Beruflich Dokumente
Kultur Dokumente
Proteins
Hochgeladen von
Chris_Barber090 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
544 Ansichten1 SeiteProteins
Copyright
© © All Rights Reserved
Verfügbare Formate
PDF, TXT oder online auf Scribd lesen
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenProteins
Copyright:
© All Rights Reserved
Verfügbare Formate
Als PDF, TXT herunterladen oder online auf Scribd lesen
0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
544 Ansichten1 SeiteProteins
Hochgeladen von
Chris_Barber09Proteins
Copyright:
© All Rights Reserved
Verfügbare Formate
Als PDF, TXT herunterladen oder online auf Scribd lesen
Sie sind auf Seite 1von 1
Biochemistry - Core Concept Cheat Sheet
07: All about Proteins
Key Terms
Antibody: A specific protein that interacts with a foreign
substance (antigen) in a specific way.
Beta-sheet (-sheet): A sheet like structure formed by
the interaction between two or more extended
polypeptide chains.
Cytoskeleton: The filamentous skeleton, formed in the
eukaryotic cytoplasm that is largely responsible for
controlling cell shape.
Dalton: A unit of mass equivalent to the mass of a
hydrogen atom (1.66 x 10-24 g)
Disulfide Bridge: A covalent linkage formed between
two cysteine-SH groups either in the same polypeptide
chain or in different polypeptide chains.
Enzyme: A molecule, most often a protein that contains a
catalytic site for a biochemical reaction.
Globular protein: A folded protein that adopts an
approximately globular shape. May also be called soluble
proteins.
Glycoprotein: A protein linked to an oligosaccharide or a
polysaccharide. Glycosaminoglycans. Long, unbranched
polysaccharide chains composed of repeating disaccharide
subunits in which one of the two sugars is either Nacetylglucosamine or N-acetylgalactosamine.
Nuclear Magnetic Resonance: NMR is a phenomenon
which occurs when the nuclei of certain atoms are
immersed in a static magnetic field and exposed to a
second oscillating magnetic field. Some nuclei experience
this phenomenon, and others do not, dependent upon
whether they possess a property called spin.
Polypeptide:
A linear polymer of amino acids held
together by peptide bonds.
Inducible proteins: Those which are synthesized in
different amounts depending on cellular signals.
Isoelectric point or pH: The pH at which a protein has
no net charge.
Membrane Protein: A protein associated with a
membrane, rather than found free in the cell. A membrane
protein may be integral (embedded or buried) in the
membrane, or peripheral (attached more loosely to the
membrane.
Myosin: The main protein of the thick filaments in a
muscle myofibril. It is composed of two coiled subunits (Mr
about 220,000) that can aggregate to form a thick
filament, which is globular at each end.
Structural Protein: A protein that serves a structural
function.
Transport Protein: A protein whose primary function is
to transport a substance from one part of the cell to
another, from one cell to another, or from one tissue to
another.
Unwinding Proteins: Proteins that help to unwind
double-stranded DNA during DNA replication.
X-ray crystallography: is an experimental technique that
exploits the fact that X-rays are diffracted by crystals.
Zymogen: An inactive precursor of an enzyme. For
example, trypsin exists in the inactive form trypsinogen
before it is converted to its active form, trypsin.
Amino Acids Building Blocks
pKa: is defined as negative logarithm of Ka (the
dissociation constant for a acid). Since there are two
groups viz. amino and carboxyl. In an amino acid both of
them will dissociate at a particular pH. Therefore we get
two pKa values (pK1 and pK2).
pI: is the isoelectric point where the net charge on the
amino acid is zero and is the average of pK1 and pK2.
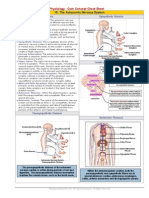
The box below explains about the proteins with hemoglobin as example
The picture shows the 4 monomeric
subunits of Hemoglobin; they are
colored differently for clarity.
The individual subunits are
polypeptide chains.
This picture represents the quaternary
structure of the protein.
There are two alpha and two beta
subunits.
The 3D structure is important for its
function.
3D structure of Hemoglobin
The 3D structure of the protein is dependent on the
amino acids in the sequence.
The nature of the side chain of the amino acid and
its dissociation under a particular pH is also
important for the 3D structure.
If there be any change in its structure (e.g. a
mutation as in SCA it results in defective binding of
oxygen resulting in severe anemia.
Four molecules of oxygen bind to hemoglobin.
The binding of first oxygen enhances the binding of
successive molecules a property called positive
cooperativity.
