Beruflich Dokumente
Kultur Dokumente
Chemical Basis of Life
Hochgeladen von
Chris_Barber09Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Chemical Basis of Life
Hochgeladen von
Chris_Barber09Copyright:
Verfügbare Formate
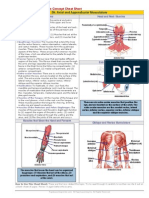
CLEP Biology - Core Concept Cheat Sheet
02: Chemical Basis of Life
Key Terms
Organic Chemicals
Atoms:
o Electrons: both energy and substance particles
o Neutrons
o Protons
Classified by the functional groups:
Alcohols, R-OH
Aldehydes, R-CHO, R-C=O
|
O
H
||
Ketones, R-C-R, R-CO-R
O
||
Carboxylic Acids, R-COOH, R-C-OH
Amines, R-NH2, R-N-H
|
H
Molecules:
o Formed by atoms
o Joined by chemical bonds
o Molecular formula and structure formula
Organic Molecules/macromolecules:
o Amino acids --> proteins
o Monosacchrides --> polysaccharides
o Fatty acids --> lipids
o Nucleotides --> nucleic acids
Isotope: Atoms have same proton numbers but may differ
in neutron numbers
Energy shell: Electrons occupy orbital around nucleus, these
are called energy shells. The most inner shell (K) contains 2
electron maximum, the L and M shell contain 8 maximum
each.
Organic chemicals: Chemicals are made from living
organisms and contain carbon backbones.
Isomers: Chemicals that have same molecular formula but
different structure formula.
Buffers: Solutions which resist change in pH upon addition
of small amounts of acid or base.
Electrolytes: Chemicals that can release ions into solutions
pH: pH represents the concentration of hydrogen ions [H+] in
solution.
pH = -log [H+]
Enzymes: Proteins that serve as catalysts for biochemical
reactions.
Enthropy: A measure for a system's degree of disorder. It
increases with increasing disorder.
Law of thermodynamics:
The first Law: The total energy of the universe is always
conserved. Energy can neither be created nor destroyed.
The second Law: The universe tends towards maximum
disorder, or, in other words: the direction of all
spontaneous processes is such as to increase the
entropy of a system plus its surroundings
G: Change of free energy of a system.
G negative reaction: spontaneous
G positive reaction: non-spontaneous
Chemical Bonds
O|
Organic Phosphates, R-OPO32-, R-O-P=O
|
OThiols, R-SH
Important Biochemical Molecules
Organic Molecules/macromolecules:
o
o
Polysaccharides
Monomer unit: monosaccharide
Store energy, provide building unit
o
o
Lipids
Monomer unit: fatty acids, glycerol
Store energy, membrane construction, hormones
o
o
Proteins:
Monomer unit: amino acids
Structure protein, enzymes
o
o
Nucleic Acids:
Monomer unit: nucleotides
Genetic material
Chemical Reactions
Coupled reactions: Many biosynthesis reactions are coupled
to ATP hydrolysis which can provide energy and therefore the
overall reaction can be delta G negative.
G negative reaction: spontaneous
G positive reaction: non-spontaneous
Enzyme catalyzed reactions: Lower the activation free
energy but do not change the G.
Biochemical Reaction Types and Enzymes
Oxidation-reduction reactions: oxidoreductase
Chemical bonds store energy. For covalent bonds, the more
electrons a bond share, the more energy it stores.
Ionic bond: ionic bond forms when atoms lose or gain
electrons.
Covalent bond: Covalent bonds form when atoms share
electrons, very strong bonds. The major one in organic
chemicals.
Hydrogen bond: Weak electrical attraction between the
positive end of one molecule and the negative end of another
Intramolecular or intermolecular functional group-transfer
reactions: transfease
Hydrolysis of esters, ethers, and amides: hydrolase
Elimination or addition reactions: lyase.
Isomerization reactions: isomerase
Formation of ester, thiol ester, and amide linkages: ligase
How to Use This Cheat Sheet: These are the keys related this topic. Try to read through it carefully twice then rewrite it out on
a blank sheet of paper. Review it again before the exams.
