Beruflich Dokumente
Kultur Dokumente
Common Questions Heat Pipes PDF
Hochgeladen von
Teo Pei San0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
250 Ansichten2 SeitenHeat pipes are evacuated vessels, typically circular in cross sections, which are back-filled with a small quantity of a working fluid. Heat pipes are totally passive and are used to transfer heat from a heat source to a heat sink with minimal temperature gradients, or to isothermalize surfaces. Heat pipe working fluids range from Helium and Nitrogen, for cryogenic temperatures, to liquid metals like Sodium and Potassium for high temperature applications.
Originalbeschreibung:
Originaltitel
common-questions-heat-pipes.pdf
Copyright
© © All Rights Reserved
Verfügbare Formate
PDF, TXT oder online auf Scribd lesen
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenHeat pipes are evacuated vessels, typically circular in cross sections, which are back-filled with a small quantity of a working fluid. Heat pipes are totally passive and are used to transfer heat from a heat source to a heat sink with minimal temperature gradients, or to isothermalize surfaces. Heat pipe working fluids range from Helium and Nitrogen, for cryogenic temperatures, to liquid metals like Sodium and Potassium for high temperature applications.
Copyright:
© All Rights Reserved
Verfügbare Formate
Als PDF, TXT herunterladen oder online auf Scribd lesen
0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
250 Ansichten2 SeitenCommon Questions Heat Pipes PDF
Hochgeladen von
Teo Pei SanHeat pipes are evacuated vessels, typically circular in cross sections, which are back-filled with a small quantity of a working fluid. Heat pipes are totally passive and are used to transfer heat from a heat source to a heat sink with minimal temperature gradients, or to isothermalize surfaces. Heat pipe working fluids range from Helium and Nitrogen, for cryogenic temperatures, to liquid metals like Sodium and Potassium for high temperature applications.
Copyright:
© All Rights Reserved
Verfügbare Formate
Als PDF, TXT herunterladen oder online auf Scribd lesen
Sie sind auf Seite 1von 2
TECHNICAL DATA SHEET
COMMON QUESTIONS ABOUT HEAT PIPES
Heat
In
Heat Out
Wick
Liquid Turning to Vapor
What is a heat pipe?
A heat pipe is a heat transfer device with an extremely high effective thermal conductivity. Heat pipes are evacuated
vessels, typically circular in cross sections, which are back-filled with a small quantity of a working fluid. They are totally
passive and are used to transfer heat from a heat source to a heat sink with minimal temperature gradients, or to
isothermalize surfaces.
How do they work?
Heat pipes transfer heat by the evaporation and condensation of a working fluid. As stated above, a heat pipe is a vacuum
tight vessel which is evacuated and partially back-filled with a working fluid. As heat is input at the evaporator, fluid is
vaporized, creating a pressure gradient in the pipe. This pressure gradient forces the vapor to flow along the pipe to the
cooler section where it condenses, giving up its latent heat of vaporization. The working fluid is then returned to the
evaporator by capillary forces developed in the porous wick structure or by gravity.
Do heat pipes work against gravity?
Yes, a heat pipe is said to be operating against gravity when the evaporator is located above the condenser. In this
orientation, the working fluid must be pumped against gravity back to the evaporator. All heat pipes have wick structures
that pump the working fluid back to the evaporator using the capillary pressure developed in the porous wick. The finer the
pore radius of a wick structure, the higher against gravity the heat pipe can operate. A thermosyphon is similar to a heat
pipe, but has no wick structure and will only operate gravity aided.
What fluids are used in heat pipes?
Heat pipe working fluids range from Helium and Nitrogen, for cryogenic temperatures, to liquid metals like Sodium and
Potassium for high temperature applications. Some of the more common heat pipe fluids used for electronics cooling
applications are ammonia, water, acetone, and methanol. Thermacore has experience designing, developing, and
manufacturing heat pipes with over 22 different working fluids for a variety of applications from cryogenic (-250C) to high
temperature (>1000C).
Corporate Headquarters
780 Eden Road
Lancaster, PA 17601-4794 USA
Phone: 717-569-6551
info@thermacore.com
www.thermacore.com
Page 1 of 2
Thermacore 2010. All rights reserved.
Subject to change without noticeNovember 2010
TECHNICAL DATA SHEET
Why does a water heat pipe work below 100C?
Water at atmospheric pressure boils at 100C. Inside a heat pipe, the working fluid (water) is not at atmospheric pressure.