The main function of Hemoglobin is to
transport oxygen to the tissues in the
body and to remove the carbon
dioxide from them.
The binding of hemoglobin to oxygen
is very much related to its native
structure.
If there be any change in its structure
(e.g. A mutation as in SCA, it results
in defective binding of oxygen
resulting in severe anemia.
The angles between various atoms in a peptide
bond are important.
Because those angles and their freedom of
rotation only determines the type of secondary
structure.
A typical example is collagen; a triple helical
protein is with a predominantly alpha helical
structure.
Its function is fully dependent on its triple helical
structure.
Ramachandrans plot explains about the type of
secondary structure formed with given set of psi
and phi angles.
How to Use This Cheat Sheet: These are the keys related to this topic. Try to read through it carefully twice then recite it out on
a blank sheet of paper. Review it again before the exams.
RapidLearningCenter.com
Rapid Learning Inc. All Rights Reserved
Das könnte Ihnen auch gefallen
- Physics Rules 5Dokument10 SeitenPhysics Rules 5Chris_Barber09100% (6)
- Fundamental of Organic ChemistryDokument11 SeitenFundamental of Organic ChemistryBernie Suarez100% (1)
- MCAT Hormones and EnzymesDokument2 SeitenMCAT Hormones and EnzymesDevie Saha100% (3)
- Organic Chemistry 1Dokument324 SeitenOrganic Chemistry 1Bellony Sanders100% (7)
- Microbiology MasterDokument13 SeitenMicrobiology MasterDelixae Phoinix100% (6)
- Hormone regulation and the hypothalamus-pituitary axisDokument16 SeitenHormone regulation and the hypothalamus-pituitary axisCal GoNoch keine Bewertungen
- BiochemistryDokument6 SeitenBiochemistryBlaine Rogalski100% (14)
- 01: Introduction To Evolutionary Thought: Key Evolution TermsDokument1 Seite01: Introduction To Evolutionary Thought: Key Evolution TermsBlaine Rogalski100% (2)
- High School Biology Core ConceptsDokument7 SeitenHigh School Biology Core ConceptsChris_Barber0986% (7)
- Cell Biology IntroductionDokument1 SeiteCell Biology IntroductionChris_Barber09100% (1)
- Biochemistry Notes PDFDokument11 SeitenBiochemistry Notes PDFChris_Barber0950% (6)
- Organic Chemistry Compounds 4Dokument33 SeitenOrganic Chemistry Compounds 4silvio1980Noch keine Bewertungen
- Mitosis and MeiosisDokument1 SeiteMitosis and MeiosisChris_Barber09100% (1)
- Chemistry MnemonicsDokument16 SeitenChemistry MnemonicsrishikeshkallaNoch keine Bewertungen
- Amino Acid NotesDokument15 SeitenAmino Acid NotesChris_Barber09Noch keine Bewertungen
- ImmunologyDokument1 SeiteImmunologyChris_Barber0986% (7)
- Molecular Cell Biology MasterDokument6 SeitenMolecular Cell Biology Masterdealt100% (5)
- MCAT Organic Summary SheetDokument6 SeitenMCAT Organic Summary SheetSpencer Thomas100% (2)
- Anatomy and PhysiologyDokument6 SeitenAnatomy and PhysiologyDelixae Phoinix80% (5)
- VirusesDokument1 SeiteVirusesChris_Barber09100% (2)
- Cheat Sheet For Organic Chemistry Midterm 1 2015 - 1Dokument1 SeiteCheat Sheet For Organic Chemistry Midterm 1 2015 - 1Norma Leticia Ramos33% (3)
- CHEM Cheat Sheet 3Dokument2 SeitenCHEM Cheat Sheet 3Ruby Rodriguez100% (2)
- Selective Attention and Problem Solving TechniquesDokument28 SeitenSelective Attention and Problem Solving TechniquesMahdeeHaqueSyed100% (2)
- MCAT Gen Chem NotesDokument8 SeitenMCAT Gen Chem NotesViviana PerezNoch keine Bewertungen
- Protein Synthesis Notes PDFDokument3 SeitenProtein Synthesis Notes PDFChris_Barber09Noch keine Bewertungen
- Protein Synthesis Notes PDFDokument3 SeitenProtein Synthesis Notes PDFChris_Barber09Noch keine Bewertungen