RapidLearningCenter.com.com
Rapid Learning Inc. All Rights Reserved
Das könnte Ihnen auch gefallen
- ProteinsDokument1 SeiteProteinsChris_Barber09Noch keine Bewertungen
- Cell Biology IntroductionDokument1 SeiteCell Biology IntroductionChris_Barber09100% (1)
- Molecular Cell Biology MasterDokument6 SeitenMolecular Cell Biology Masterdealt100% (5)
- VirusesDokument1 SeiteVirusesChris_Barber09100% (2)
- Anatomy and PhysiologyDokument6 SeitenAnatomy and PhysiologyDelixae Phoinix80% (5)
- Human Physiology IntroductionDokument1 SeiteHuman Physiology IntroductionChris_Barber09Noch keine Bewertungen
- Muscular SystemDokument1 SeiteMuscular SystemChris_Barber09100% (1)
- 01: Introduction To Evolutionary Thought: Key Evolution TermsDokument1 Seite01: Introduction To Evolutionary Thought: Key Evolution TermsBlaine Rogalski100% (2)
- Mitosis and MeiosisDokument1 SeiteMitosis and MeiosisChris_Barber09100% (1)
- Bio Cheat Sheet MasterDokument7 SeitenBio Cheat Sheet MasterChris_Barber0986% (7)
- Peripheral Nervous SystemDokument1 SeitePeripheral Nervous SystemChris_Barber09Noch keine Bewertungen
- Urinary SystemDokument1 SeiteUrinary SystemChris_Barber09100% (1)
- Biotechnoloy Introduction and Application: Table of ContentDokument40 SeitenBiotechnoloy Introduction and Application: Table of ContentnescafeforeverNoch keine Bewertungen
- Autonomic Nervous SystemDokument1 SeiteAutonomic Nervous SystemChris_Barber09Noch keine Bewertungen
- Microbiology MasterDokument13 SeitenMicrobiology MasterDelixae Phoinix100% (6)
- Rate Law GraphsDokument2 SeitenRate Law GraphsChris_Barber09Noch keine Bewertungen
- Biochemistry Notes PDFDokument11 SeitenBiochemistry Notes PDFChris_Barber0950% (6)
- Chemistry MnemonicsDokument16 SeitenChemistry MnemonicsrishikeshkallaNoch keine Bewertungen
- BIOCHEMISTRY, CELL AND MOLECULAR BIOLOGY: Passbooks Study GuideVon EverandBIOCHEMISTRY, CELL AND MOLECULAR BIOLOGY: Passbooks Study GuideNoch keine Bewertungen
- Cheat Sheet For Organic Chemistry Midterm 1 2015 - 1Dokument1 SeiteCheat Sheet For Organic Chemistry Midterm 1 2015 - 1Norma Leticia Ramos33% (3)
- Chemistry MasterDokument7 SeitenChemistry MasterDelixae Phoinix100% (2)
- Practical Biology: For Advanced Level, Medical and Intermediate StudentsVon EverandPractical Biology: For Advanced Level, Medical and Intermediate StudentsNoch keine Bewertungen
- ImmunologyDokument1 SeiteImmunologyChris_Barber0986% (7)
- CHEM Cheat Sheet 3Dokument2 SeitenCHEM Cheat Sheet 3Ruby Rodriguez100% (2)
- OCR Biology F211 Cells, Exchange and Transport Revision NotesDokument4 SeitenOCR Biology F211 Cells, Exchange and Transport Revision Notes24wrightphilip100% (1)
- MCAT Biology Notes 2 PDFDokument23 SeitenMCAT Biology Notes 2 PDFChris_Barber09100% (1)
- BIOL 200 Molecular Biology Lecture NotesDokument44 SeitenBIOL 200 Molecular Biology Lecture NotesDantong JiaNoch keine Bewertungen
- Bacteria: Type of Food Depends On Organism)Dokument5 SeitenBacteria: Type of Food Depends On Organism)Chris_Barber09Noch keine Bewertungen
- Biology NotesDokument6 SeitenBiology NotesElizaNoch keine Bewertungen
- Genetic Cheat SheetDokument11 SeitenGenetic Cheat SheetLeah MaloneyNoch keine Bewertungen
- Preliminary Instructions: Cu Ni CR Fe FeDokument4 SeitenPreliminary Instructions: Cu Ni CR Fe FeEmmanuel Ryan100% (1)
- Cells Cheat SheetDokument2 SeitenCells Cheat SheetUlka Sutar100% (2)
- Naming Organic MoleculesDokument47 SeitenNaming Organic MoleculesSandeep BadarlaNoch keine Bewertungen
