Beruflich Dokumente
Kultur Dokumente
Fugacity Coefficient
Hochgeladen von
sigit10580 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
284 Ansichten4 Seiten1) The fugacity of a species in solution is defined analogously to the pure species fugacity, with fugacity (fi) replacing partial pressure (pi). This allows fugacity to be used as the criterion for phase equilibrium between mixtures and solutions.
2) The fugacity coefficient (φi) is defined as the ratio of fugacity to ideal gas partial pressure. It accounts for non-ideal behavior and reduces to 1 for an ideal gas. Equations of state can be used to calculate fugacity coefficients.
3) The virial equation of state allows derivation of expressions for the fugacity coefficients of species in a binary gas mixture in terms of temperature, pressure, and virial coefficients.
Originalbeschreibung:
bab 3
Originaltitel
fugacity
Copyright
© © All Rights Reserved
Verfügbare Formate
DOCX, PDF, TXT oder online auf Scribd lesen
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument melden1) The fugacity of a species in solution is defined analogously to the pure species fugacity, with fugacity (fi) replacing partial pressure (pi). This allows fugacity to be used as the criterion for phase equilibrium between mixtures and solutions.
2) The fugacity coefficient (φi) is defined as the ratio of fugacity to ideal gas partial pressure. It accounts for non-ideal behavior and reduces to 1 for an ideal gas. Equations of state can be used to calculate fugacity coefficients.
3) The virial equation of state allows derivation of expressions for the fugacity coefficients of species in a binary gas mixture in terms of temperature, pressure, and virial coefficients.
Copyright:
© All Rights Reserved
Verfügbare Formate
Als DOCX, PDF, TXT herunterladen oder online auf Scribd lesen
0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
284 Ansichten4 SeitenFugacity Coefficient
Hochgeladen von
sigit10581) The fugacity of a species in solution is defined analogously to the pure species fugacity, with fugacity (fi) replacing partial pressure (pi). This allows fugacity to be used as the criterion for phase equilibrium between mixtures and solutions.
2) The fugacity coefficient (φi) is defined as the ratio of fugacity to ideal gas partial pressure. It accounts for non-ideal behavior and reduces to 1 for an ideal gas. Equations of state can be used to calculate fugacity coefficients.
3) The virial equation of state allows derivation of expressions for the fugacity coefficients of species in a binary gas mixture in terms of temperature, pressure, and virial coefficients.
Copyright:
© All Rights Reserved
Verfügbare Formate
Als DOCX, PDF, TXT herunterladen oder online auf Scribd lesen
Sie sind auf Seite 1von 4
11.
6 FUGACITY AND FUGACITY COEFFICIENT:
SPECIES IN SOLUTION
The definition of the fugacity of a species in solution is parallel to the definition of the purespecies
fugacity. For species i in a mixture of real gases or in a solution of liquids, the equation
analogous to Eq. (1 1.28), the ideal-gas expression, is:
pi -- ri (T) + RT In ti (1 1.42)
where fi is the fugacity of species i in solution, replacing the partial pressure yi P. This
definition of ti does not make it a partial molar property, and it is therefore identified by a
circumflex rather than by an overbar.
A direct application of this definition indicates its potential utility. Equation 11.6 is the
fundamental criterion for phase equilibrium. Since all phases in equilibrium are at the same
temperature, an alternative and equally general criterion follows immediately from Eq. (1 1.42):
Thus, multiple phases at the same T and P are in equilibrium when
the fugacity of each constituent species is the same in all phases.
This criterion of equilibrium is the one usually applied by chemical engineers in the solution
of phase-equilibrium problems.
For the specific case of multicomponent vaporlliquid equilibrium, Eq. (1 1.43) becomes:
Equation (1 1.38) results as a special case when this relation is applied to the vaporAiquid
equilibrium of pure species i .
The definition of a residual property is given in Sec. 6.2:
MR - M - I,@ (6.41)
where M is the molar (or unit-mass) value of a thermodynamic property and M'g is the value
that the property would have for an ideal gas of the same composition at the same T and P. The
defining equation for apartial residual property M: follows from this equation. Multiplied by
n mol of mixture, it becomes:
Differentiation with respect to ni at constant T, P, and nj gives:
Reference to Eq. (1 1.7) shows that each term has the form of a partial molar property.