- College PhysicsDokument6 SeitenCollege PhysicsDelixae Phoinix100% (1)
- Autonomic Nervous System Cheat Sheet - Key Terms & DivisionsDokument1 SeiteAutonomic Nervous System Cheat Sheet - Key Terms & DivisionsChris_Barber09Noch keine Bewertungen
- Types of Organic ReactionsDokument31 SeitenTypes of Organic ReactionsNurulMAprilia80% (5)
- Biochemistry NotesDokument90 SeitenBiochemistry Notespatialokkumar100% (2)
- Biology NotesDokument42 SeitenBiology NotesNate VojtikNoch keine Bewertungen
- Molecular BiologyDokument54 SeitenMolecular BiologyAnonymous GhQ1eIyxunNoch keine Bewertungen
- A2 Biology Notes Cellular RespirationDokument20 SeitenA2 Biology Notes Cellular RespirationArnel100% (1)
- General Chemistry MCAT - 1Dokument63 SeitenGeneral Chemistry MCAT - 1pparik10100% (2)
- Chemical Basis of LifeDokument1 SeiteChemical Basis of LifeChris_Barber09100% (2)
- MCAT ReviewDokument114 SeitenMCAT Reviewjustinwendel100% (4)
- MCAT Biology Complete OutlinesDokument34 SeitenMCAT Biology Complete OutlinesJacob Mikhail90% (10)
- MCAT Prep Organic Equation SheetDokument6 SeitenMCAT Prep Organic Equation SheetChris_Barber09Noch keine Bewertungen
- Rate Law GraphsDokument2 SeitenRate Law GraphsChris_Barber09Noch keine Bewertungen
- Molecular Biology - DNA and Protein SynthesisDokument23 SeitenMolecular Biology - DNA and Protein SynthesisChris_Barber09100% (1)
- MCAT Biology Notes 3 PDFDokument16 SeitenMCAT Biology Notes 3 PDFChris_Barber09Noch keine Bewertungen
- Human Physiology IntroductionDokument1 SeiteHuman Physiology IntroductionChris_Barber09Noch keine Bewertungen
- 6 Biochemistry Map PDFDokument2 Seiten6 Biochemistry Map PDFDipesh ShresthaNoch keine Bewertungen
- Notes CarbohydratesDokument21 SeitenNotes CarbohydratesChris_Barber09100% (1)
- Summary Essential Cell Biology Chapters 4678121516 1718 and 20Dokument36 SeitenSummary Essential Cell Biology Chapters 4678121516 1718 and 20mohamed arbi taifNoch keine Bewertungen
- Cell Biology Exam 1 ReaDokument7 SeitenCell Biology Exam 1 ReaCsa At Uh100% (3)
- Day 3 - Transcription and RNA ProcessingDokument50 SeitenDay 3 - Transcription and RNA ProcessingAniket ParabNoch keine Bewertungen
- Physics Rules 4Dokument6 SeitenPhysics Rules 4Chris_Barber09Noch keine Bewertungen
- Muscular SystemDokument1 SeiteMuscular SystemChris_Barber09100% (1)
- Urinary SystemDokument1 SeiteUrinary SystemChris_Barber09100% (1)
- Peripheral Nervous SystemDokument1 SeitePeripheral Nervous SystemChris_Barber09Noch keine Bewertungen
- Biotechnoloy Introduction and Application: Table of ContentDokument40 SeitenBiotechnoloy Introduction and Application: Table of ContentnescafeforeverNoch keine Bewertungen
- MCAT Physics Equation SheetDokument3 SeitenMCAT Physics Equation SheetYui YuiNoch keine Bewertungen
- Bio MCAT NotesDokument2 SeitenBio MCAT NotesJuan DeSantosNoch keine Bewertungen
- Final Exam Study Guide TipsDokument22 SeitenFinal Exam Study Guide Tipsrazgriz1211Noch keine Bewertungen
- Cheat Sheet (Bio) 1Dokument1 SeiteCheat Sheet (Bio) 1Kelvin KohNoch keine Bewertungen
- Khan Academy Notes - CellsDokument42 SeitenKhan Academy Notes - Cellsmememe123123100% (1)
- Bacteria: Type of Food Depends On Organism)Dokument5 SeitenBacteria: Type of Food Depends On Organism)Chris_Barber09Noch keine Bewertungen
- DNA Structure and Replication Lecture QuestionsDokument3 SeitenDNA Structure and Replication Lecture QuestionsAyodejiES1Noch keine Bewertungen
- BiochemistryDokument113 SeitenBiochemistryMohammed Faizuddin siddiquiNoch keine Bewertungen
- Viruses, Cells, Immune System DefencesDokument5 SeitenViruses, Cells, Immune System DefencesGlaukaNoch keine Bewertungen