- 16: Work, Power, and Energy: Key Physics Terms Key ConceptsDokument1 Seite16: Work, Power, and Energy: Key Physics Terms Key ConceptsBlaine RogalskiNoch keine Bewertungen
- Introduction To Biology ReviewerDokument22 SeitenIntroduction To Biology ReviewerAnton Miguel Jordan83% (6)
- College PhysicsDokument6 SeitenCollege PhysicsDelixae Phoinix100% (1)
- MCAT Biology Notes 3 PDFDokument16 SeitenMCAT Biology Notes 3 PDFChris_Barber09Noch keine Bewertungen
- Phylogenetic Tree Creation Morphological and Molecular Methods For 07-JohnsonDokument35 SeitenPhylogenetic Tree Creation Morphological and Molecular Methods For 07-JohnsonCHRISTEROP100% (2)
- AP Biology - Chapter 30 Discussion AnswersDokument3 SeitenAP Biology - Chapter 30 Discussion Answersangel91me6371100% (6)
- Surviving Chemistry: A Guided Study Book For High School ChemistryDokument83 SeitenSurviving Chemistry: A Guided Study Book For High School ChemistryE3 Scholastic Publishing100% (2)
- MCAT MetabolismDokument4 SeitenMCAT MetabolismNawledge9308100% (1)
- Notes CarbohydratesDokument21 SeitenNotes CarbohydratesChris_Barber09100% (1)
- Genetics NotesDokument6 SeitenGenetics NotesShane Tang100% (2)
- Zoology Notes Class 11Dokument109 SeitenZoology Notes Class 11Suresh chand50% (2)
- Molecular Biology of The Cell Cumulative Final Exam Study GuideDokument22 SeitenMolecular Biology of The Cell Cumulative Final Exam Study Guiderazgriz1211Noch keine Bewertungen
- Chemistry Cheat SheetDokument10 SeitenChemistry Cheat Sheetbrook92% (39)
- Microbiology NotesDokument177 SeitenMicrobiology NotesMarichu Rogas91% (11)
- Organic Chem Midterm 1 Multi+keyDokument1 SeiteOrganic Chem Midterm 1 Multi+keyNorma Leticia RamosNoch keine Bewertungen
- Organic Chemistry NotesDokument9 SeitenOrganic Chemistry NotesBuana SandilaNoch keine Bewertungen
- Phylogenetic Trees Click Learn WorksheetDokument4 SeitenPhylogenetic Trees Click Learn Worksheetfcm31450% (1)
- MCAT Organic Summary SheetDokument6 SeitenMCAT Organic Summary SheetSpencer Thomas100% (2)
- Physics2A CheatSheetDokument1 SeitePhysics2A CheatSheetNastassja LopezNoch keine Bewertungen
- Nmat Biology Cell Biology 1.1 Eukaryotic & Prokaryotic CellsDokument12 SeitenNmat Biology Cell Biology 1.1 Eukaryotic & Prokaryotic Cellssavina100% (1)
- Audio Osmosis - Organic ChemistryDokument9 SeitenAudio Osmosis - Organic ChemistryddNoch keine Bewertungen
- Lecture Notes On Basic Chemistry and Biochemistry (Chapter 2)Dokument10 SeitenLecture Notes On Basic Chemistry and Biochemistry (Chapter 2)Helen ShuangNoch keine Bewertungen
- Jack Westin MCAT Content BiochemistryDokument52 SeitenJack Westin MCAT Content BiochemistryLora100% (2)
- Chapter 2 Chemical Bsis of LifeDokument9 SeitenChapter 2 Chemical Bsis of LifeMary Ann SacramentoNoch keine Bewertungen
- Biochemistry Fundamentals: An Overview of Enzymes, Energy and BioenergeticsDokument58 SeitenBiochemistry Fundamentals: An Overview of Enzymes, Energy and BioenergeticsMarc Imhotep Cray, M.D.100% (1)
- Schaum's Easy Outline of Organic Chemistry, Second EditionVon EverandSchaum's Easy Outline of Organic Chemistry, Second EditionBewertung: 3.5 von 5 Sternen3.5/5 (2)
- Protein Synthesis Notes PDFDokument3 SeitenProtein Synthesis Notes PDFChris_Barber09Noch keine Bewertungen
- Heart Notes PDFDokument2 SeitenHeart Notes PDFChris_Barber09Noch keine Bewertungen