Thus, Since residual properties measure departure from ideal-gas values, their most logical use is as
gas-phase properties, but in fact they also find use as liquid-phase properties.
Written for the residual Gibbs energy, Eq. (1 1.45) becomes:
an equation which defines the partial residual Gibbs energy.
Subtracting Eq. (1 1.28) from Eq. (1 1.42), both written for the same T and P, yields:
This result combined with Eq. (1 1.46) and the identity pi E Ci gives:
where by definition
The dimensionless ratio $i is called the fugacity coefJicient of species i in solution. Although
most commonly applied to gases, the fugacity coefficient may also be used for liquids, and in
this case mole fraction yi is replaced by xi.
Equation (1 1.47) is the analog of Eq. (1 1.31), which relates $i to G;. For an ideal gas,
G: is necessarily zero; therefore $jg = 1, and
Thus the fugacity of species i in an ideal-gas mixture is equal to its partial pressure.
The Fundamental Residual-Property Relation
In order to extend the fundamental property relation to residual properties, we transform
Eq. (1 1.2) into an alternative form through the mathematical identity (also used in Sec. 6.1):
1 nG
d (1-;) - --d(nG)- -dT Ri" RT2
In this equation d(nG) is eliminated by Eq. (1 1.2) and G is replaced by its definition, H - TS.
The result, after algebraic reduction, is:
All terms in Eq. (1 1.50) have the units of moles; moreover, in contrast to Eq. (1 1.2), the
enthalpy rather than the entropy appears on the right side.
Equation (1 1.50) is a general relation expressing nGIRT as a function of all of its canonical variables, T, P, and the
mole numbers. It
reduces to Eq. (6.37) for the special case of 1 mol of a constant-composition phase. Equations
(6.38) and (6.39) follow from either equation, and equations for the other thermodynamic
properties then come from appropriate defining equations. Knowledge of GIRT as a function
of its canonical variables allows evaluation of all other thermodynamic properties, and therefore
implicitly contains complete property information. However, we cannot directly exploit this
characteristic, and for this reason develop an analogous equation relating residual properties.
Since Eq. (1 1 SO) is general, it may be written for the special case of an ideal gas:
In view of Eqs. (6.41) and (1 1.46), subtracting this equation from Eq. (1 1.50) gives:
Equation (1 1.51) is thefundamental residual-property relation. Its derivation from Eq. (1 1.2)
parallels the derivation in Chap. 6 that led from Eq. (6.10) to Eq. (6.42). Indeed, Eqs. (6.10) and
(6.42) are special cases of Eqs. (1 1.2) and (1 1.51), valid for 1 mol of a constant-composition
fluid. An alternative form of Eq. (1 1.51) follows by introduction of the fugacity coefficient as
given by Eq. (1 1.47):
Equations so general as Eqs. (1 1.51) and (1 1.52) are useful for practical application only
in their restricted forms. Division of Eqs. (1 1.5 1) and (1 1.52) by d P and restriction to constant
T and composition leads to:
Similarly, division by dT and restriction to constant P and composition gives:
These equations are restatements of Eqs. (6.43) and (6.44) wherein the restriction of the derivatives
to constant composition is shown explicitly. They lead to Eqs. (6.46), (6.47), and (6.48)
for the calculation of residual properties from volumetric data. Moreover, Eq. (1 1.53) is the
basis for the direct derivation of Eq. (1 1.34), which yields fugacity coefficients from volumetric
data. It is through the residual properties that this kind of experimental information enters into
the practical application of thermodynamics. In addition, from Eq. (1 1.52),
This equation demonstrates that In ai is a partial property with respect to G R / ~
Fugacity Coefficients from the Virial Equation of State
Values of )i for species i in solution are readily found from equations of state. The simplest
form of the virial equation provides a useful example. Written for a gas mixture it is exactly
the same as for a pure species: The mixture second virial coefficient B is a function of temperature and composition.
Its exact
composition dependence is given by statistical mechanics, and this makes the virial equation
preeminent among equations of state where it is applicable, i.e., to gases at low to moderate
pressures. The equation giving this composition dependence is:
where y represents mole fractions in a gas mixture. The indices i and j identify species,
and both run over all species present in the mixture. The virial coefficient Bij characterizes
a bimolecular interaction between molecule i and molecule j , and therefore Bij = Bji. The
summations account for all possible bimolecular interactions.