- Examkrackers General Chemistry NotesDokument16 SeitenExamkrackers General Chemistry NotesddNoch keine Bewertungen
- Sinh Hóa HọcDokument375 SeitenSinh Hóa HọcCẩm TúNoch keine Bewertungen
- He Cell 1Dokument34 SeitenHe Cell 1Le Phuong LyNoch keine Bewertungen
- Biosynthesis of CollagenDokument40 SeitenBiosynthesis of CollagenNausheerNoch keine Bewertungen
- Biochemistry LN05-2Dokument12 SeitenBiochemistry LN05-2Rahaf Al-muhtasebNoch keine Bewertungen
- Solved BiochemDokument24 SeitenSolved Biochemrashminagar611Noch keine Bewertungen
- Tutorial 4: Abhijeet Das J070Dokument5 SeitenTutorial 4: Abhijeet Das J070Abhijeet DasNoch keine Bewertungen
- BSC 2010: Explore Macromolecules and Their FunctionsDokument11 SeitenBSC 2010: Explore Macromolecules and Their FunctionsChris_Barber09Noch keine Bewertungen
- Heart Notes PDFDokument2 SeitenHeart Notes PDFChris_Barber09Noch keine Bewertungen
- Reproductive System NotesDokument3 SeitenReproductive System NotesChris_Barber09Noch keine Bewertungen
- Digestive System PDFDokument3 SeitenDigestive System PDFChris_Barber09Noch keine Bewertungen
- Bacteria: Type of Food Depends On Organism)Dokument5 SeitenBacteria: Type of Food Depends On Organism)Chris_Barber09Noch keine Bewertungen
- Cardiovasculary System PDFDokument5 SeitenCardiovasculary System PDFChris_Barber09Noch keine Bewertungen
- Notes CarbohydratesDokument3 SeitenNotes CarbohydratesChris_Barber09Noch keine Bewertungen
- Nucleic Acids and Their Structure: Information?Dokument4 SeitenNucleic Acids and Their Structure: Information?Chris_Barber09Noch keine Bewertungen
- Notes RNA and DNADokument23 SeitenNotes RNA and DNAChris_Barber09Noch keine Bewertungen
- MCAT O-Chem NotesDokument1 SeiteMCAT O-Chem NotesChris_Barber09Noch keine Bewertungen
- Aromatic Notes 2 PDFDokument6 SeitenAromatic Notes 2 PDFChris_Barber09100% (1)
- Extra Chirality ProblemsDokument21 SeitenExtra Chirality ProblemsChris_Barber09Noch keine Bewertungen
- Carbs: Nature's Most Abundant MoleculesDokument24 SeitenCarbs: Nature's Most Abundant MoleculesGulus CfNoch keine Bewertungen
- AP Physics 1 Review: Essential Equations and ConceptsDokument9 SeitenAP Physics 1 Review: Essential Equations and ConceptsChris_Barber09Noch keine Bewertungen
- Leaving GroupsDokument1 SeiteLeaving GroupsChris_Barber09Noch keine Bewertungen
- Chapter 16:substituent Effects in Aromatic SubstitutionDokument2 SeitenChapter 16:substituent Effects in Aromatic SubstitutionChris_Barber09Noch keine Bewertungen
- Physics Equation SheetDokument2 SeitenPhysics Equation SheetChris_Barber09Noch keine Bewertungen
- MCAT Physics Equation SheetDokument6 SeitenMCAT Physics Equation SheetChris_Barber09Noch keine Bewertungen
- Regents Physics Exam Prep: 101 Facts You Should Know: MechanicsDokument3 SeitenRegents Physics Exam Prep: 101 Facts You Should Know: MechanicsChris_Barber09Noch keine Bewertungen
- Physics Rules 1Dokument2 SeitenPhysics Rules 1Chris_Barber09Noch keine Bewertungen
- Physics Rules 2Dokument4 SeitenPhysics Rules 2Chris_Barber09Noch keine Bewertungen
- 107 Rules in PhysicsDokument2 Seiten107 Rules in PhysicsChris_Barber09Noch keine Bewertungen
- AAMC 11 Essay 1Dokument6 SeitenAAMC 11 Essay 1Chris_Barber09Noch keine Bewertungen
- AAMC 11 Essay 2Dokument5 SeitenAAMC 11 Essay 2Chris_Barber09Noch keine Bewertungen
- Palmea Cooking Oil Nutrient AnalysisDokument5 SeitenPalmea Cooking Oil Nutrient AnalysisLeslie Darwin DumasNoch keine Bewertungen
- Biological MoleculesDokument38 SeitenBiological MoleculesmuzammalNoch keine Bewertungen