- Reproductive System NotesDokument3 SeitenReproductive System NotesChris_Barber09Noch keine Bewertungen
- Digestive System PDFDokument3 SeitenDigestive System PDFChris_Barber09Noch keine Bewertungen
- Macromolecules PDFDokument11 SeitenMacromolecules PDFChris_Barber09Noch keine Bewertungen
- Rate Law GraphsDokument2 SeitenRate Law GraphsChris_Barber09Noch keine Bewertungen
- Cardiovasculary System PDFDokument5 SeitenCardiovasculary System PDFChris_Barber09Noch keine Bewertungen
- MCAT O-Chem NotesDokument1 SeiteMCAT O-Chem NotesChris_Barber09Noch keine Bewertungen
- Notes CarbohydratesDokument21 SeitenNotes CarbohydratesChris_Barber09100% (1)
- Nucleic Acids and Their Structure: Information?Dokument4 SeitenNucleic Acids and Their Structure: Information?Chris_Barber09Noch keine Bewertungen
- Bacteria: Type of Food Depends On Organism)Dokument5 SeitenBacteria: Type of Food Depends On Organism)Chris_Barber09Noch keine Bewertungen
- CARBSDokument24 SeitenCARBSGulus CfNoch keine Bewertungen
- 107 Rules in PhysicsDokument2 Seiten107 Rules in PhysicsChris_Barber09Noch keine Bewertungen
- Regents Physics Exam Prep: 101 Facts You Should Know: MechanicsDokument3 SeitenRegents Physics Exam Prep: 101 Facts You Should Know: MechanicsChris_Barber09Noch keine Bewertungen
- Aromatic Notes 2 PDFDokument6 SeitenAromatic Notes 2 PDFChris_Barber09100% (1)
- Extra Chirality ProblemsDokument21 SeitenExtra Chirality ProblemsChris_Barber09Noch keine Bewertungen
- Chapter 16:substituent Effects in Aromatic SubstitutionDokument2 SeitenChapter 16:substituent Effects in Aromatic SubstitutionChris_Barber09Noch keine Bewertungen
- Physics Rules 5Dokument10 SeitenPhysics Rules 5Chris_Barber09100% (6)
- Amino Acid NotesDokument15 SeitenAmino Acid NotesChris_Barber09Noch keine Bewertungen
- Physics Rules 2Dokument4 SeitenPhysics Rules 2Chris_Barber09Noch keine Bewertungen
- Physics Rules 1Dokument2 SeitenPhysics Rules 1Chris_Barber09Noch keine Bewertungen
- Advanced Placement Physics Physics OverviewDokument9 SeitenAdvanced Placement Physics Physics OverviewChris_Barber09Noch keine Bewertungen
- MCAT Physics Equation SheetDokument6 SeitenMCAT Physics Equation SheetChris_Barber09Noch keine Bewertungen
- Peripheral Nervous SystemDokument1 SeitePeripheral Nervous SystemChris_Barber09Noch keine Bewertungen
- Sample Essay #1: Progress Often Complicates As Much As It SimplifiesDokument4 SeitenSample Essay #1: Progress Often Complicates As Much As It SimplifiesChris_Barber09Noch keine Bewertungen
- AAMC 9 Essay 1Dokument4 SeitenAAMC 9 Essay 1Chris_Barber09Noch keine Bewertungen
- Essay #1: A Politician's Lifestyle Should Reflect His or Her Political ViewsDokument4 SeitenEssay #1: A Politician's Lifestyle Should Reflect His or Her Political ViewsChris_Barber09Noch keine Bewertungen
- Sample Essay #1: Education Makes Everyone EqualDokument4 SeitenSample Essay #1: Education Makes Everyone EqualChris_Barber09Noch keine Bewertungen
- Sample Essay #1: Advancements in Communication Technology Have Reduced The Quality of Human InteractionDokument6 SeitenSample Essay #1: Advancements in Communication Technology Have Reduced The Quality of Human InteractionChris_Barber09Noch keine Bewertungen
- IS4242 W3 Regression AnalysesDokument67 SeitenIS4242 W3 Regression Analyseswongdeshun4Noch keine Bewertungen
- Calculation Eurocode 2Dokument4 SeitenCalculation Eurocode 2rammirisNoch keine Bewertungen
- 8 Coil PWM Drivers PDFDokument4 Seiten8 Coil PWM Drivers PDFDuzng Hoang TriNoch keine Bewertungen