For a binary mixture i = l , 2 and j = l,2; the expansion of Eq. (1 1.57) then gives:
Two types of virial coefficients have appeared: Bll and B22, for which the successive subscripts
are the same, and BI2, for which the two subscripts are different. The first type is a purespecies
virial coefficient; the second is a mixture property, known as a cross coeficient. Both
are functions of temperature only. Expressions such as Eqs. (1 1.57) and (1 1.58) relate mixture
coefficients to pure-species and cross coefficients. They are called mixing rules.
Equation (1 1.58) allows derivation of expressions for In $1 and In $2 for a binary gas
mixture that obeys Eq. (3.37). Written for n mol of gas mixture, it becomes:
Differentiation with respect to nl gives:
Substitution for in Eq. (1 1.56) yields:
where the integration is elementary, because B is not a function of pressure. All that remains
is evaluation of the derivative.
Equation (1 1.58) for the second virial coefficient may be written:
where 812 -- 2B12 - Bll - B22
Since yi = ni/n,
n B = ~ ~ B+ In2BI 22 + -1a21l122 2 n
Differentiation gives:
A P
Therefore, In41 = -R(&TI + ~22812) ( 11 .59)
. P
Similarly, In42 = -R(BT2 2 + yf612)
(1 1.60)
Equations (1 1.59) and ( 1 1.60) are readily extended for application to multicomponent gas
mixtures; the general equation is:9
where the dummy indices i and j run over all species, and
Sik -- 2Bik - Bii - Bkk &' J . -= 2 B'.J . - B .1.1- B . .J J
with Sii = 0, 8kk = 0, etc., and Ski = aik, etc.
11.7 GENERALIZED CORRELATIONS FOR THE
FUGACITY COEFFICIENT
The generalized methods developed in Sec. 3.6 for the compressibility factor Z and in Sec. 6.7
for the residual enthalpy and entropy of pure gases are applied here to the fugacity coefficient.
Equation (1 1.34) is put into generalized form by substitution of the relations,
pr
Hence, d P,
ln4i = (z' - 1)- (1 1.62)
P,
where integration is at constant T,. Substitution for Zi by Eq. (3.54) yields:
where for simplicity we have dropped subscript i. This equation may be written in alternative
form:
In4 = ln@O + w ln4' (1 1.63)
p,
where 1 n 4O- - l (Z, , -1)- dP, and l n d =
p,
The integrals in these equations may be evaluated numerically or graphically for various values
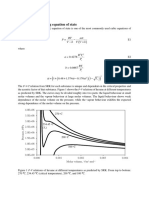
of T, and P, from the data for Z0 and Z' given in Tables E.l through E.4 (App. E).
Another method, and the one adopted by Lee and Kesler to extend their correlation to fugacity
coefficients, is based on an equation of state.
Since Eq. (1 1.63) may also be written,
4 = (4O)(4'>" (1 1.64)
we have the option of providing correlations for 4' and 4' rather than for their logarithms.
This is the choice made here, and Tables E. 13 through E. 16 present values for these quantities
as derived from the LeeIKesler correlation as functions of T, and P,, thus providing a threeparameter
generalized correlation for fugacity coefficients. Tables E. 13 and E. 15 for #' can be
used alone as a two-parameter correlation which does not incorporate the refinement introduced
by the acentric factor.
A useful generalized correlation for In # results when the simplest form of the virial
equation is valid. Equations (3.58) and (3.59) combine to give:
Substitution in Eq. (1 1.62) and integration yield:
This equation, used in conjunction with Eqs. (3.61) and (3.62), provides reliable values of 4
for any nonpolar or slightly polar gas when applied at conditions where
Named functions, HRB(TR,PR,OMEGA) and SRB(TR,PR,OMEGA), for evaluation
of H R/ RT, and S R/ R by the generalized virial-coefficient correlation were described in
Sec. 6.7. Similarly, we introduce here a function named PHIB(TR,PR,OMEGA) for evaluation
of 4:
It combines Eq. (1 1.65) with Eqs. (3.61) and (3.62), as indicated in the representative computer
programs given in App. D. For example, the value of 4 for 1-butene vapor at the conditions of
Example 6.8, Step (b), is:
The generalized correlation just described is for pure gases only. The remainder of this
section shows how the virial equation may be generalized to allow calculation of fugacity
coefficients Ji for species in gas mixtures.