- Biomolecule ChartDokument5 SeitenBiomolecule ChartRachel WrightNoch keine Bewertungen
- ECS129 Quiz 8 Secondary Structure PredictionDokument5 SeitenECS129 Quiz 8 Secondary Structure PredictionSasikala RajendranNoch keine Bewertungen
- Structure and Function of DNA Presentation in Colorful Retro Illustrative StyleDokument51 SeitenStructure and Function of DNA Presentation in Colorful Retro Illustrative Stylekimmymantos022Noch keine Bewertungen
- Letter: de Novo Protein Design by Citizen ScientistsDokument19 SeitenLetter: de Novo Protein Design by Citizen ScientistsLivsNoch keine Bewertungen
- Lactobaciluss LactisDokument12 SeitenLactobaciluss LactisHary Peace LoveerNoch keine Bewertungen
- Amino Acids and Proteins ReviewerDokument5 SeitenAmino Acids and Proteins ReviewerDaine MarconNoch keine Bewertungen
- Experiment 10 Properties of Lipids Fats and OilsDokument8 SeitenExperiment 10 Properties of Lipids Fats and OilsMark JNoch keine Bewertungen
- Amino Acids and ProteinsDokument58 SeitenAmino Acids and ProteinsJAPASHANoch keine Bewertungen
- LipidsDokument13 SeitenLipidsFormulae GurujiNoch keine Bewertungen
- B.Sc. Degree Examination, NOVEMBER-2020 Third Semester Catering and Hotel Administration Nutrition and Food Science (2018 Onwards)Dokument3 SeitenB.Sc. Degree Examination, NOVEMBER-2020 Third Semester Catering and Hotel Administration Nutrition and Food Science (2018 Onwards)Kumar UdayaNoch keine Bewertungen
- BCHMDokument2 SeitenBCHMKaren CasaulNoch keine Bewertungen
- LIPIDSDokument2 SeitenLIPIDSAucel Jade ZafraNoch keine Bewertungen
- Pengujian Sifat Fisik Dan Kimiawi ProteinDokument12 SeitenPengujian Sifat Fisik Dan Kimiawi ProteinNurfy LuthfiatiNoch keine Bewertungen
- Lecture 1 Pendahuluan BiokatalisDokument18 SeitenLecture 1 Pendahuluan BiokatalisSabar SitioNoch keine Bewertungen
- Inbound 2949823744268348325Dokument15 SeitenInbound 2949823744268348325Tristan SiocoNoch keine Bewertungen
- Dna, Rna, Dan Protein 1Dokument26 SeitenDna, Rna, Dan Protein 1R yNoch keine Bewertungen
- Mangrovite Belting Limited, Tanners and Manufacturers of Leather and Raw Hide, Belting, Belt Laces, Mechanical, Leathers: Price ListDokument30 SeitenMangrovite Belting Limited, Tanners and Manufacturers of Leather and Raw Hide, Belting, Belt Laces, Mechanical, Leathers: Price ListAndrea GrazioliNoch keine Bewertungen
- Compare top fish oil brands by price, concentration and reviewsDokument137 SeitenCompare top fish oil brands by price, concentration and reviewsLim SoonhoeNoch keine Bewertungen
- Crash Course KEY NOTES on BiomoleculesDokument6 SeitenCrash Course KEY NOTES on BiomoleculesDebkanta Roy0% (1)
- Farmakognosi: Dyke Gita Wirasisya, S.Farm., M.SC., AptDokument24 SeitenFarmakognosi: Dyke Gita Wirasisya, S.Farm., M.SC., AptRandi RasyidNoch keine Bewertungen
- List of Ingredients - OrganicDokument13 SeitenList of Ingredients - OrganicedmondjoshiNoch keine Bewertungen
- Beard - Oil FormulationDokument1 SeiteBeard - Oil Formulationhasib.m1040Noch keine Bewertungen
- Functions of Each of The Following PeptidesDokument3 SeitenFunctions of Each of The Following Peptidesj9Noch keine Bewertungen
- Catalase Activity WorksheetDokument94 SeitenCatalase Activity WorksheetAbuzettin BakiroğluNoch keine Bewertungen
- MPOA Gala Dinner 2023 Joseph Tek Tribute To OPDokument31 SeitenMPOA Gala Dinner 2023 Joseph Tek Tribute To OPMohammad AisamuddinNoch keine Bewertungen
- 12 Chap 2 (Biological Molecules) F.SC 1st Year Biology Helping Notes PDFDokument10 Seiten12 Chap 2 (Biological Molecules) F.SC 1st Year Biology Helping Notes PDFZainab fatimaNoch keine Bewertungen