- Plate - 3 (FLOT)Dokument2 SeitenPlate - 3 (FLOT)patrick dgNoch keine Bewertungen
- ImmunologyDokument8 SeitenImmunologyማላያላም ማላያላም89% (9)
- High Pressure Jet Grouting in TunnelsDokument8 SeitenHigh Pressure Jet Grouting in TunnelsSandeep AggarwalNoch keine Bewertungen
- Unit 6 - EarthingDokument26 SeitenUnit 6 - Earthinggautam100% (1)
- Momus Design CNC Router Manual Version 2.1Dokument178 SeitenMomus Design CNC Router Manual Version 2.1Francisco Teruel100% (8)
- A B C D: Choose Only One Answer For Each QuestionDokument10 SeitenA B C D: Choose Only One Answer For Each QuestionAchitt AchitNoch keine Bewertungen
- Dbms-Lab Assignment - 1: Name - VIKAS SINGH Roll No - 4257Dokument3 SeitenDbms-Lab Assignment - 1: Name - VIKAS SINGH Roll No - 4257Vikas SinghNoch keine Bewertungen
- Leonardo Romero SR High School: Republic of The Philippines Region Xii - Soccsksargen Schools Division Office of CotabatoDokument4 SeitenLeonardo Romero SR High School: Republic of The Philippines Region Xii - Soccsksargen Schools Division Office of CotabatoDulce M. LupaseNoch keine Bewertungen
- FP - ES - 28 - Rindu Grahabhakti Intani - PERMEABLE ENTRY CHARACTERIZATION AT DARAJAT FIELD, WEST JAVA PDFDokument4 SeitenFP - ES - 28 - Rindu Grahabhakti Intani - PERMEABLE ENTRY CHARACTERIZATION AT DARAJAT FIELD, WEST JAVA PDFrindu_intaniNoch keine Bewertungen
- Astm A394 2008 PDFDokument6 SeitenAstm A394 2008 PDFJavier Ricardo Romero BohorquezNoch keine Bewertungen
- Hiley TableDokument5 SeitenHiley TableHanafiahHamzahNoch keine Bewertungen
- 00.concrete Mix Design-RailwayDokument38 Seiten00.concrete Mix Design-RailwaySoundar PachiappanNoch keine Bewertungen
- 2010 Jan-01Dokument32 Seiten2010 Jan-01Shine PrabhakaranNoch keine Bewertungen
- Engg Mechanics Ques BankDokument68 SeitenEngg Mechanics Ques BankUtkalNoch keine Bewertungen
- Nozzle Loads - Part 2 - Piping-EngineeringDokument3 SeitenNozzle Loads - Part 2 - Piping-EngineeringShaikh AftabNoch keine Bewertungen
- Typical Detailing of Reinforcements in Beams and SlabsDokument2 SeitenTypical Detailing of Reinforcements in Beams and SlabsNaveen BansalNoch keine Bewertungen
- Auto-Tune Pid Temperature & Timer General Specifications: N L1 L2 L3Dokument4 SeitenAuto-Tune Pid Temperature & Timer General Specifications: N L1 L2 L3sharawany 20Noch keine Bewertungen
- G3412 - 450 KW Performance DataDokument3 SeitenG3412 - 450 KW Performance DataJacob De CasillasNoch keine Bewertungen
- OcrDokument16 SeitenOcrBeena JaiswalNoch keine Bewertungen
- Python Unit 1Dokument18 SeitenPython Unit 1Rtr. Venkata chetan Joint secretaryNoch keine Bewertungen
- API ISCAN-LITE ScannerDokument4 SeitenAPI ISCAN-LITE Scannergrg_greNoch keine Bewertungen
- Magnetophoresis and Electromagnetophoresis of Microparticles in LiquidsDokument7 SeitenMagnetophoresis and Electromagnetophoresis of Microparticles in Liquids3issazakaNoch keine Bewertungen
- Asme Ix Test 1Dokument8 SeitenAsme Ix Test 1RedzuanNoch keine Bewertungen
- A Report On Traffic Volume StudyDokument33 SeitenA Report On Traffic Volume StudyManoj Durairaj100% (1)
- Glpi Developer DocumentationDokument112 SeitenGlpi Developer Documentationvictorlage7Noch keine Bewertungen
- App NandDokument30 SeitenApp NandRajesh MedampudiNoch keine Bewertungen
- Configuration A: Unloaded BJT Transistor AmplifiersDokument3 SeitenConfiguration A: Unloaded BJT Transistor AmplifiersdasdNoch keine Bewertungen