SeitenPNGE 332 Lecture NotesZack Densmore100% (4)
- Bergman 2007Dokument30 SeitenBergman 2007Miguel Flores JimenezNoch keine Bewertungen
- Flash Calculations NewDokument8 SeitenFlash Calculations NewSantosh SakhareNoch keine Bewertungen
- SPE30714 Fevang WhitsonDokument16 SeitenSPE30714 Fevang WhitsonMohamed El KikiNoch keine Bewertungen
- Spe 15835 PaDokument14 SeitenSpe 15835 Pacamelion3100% (1)
- MULTIPHASE CORRELATION TECHNICAL REPORTDokument10 SeitenMULTIPHASE CORRELATION TECHNICAL REPORTGarion CharlesNoch keine Bewertungen
- Day 2 - Part 2: Equation of State ModelsDokument42 SeitenDay 2 - Part 2: Equation of State Modelsfoxnew11Noch keine Bewertungen
- 69-Chiara Gilardi Foster Wheeler Italiana-Relief-EDokument16 Seiten69-Chiara Gilardi Foster Wheeler Italiana-Relief-EKamil MarszałekNoch keine Bewertungen
- 1992 BenderDokument12 Seiten1992 BenderJohn PACHON MORALESNoch keine Bewertungen
- Gas Hydrates - PCDokument8 SeitenGas Hydrates - PCAashish DwivediNoch keine Bewertungen
- Properties of Dry GasDokument10 SeitenProperties of Dry GasChia Boon ChungNoch keine Bewertungen
- A Prediction of Water Content in Sour NGDokument70 SeitenA Prediction of Water Content in Sour NGMartin Šoltýs100% (1)
- Riazi Daubert 1980Dokument6 SeitenRiazi Daubert 1980Jeiel FrançaNoch keine Bewertungen
- Constant-Composition Expansion Test (CCE) : Tập Đoàn Dầu Khí Việt NamDokument19 SeitenConstant-Composition Expansion Test (CCE) : Tập Đoàn Dầu Khí Việt NamTruong1102100% (1)
- REN5415 - Y19 - Lec13, 14 &15Dokument87 SeitenREN5415 - Y19 - Lec13, 14 &15Abdulla MohammadNoch keine Bewertungen
- Guideline For Prevention and Safe Handling of HydratesDokument11 SeitenGuideline For Prevention and Safe Handling of Hydrateshitm357Noch keine Bewertungen
- Fluid Properties: PVT Properties of Crude Oils: 1. Oil Formation Volume Factor, BDokument6 SeitenFluid Properties: PVT Properties of Crude Oils: 1. Oil Formation Volume Factor, BAli HijaziNoch keine Bewertungen
- Joule Thomson ExpansionDokument2 SeitenJoule Thomson ExpansiondndudcNoch keine Bewertungen
- SC RE Chap4 - Phase BehaviourDokument68 SeitenSC RE Chap4 - Phase BehaviourweldsvNoch keine Bewertungen
- Spe 9235Dokument10 SeitenSpe 9235Anonymous 2MI1EjFN3cNoch keine Bewertungen
- COSTALD Correlation For Saturated Liquid DensitiesDokument3 SeitenCOSTALD Correlation For Saturated Liquid DensitiesPrasad patgaonkarNoch keine Bewertungen
- Multiphase Flow 1995Von EverandMultiphase Flow 1995A. SerizawaNoch keine Bewertungen
- Phase Equilibria: Basic Principles, Applications, Experimental TechniquesVon EverandPhase Equilibria: Basic Principles, Applications, Experimental TechniquesNoch keine Bewertungen
- Development and Application of Classical Capillary Number Curve TheoryVon EverandDevelopment and Application of Classical Capillary Number Curve TheoryNoch keine Bewertungen
- Hydrocarbon Fluid Inclusions in Petroliferous BasinsVon EverandHydrocarbon Fluid Inclusions in Petroliferous BasinsNoch keine Bewertungen
- Diemax XL: Maximum Life SpringsDokument44 SeitenDiemax XL: Maximum Life SpringsAnonymous yjF4yygpPbNoch keine Bewertungen
- 1409.7977 Review On The Quantization of Gravity Benjamin SchulzDokument100 Seiten1409.7977 Review On The Quantization of Gravity Benjamin SchulzAnonymous yjF4yygpPbNoch keine Bewertungen
- 2015-03-einstein-scientists-spacetime-foamDokument3 Seiten2015-03-einstein-scientists-spacetime-foamAnonymous yjF4yygpPbNoch keine Bewertungen
- The Weaponization of Increasingly Autonomous Technologies Artificial Intelligence en 700Dokument16 SeitenThe Weaponization of Increasingly Autonomous Technologies Artificial Intelligence en 700Anonymous yjF4yygpPbNoch keine Bewertungen