The general expression for calculation of In Jk from second-virial-coefficient data is given
by Eq. (1 1.61). Values of the pure-species virial coefficients Bkk, Bii, etc., are found from the
generalized correlation represented by Eqs. (3.59), (3.61), and (3.62). The cross coefficients
Bik, Bij, etc., are found from an extension of the same correlation. For this purpose, Eq. (3.59)
is rewritten in the more general form: lo
where BO and B' are the same functions of Tr as given by Eqs. (3.61) and (3.62). The combining
rules proposed by Prausnitz for calculation of wij, Tcij,a nd PCija re:
In Eq. (1 1.68), kij is an empirical interaction parameter specific to an i- j molecular pair.
When i = j and for chemically similar species, kij = 0. Otherwise, it is a small positive
number evaluated from minimal P VT data or in the absence of data set equal to zero.
When i = j, all equations reduce to the appropriate values for a pure species. When
i # j, these equations define a set of interaction parameters having no physical significance.
Reduced temperature is given for each i j pair by TTi-j-= T/T cij.
For a mixture, values of Bij from Eq. (1 1.66) substituted into Eq. (1 1.57) yield the
mixture second virial coefficient B, and substituted into Eq. (1 1.61) [Eqs. (1 1.59) and (1 1.60)
for a binary] they yield values of In $i.
The primary virtue of the generalized correlation for second virial coefficients presented
here is simplicity; more accurate, but more complex, correlations appear in the literature."
Das könnte Ihnen auch gefallen
- Chapter 5Dokument36 SeitenChapter 5Lucy BrownNoch keine Bewertungen
- Equation of StateDokument6 SeitenEquation of StateJanardhan CnNoch keine Bewertungen
- EOSDokument9 SeitenEOSgoicoxNoch keine Bewertungen
- 7 1. Vapor Liquid EquilibriumDokument9 Seiten7 1. Vapor Liquid Equilibriumwaseemkhan49Noch keine Bewertungen
- CH 7 AssignmentDokument3 SeitenCH 7 AssignmentUday Prakash SahuNoch keine Bewertungen
- Correlation of Ideal Gas Enthalpy, Heat Capacity, and EntropyDokument4 SeitenCorrelation of Ideal Gas Enthalpy, Heat Capacity, and EntropyJosé Luís Díaz VargasNoch keine Bewertungen
- Mixing RulesDokument15 SeitenMixing RulesCremorlabNoch keine Bewertungen
- Practical Chemical Thermodynamics for GeoscientistsVon EverandPractical Chemical Thermodynamics for GeoscientistsNoch keine Bewertungen
- Phase Equilibria: Basic Principles, Applications, Experimental TechniquesVon EverandPhase Equilibria: Basic Principles, Applications, Experimental TechniquesNoch keine Bewertungen
- Week 2 - Vle Part 1Dokument35 SeitenWeek 2 - Vle Part 1Syed Hassan Syed Hashim100% (1)
- SRK Equation of State ExplainedDokument5 SeitenSRK Equation of State Explainednutnicha suwannangkoonNoch keine Bewertungen
- Isobaric Vapor-Liquid Equilibrium For Binary System of Methanol and AcetonitrileDokument4 SeitenIsobaric Vapor-Liquid Equilibrium For Binary System of Methanol and AcetonitrileAlejandra Rodríguez MorenoNoch keine Bewertungen
- Phase Diagrams in Chemical EngineeringDokument23 SeitenPhase Diagrams in Chemical Engineeringchemsac2Noch keine Bewertungen
- Chapter 10Dokument24 SeitenChapter 10Lucy BrownNoch keine Bewertungen
- Advanced Thermodynamics: Vapor/Liquid EquilibriumDokument28 SeitenAdvanced Thermodynamics: Vapor/Liquid Equilibriumdo_overNoch keine Bewertungen