- AGNIR Report 2012 PDFDokument348 SeitenAGNIR Report 2012 PDFSpiros PolidorosNoch keine Bewertungen
- Quantum Vibrations in Brain Cells - 1707.0124v1Dokument41 SeitenQuantum Vibrations in Brain Cells - 1707.0124v1Anonymous yjF4yygpPbNoch keine Bewertungen
- Access the Universe of Online Information in 40 CharactersDokument6 SeitenAccess the Universe of Online Information in 40 CharactersLânNoch keine Bewertungen
- Knot Physics - Spacetime in Co - Dimension 2 1405 - 0223 v1Dokument31 SeitenKnot Physics - Spacetime in Co - Dimension 2 1405 - 0223 v1Anonymous yjF4yygpPbNoch keine Bewertungen
- SM2 MediaGuide PDFDokument141 SeitenSM2 MediaGuide PDFAnonymous yjF4yygpPbNoch keine Bewertungen
- Quantum MechanicsDokument116 SeitenQuantum MechanicsBruno Kongawi100% (1)
- Near-Earth Asteroid Mining: Shane D. Ross Control and Dynamical Systems Caltech 107-81, Pasadena, CA 91125Dokument24 SeitenNear-Earth Asteroid Mining: Shane D. Ross Control and Dynamical Systems Caltech 107-81, Pasadena, CA 91125Madalina BivolaruNoch keine Bewertungen
- An Introduction To CRISPR Technology For Genome Activation and Repression in Mammalian CellsDokument16 SeitenAn Introduction To CRISPR Technology For Genome Activation and Repression in Mammalian CellsAnonymous yjF4yygpPbNoch keine Bewertungen
- Hubble Error: Time, Money and Millionths of an InchDokument15 SeitenHubble Error: Time, Money and Millionths of an InchLester Dong Jara FerreyraNoch keine Bewertungen
- Asteroid Retrieval FeasibilityDokument16 SeitenAsteroid Retrieval FeasibilityAnonymous yjF4yygpPbNoch keine Bewertungen
- Satellite Remote SensingDokument52 SeitenSatellite Remote SensingAnonymous yjF4yygpPbNoch keine Bewertungen
- 6th Central Pay Commission Salary CalculatorDokument15 Seiten6th Central Pay Commission Salary Calculatorrakhonde100% (436)
- Corresponding Author: E-Mail: Kenath - Arun@cjc - Christcollege.edu Telephone: +91-80-4012 9292 Fax: +91-80-4012 9222Dokument55 SeitenCorresponding Author: E-Mail: Kenath - Arun@cjc - Christcollege.edu Telephone: +91-80-4012 9292 Fax: +91-80-4012 9222Anonymous yjF4yygpPbNoch keine Bewertungen
- Crack PropagationDokument3 SeitenCrack PropagationAnonymous yjF4yygpPbNoch keine Bewertungen
- Is Faster-Than-Light Travel or Communication Possible - Philip Gibbs 1997Dokument12 SeitenIs Faster-Than-Light Travel or Communication Possible - Philip Gibbs 1997Anonymous yjF4yygpPbNoch keine Bewertungen
- PositronDokument7 SeitenPositronAna SpataruNoch keine Bewertungen
- Aflas Processing Recommendations Rev 0 Jan 2013Dokument11 SeitenAflas Processing Recommendations Rev 0 Jan 2013Anonymous yjF4yygpPbNoch keine Bewertungen
- Time Dilation As Quantum Tunneling Time PDFDokument6 SeitenTime Dilation As Quantum Tunneling Time PDFAnonymous yjF4yygpPbNoch keine Bewertungen
- Summary - QuadraticFormulaDokument1 SeiteSummary - QuadraticFormulaAnonymous yjF4yygpPbNoch keine Bewertungen
- 6th Central Pay Commission Salary CalculatorDokument15 Seiten6th Central Pay Commission Salary Calculatorrakhonde100% (436)
- Time Dilation As Quantum Tunneling Time PDFDokument6 SeitenTime Dilation As Quantum Tunneling Time PDFAnonymous yjF4yygpPbNoch keine Bewertungen
- 2012 03 04 - Fermilab - Ask A Scientist PDFDokument51 Seiten2012 03 04 - Fermilab - Ask A Scientist PDFAnonymous yjF4yygpPbNoch keine Bewertungen
- Mechanical Properties of Graphene PapersDokument23 SeitenMechanical Properties of Graphene PapersAnonymous yjF4yygpPbNoch keine Bewertungen
- API Buttress Running Guidelines PDFDokument8 SeitenAPI Buttress Running Guidelines PDFEdwin MPNoch keine Bewertungen