- Thermophysical Properties-Industrial DirectionsDokument15 SeitenThermophysical Properties-Industrial DirectionsfabiopdnNoch keine Bewertungen
- A MODIFIED PENG-ROBINSON EQUATION OF STATE FOR - ELV - 20519 - FTP PDFDokument12 SeitenA MODIFIED PENG-ROBINSON EQUATION OF STATE FOR - ELV - 20519 - FTP PDFLuiz Roberto TerronNoch keine Bewertungen
- Gas Laws and Equations Chapter SummaryDokument57 SeitenGas Laws and Equations Chapter SummaryEbook Download0% (1)
- Vapor-Liquid Equilibrium Data For The SystemDokument4 SeitenVapor-Liquid Equilibrium Data For The Systemapi-3817782100% (1)
- Improvement in Patel Teja Eqn of StatesDokument10 SeitenImprovement in Patel Teja Eqn of StatesSumukh VermaNoch keine Bewertungen
- P4E2: Kinetics of Homogeneous Reaction in Batch and Continuous Stirred-Tank Reactor at Two Different TemperatureDokument7 SeitenP4E2: Kinetics of Homogeneous Reaction in Batch and Continuous Stirred-Tank Reactor at Two Different TemperaturejayaprinaNoch keine Bewertungen
- Chapter 8Dokument64 SeitenChapter 8Lucy BrownNoch keine Bewertungen
- A Comparison of The Peng-Robinson and Soave-Redlich-Kwong Equations of StateDokument18 SeitenA Comparison of The Peng-Robinson and Soave-Redlich-Kwong Equations of StateVettidog100% (1)
- K values and relative volatility diagram for chloroform/methanol mixtureDokument2 SeitenK values and relative volatility diagram for chloroform/methanol mixtureIbrar KhanNoch keine Bewertungen
- A Method To Estimate The Patel-Teja Equation of State ConstantsDokument7 SeitenA Method To Estimate The Patel-Teja Equation of State Constants1940LaSalleNoch keine Bewertungen
- L13 Equilibrium ConversionDokument21 SeitenL13 Equilibrium ConversionLukas EstivaresNoch keine Bewertungen
- 1992 BenderDokument12 Seiten1992 BenderJohn PACHON MORALESNoch keine Bewertungen
- VLE Calculations for Distillation DesignDokument4 SeitenVLE Calculations for Distillation Designsandeep lal0% (1)
- 0378 3812 2889 2980369 3 PDFDokument12 Seiten0378 3812 2889 2980369 3 PDFsaeedt538Noch keine Bewertungen
- Combining Pinch and Exergy Analysis ForDokument13 SeitenCombining Pinch and Exergy Analysis Forasad0071Noch keine Bewertungen
- A New Generalized Alpha Function For A Cubic Equation of StateDokument11 SeitenA New Generalized Alpha Function For A Cubic Equation of StateJenn QuintoNoch keine Bewertungen
- Separation Process Engineering CHEN 312: Ys18@aub - Edu.lbDokument28 SeitenSeparation Process Engineering CHEN 312: Ys18@aub - Edu.lbsoe0303Noch keine Bewertungen
- Module 1. Vapor - Liquid EquilibriumDokument87 SeitenModule 1. Vapor - Liquid EquilibriumVanNoch keine Bewertungen
- Oliveira Et Al 2012Dokument10 SeitenOliveira Et Al 2012Daniela De Araujo SampaioNoch keine Bewertungen
- Solution Thermodynamics: Theory: Fundamental Property RelationDokument6 SeitenSolution Thermodynamics: Theory: Fundamental Property RelationLim Ying PeiNoch keine Bewertungen
- Phase Equilibrium Properties of The Ternary Mixture Dibutylether + Toluene + Heptane at 313.15 KDokument5 SeitenPhase Equilibrium Properties of The Ternary Mixture Dibutylether + Toluene + Heptane at 313.15 Kmurdanetap957Noch keine Bewertungen
- Vapor-Liquid Equilibrium of Systems Containing Alcohols, Water, CarbonDokument8 SeitenVapor-Liquid Equilibrium of Systems Containing Alcohols, Water, CarbonJessica CehNoch keine Bewertungen