- Corresponding Author: E-Mail: Kenath - Arun@cjc - Christcollege.edu Telephone: +91-80-4012 9292 Fax: +91-80-4012 9222Dokument55 SeitenCorresponding Author: E-Mail: Kenath - Arun@cjc - Christcollege.edu Telephone: +91-80-4012 9292 Fax: +91-80-4012 9222Anonymous yjF4yygpPbNoch keine Bewertungen
- Fluid Mechanics 2Dokument10 SeitenFluid Mechanics 2Jeffward Jaguio100% (1)
- Experiment No. 6 Specific Gravity of Coarse Aggregate: Department of Civil EngineeringDokument8 SeitenExperiment No. 6 Specific Gravity of Coarse Aggregate: Department of Civil EngineeringAli EspinosaNoch keine Bewertungen
- ME380L Lab1Dokument7 SeitenME380L Lab1raylo4594Noch keine Bewertungen
- Chemicals Zetag DATA Powder Zetag 4120 - 1110Dokument2 SeitenChemicals Zetag DATA Powder Zetag 4120 - 1110PromagEnviro.comNoch keine Bewertungen
- Some Basics On OBMDokument27 SeitenSome Basics On OBMRamanamurthy PalliNoch keine Bewertungen
- Soil volumetric and mass ratiosDokument55 SeitenSoil volumetric and mass ratiosJOSE ESTEBAN SOTO TINCONoch keine Bewertungen
- Wireline ManualDokument307 SeitenWireline ManualJorge Rodriguez100% (14)
- CE215 GeoLab Manual - Determination of Specific GravityDokument2 SeitenCE215 GeoLab Manual - Determination of Specific GravityJoy MondalNoch keine Bewertungen
- 7 Lab Tests On Aggregate To Check Quality For Use in Road Work - CivilblogDokument11 Seiten7 Lab Tests On Aggregate To Check Quality For Use in Road Work - Civilblogengr.zubair34Noch keine Bewertungen
- Calibrate Blaine apparatusDokument1 SeiteCalibrate Blaine apparatusVijay Bhan100% (3)
- Breadth Exam No. 1Dokument18 SeitenBreadth Exam No. 1Random Stuff100% (2)
- ASTM D 1075 Effect of Water On Strength of Compacted BitumenDokument2 SeitenASTM D 1075 Effect of Water On Strength of Compacted BitumenRajesh Kumar100% (3)
- Properties of Crude Oil and Petroleum ProductsDokument54 SeitenProperties of Crude Oil and Petroleum ProductsMihaelaPaval0% (1)
- Jis A 1102Dokument17 SeitenJis A 1102Anonymous MYUWvUrhdbNoch keine Bewertungen
- Astm C20Dokument3 SeitenAstm C20nee2790100% (1)
- Density of Semi-Solid Bituminous Materials (Pycnometer Method)Dokument4 SeitenDensity of Semi-Solid Bituminous Materials (Pycnometer Method)ROHITNoch keine Bewertungen
- Gas Pipeline Blowdown TimeDokument3 SeitenGas Pipeline Blowdown Timeankur2061Noch keine Bewertungen
- 5-Reservoir Fluid Property Correlations State of The ArtDokument9 Seiten5-Reservoir Fluid Property Correlations State of The ArtAnonymous Vbv8SHv0bNoch keine Bewertungen
- Cement Testing ExperimentsDokument20 SeitenCement Testing ExperimentsThe BluemanNoch keine Bewertungen
- API 650 Tank Design 1foot MethodDokument4 SeitenAPI 650 Tank Design 1foot Methodjakjak67% (3)
- IX - Drilling Fluids ManualDokument332 SeitenIX - Drilling Fluids Manualjovicad100% (1)
- Basra University For OilDokument7 SeitenBasra University For OilkareemNoch keine Bewertungen
- Tank Farm AutomationDokument28 SeitenTank Farm Automationmahmoud_mustafaNoch keine Bewertungen
- Pressure Measurements NotesDokument15 SeitenPressure Measurements NotesManjunath MohiteNoch keine Bewertungen
- CAP6 Whitson Phase BehaviorDokument21 SeitenCAP6 Whitson Phase BehaviorMaría José MartínezNoch keine Bewertungen
- 2710 Tests On Sludges : 1. General DiscussionDokument8 Seiten2710 Tests On Sludges : 1. General Discussionpollux23Noch keine Bewertungen
- ASTM D 2726 - 05a Standard Test Method For Bulk Specific Gravity and DensityDokument4 SeitenASTM D 2726 - 05a Standard Test Method For Bulk Specific Gravity and DensityAzlan AbdNoch keine Bewertungen
- Astm C127Dokument5 SeitenAstm C127Isaac Correa Julcarima100% (1)
- Bulk Density Cacl2 PDFDokument2 SeitenBulk Density Cacl2 PDFRenandaBudiPratamaNoch keine Bewertungen
- PDFDokument8 SeitenPDFgobiksNoch keine Bewertungen