- A Mathematical Model For Equilibrium Solubility of Hydrogen Sulfide and Carbon Dioxide in Aqueous Alkanolamine SolutionsDokument8 SeitenA Mathematical Model For Equilibrium Solubility of Hydrogen Sulfide and Carbon Dioxide in Aqueous Alkanolamine Solutionsiitgn007100% (1)
- Riazi Daubert 1980Dokument6 SeitenRiazi Daubert 1980Jeiel FrançaNoch keine Bewertungen
- Formula RioDokument13 SeitenFormula RioNoeliaNoch keine Bewertungen
- VLE Lactic Acid Ethyl Lactate Esterification PDFDokument7 SeitenVLE Lactic Acid Ethyl Lactate Esterification PDFAseem Kashyap0% (1)
- Effect of On Equation-of-State Predictions: C Prop ErtiesDokument12 SeitenEffect of On Equation-of-State Predictions: C Prop ErtiesAllanNoch keine Bewertungen
- HENRY'S LAW IN A BINARY MIXTUREDokument22 SeitenHENRY'S LAW IN A BINARY MIXTUREFaisal El HakimNoch keine Bewertungen
- Two ComponentsDokument19 SeitenTwo ComponentsMumtaz AhmadNoch keine Bewertungen
- Clausius ClapeyronDokument2 SeitenClausius ClapeyronKarthikeyanNoch keine Bewertungen
- Matlab - Equations of StateDokument23 SeitenMatlab - Equations of StateMia Beatrice Benavidez MawiliNoch keine Bewertungen
- Ideal Gas Mixture Properties and Entropy ChangeDokument86 SeitenIdeal Gas Mixture Properties and Entropy ChangeMariaAlejandraReyesNoch keine Bewertungen
- Flash Calc 152Dokument8 SeitenFlash Calc 152Mohamed MamdouhNoch keine Bewertungen
- Thermo 1Dokument9 SeitenThermo 1notapernota101Noch keine Bewertungen
- CRE AssignmentDokument5 SeitenCRE AssignmentKuldeepChoudharyNoch keine Bewertungen
- Development of A Generalized Quartic Equation of State For Pure FDokument171 SeitenDevelopment of A Generalized Quartic Equation of State For Pure FFelipe Miguel Sánchez ClementsNoch keine Bewertungen
- Cubic Equations of State-Which Is BestDokument17 SeitenCubic Equations of State-Which Is BestvenkieeNoch keine Bewertungen
- Loufllgria: An Improved Correlation For Compressed Liquid Densities of Hydrocarbons. Part 2. MixturesDokument15 SeitenLoufllgria: An Improved Correlation For Compressed Liquid Densities of Hydrocarbons. Part 2. MixturesPrasad patgaonkarNoch keine Bewertungen
- Phase Equilibria InsightsDokument19 SeitenPhase Equilibria InsightsjacNoch keine Bewertungen
- Liquid-Liquid Extractions Lesson 7Dokument20 SeitenLiquid-Liquid Extractions Lesson 7GATOMICONoch keine Bewertungen
- NPTEL Chemical Engineering ThermodynamicsDokument2 SeitenNPTEL Chemical Engineering Thermodynamicsvinovictory8571Noch keine Bewertungen
- Vapor Pressure PDFDokument9 SeitenVapor Pressure PDFArindam BanerjeeNoch keine Bewertungen
- Tutorial DistillationDokument3 SeitenTutorial DistillationManu Indivare Nundoolall100% (1)
- High Pressure Phase Behaviour of Multicomponent Fluid MixturesVon EverandHigh Pressure Phase Behaviour of Multicomponent Fluid MixturesNoch keine Bewertungen
- High-Pressure Fluid Phase Equilibria: Phenomenology and ComputationVon EverandHigh-Pressure Fluid Phase Equilibria: Phenomenology and ComputationNoch keine Bewertungen
- Ejercicos Mentales Volumen 13Dokument10 SeitenEjercicos Mentales Volumen 13Luis TorresNoch keine Bewertungen
- 1 s2.0 S1110016822005646 MainDokument14 Seiten1 s2.0 S1110016822005646 MainEss ZeeNoch keine Bewertungen
- Assignment 1 - Simple Harmonic MotionDokument2 SeitenAssignment 1 - Simple Harmonic MotionDr. Pradeep Kumar SharmaNoch keine Bewertungen
- Bending Moment Normal Forces in Tunnel Linings PDFDokument8 SeitenBending Moment Normal Forces in Tunnel Linings PDFhendrawanNoch keine Bewertungen
- 04 Handout 1 (Feedback)Dokument11 Seiten04 Handout 1 (Feedback)Kathleen Anne MendozaNoch keine Bewertungen
- SAS Certification Practice Exam - Base ProgrammingDokument18 SeitenSAS Certification Practice Exam - Base ProgrammingArvind Shukla100% (1)
- Engg Mechanics QuestionnaireDokument15 SeitenEngg Mechanics Questionnaireacurvz2005Noch keine Bewertungen
- Mathematics HL Paper 2 TZ2 PDFDokument16 SeitenMathematics HL Paper 2 TZ2 PDFMADANNoch keine Bewertungen
- No SystemDokument15 SeitenNo SystemAjeet Singh RachhoyaNoch keine Bewertungen
- THE RIGHT APPROACH TO MF SELECTIONDokument2 SeitenTHE RIGHT APPROACH TO MF SELECTIONMahesh NarayananNoch keine Bewertungen
- Role of HypothesisDokument2 SeitenRole of Hypothesisशिवम कर्णNoch keine Bewertungen
- Intro to Algorithms & Data Structures LectureDokument30 SeitenIntro to Algorithms & Data Structures LectureSaqib SherNoch keine Bewertungen
- Detailed Lesson Plan on Subtracting 2-3 Digit NumbersDokument7 SeitenDetailed Lesson Plan on Subtracting 2-3 Digit NumbersNhor Jehan SaydinNoch keine Bewertungen
- Solved For Each of The Following Examples Draw A Representative IsoquantDokument1 SeiteSolved For Each of The Following Examples Draw A Representative IsoquantM Bilal SaleemNoch keine Bewertungen
- Design of Predictive Magic Cards.Dokument26 SeitenDesign of Predictive Magic Cards.aries25th3Noch keine Bewertungen
- Poisson's and Laplace Equations ExplainedDokument19 SeitenPoisson's and Laplace Equations ExplainedMnskSaroNoch keine Bewertungen
- Multi-Objective Optimization For Football Team Member SelectionDokument13 SeitenMulti-Objective Optimization For Football Team Member SelectionSezim Tokozhan kyzyNoch keine Bewertungen
- Divergence and CurlDokument8 SeitenDivergence and CurlaliNoch keine Bewertungen
- Soil Mechanics: Explain About The Friction Circle MethodDokument2 SeitenSoil Mechanics: Explain About The Friction Circle MethodmaniNoch keine Bewertungen
- Material Models in PlaxisDokument136 SeitenMaterial Models in PlaxismpvfolloscoNoch keine Bewertungen
- S Parameter BasicsDokument11 SeitenS Parameter Basicslancelot795Noch keine Bewertungen
- Rangka Batang RhezaDokument11 SeitenRangka Batang RhezaKABINET JALADARA NABDANoch keine Bewertungen
- Notes On The Site That Takes CalculationsDokument7 SeitenNotes On The Site That Takes CalculationsNurdin ŠabićNoch keine Bewertungen
- Journal of Food Engineering: V.R. Vasquez, A. Braganza, C.J. CoronellaDokument12 SeitenJournal of Food Engineering: V.R. Vasquez, A. Braganza, C.J. CoronellaRamiro De Aquino GarciaNoch keine Bewertungen
- Cs-Module 1 NotesDokument58 SeitenCs-Module 1 NotesSuprithaNoch keine Bewertungen
- The Relationship Between Capital Structure and Firm Performance - New Evidence From PakistanDokument12 SeitenThe Relationship Between Capital Structure and Firm Performance - New Evidence From Pakistanhammadshah786Noch keine Bewertungen
- Unit 4 DeadlocksDokument14 SeitenUnit 4 DeadlocksMairos Kunze BongaNoch keine Bewertungen
- On The Static Solutions in Gravity With Massive Scalar Field in Three DimensionsDokument11 SeitenOn The Static Solutions in Gravity With Massive Scalar Field in Three DimensionsMojeime Igor NowakNoch keine Bewertungen
- Csat Test 1 AnswersDokument11 SeitenCsat Test 1 Answersrahul kumarNoch keine Bewertungen