The internal pressure of the heat pipe is the saturation pressure of the fluid at the corresponding fluid temperature. As
such, the fluid in a heat pipe will boil at any temperature above its freezing point. Therefore, at room temperature (20C), a
water heat pipe is under partial vacuum, and the heat pipe will boil as soon as heat is input.
What are heat pipes used for?
Heat pipes are used for a wide variety of applications covering the complete spectrum of heat transfer applications. Heat
pipes are ideal for any application where heat must be transferred with a minimum thermal gradient either to increase the
size of heat sink, to relocate the sink to a remote location, or where isothermal surfaces are required. Typical applications
include electronics cooling, isothermal furnace liners, and heat transfer for space applications.
What is the thermal conductivity of a heat pipe?
Heat pipes do not have a set thermal conductivity like solid materials due to the two phase heat transfer. Instead, the
effective thermal conductivity improves with length. For example, a 4 inch long heat pipe carrying 100 watts will have
close to the same thermal gradient as a 12 inch long pipe carrying the same power. Thus the 12 inch pipe would have a
higher effective thermal conductivity. Unlike solid materials, a heat pipe's effective thermal conductivity will also change
with the amount of power being transferred and with the evaporator and condenser sizes. For a well-designed heat pipe,
effective thermal conductivities can range from 10 to 10,000 times the effective thermal conductivity of copper depending
on the length of the heat pipe.
Are heat pipes reliable?
Since heat pipes have no moving parts, they are extremely reliable. This is the main reason they are used extensively in
space applications where maintenance is not available. The main cause of heat pipe failures is gas generation in the heat
pipe. This problem is totally eliminated by proper cleaning and assembly procedures.
Are they expensive?
Compared to less effective methods of heat transfer such as aluminum extrusions and cast heat sinks, heat pipes can be
expensive, especially in small quantities. In applications where cooling requirements can be met by simple conductive
heat sinks, heat pipes are not recommended. In more demanding applications, however, the cost of heat pipes is
competitive with other alternatives. The cost of heat pipes is also partially offset by the improvement in system reliability
and the increased life of cooler running electronics. In high quantity applications, the cost of heat pipes drops significantly
and often provides the most economical solution to many cooling applications.
Do water heat pipes freeze?
Yes, heat pipe working fluids including water maintain the normal freezing point. Properly designed heat pipes, however,
will not be damaged by the freezing and thawing of the working fluid. Heat pipes will not operate until the temperature
rises above the freezing temperature of the fluid.
Corporate Headquarters
780 Eden Road
Lancaster, PA 17601-4794 USA
Phone: 717-569-6551
info@thermacore.com
www.thermacore.com
Page 2 of 2
Thermacore 2010. All rights reserved.
Subject to change without noticeNovember 2010
Das könnte Ihnen auch gefallen
- Plate Type Heat Exchanger Project Report 5Dokument26 SeitenPlate Type Heat Exchanger Project Report 5ParthivNoch keine Bewertungen
- Handbook of Thermal Conductivity, Volume 1: Organic Compounds C1 to C4Von EverandHandbook of Thermal Conductivity, Volume 1: Organic Compounds C1 to C4Bewertung: 5 von 5 Sternen5/5 (1)
- Measuring Temp Distribution in a Linear Heat BarDokument52 SeitenMeasuring Temp Distribution in a Linear Heat BarM Junaid tabassumNoch keine Bewertungen
- Designn of Heat ExchangerDokument53 SeitenDesignn of Heat ExchangerBalu BalireddiNoch keine Bewertungen
- Heat ExchangerDokument33 SeitenHeat ExchangerKhloud MadihNoch keine Bewertungen
- Refrigerant Selection CriteriaDokument7 SeitenRefrigerant Selection CriteriazetseatNoch keine Bewertungen
- Department of Mechanical Engineering Indian Institute of Engineering Science and Technology, ShibpurDokument15 SeitenDepartment of Mechanical Engineering Indian Institute of Engineering Science and Technology, ShibpurSuraj Kumar0% (1)
- More ExercisesDokument3 SeitenMore ExercisesAnas Shatnawi100% (1)
- Heat Exchanger Sample Report 2018Dokument55 SeitenHeat Exchanger Sample Report 2018RahulSrivastava100% (1)
- Heat & Mass Transfer Question BankDokument4 SeitenHeat & Mass Transfer Question BankBhavesh Kapil50% (2)
- Lecture-17: Multi-Stage Vapour Compression Refrigeration SystemsDokument13 SeitenLecture-17: Multi-Stage Vapour Compression Refrigeration SystemsMuhaamad TiloNoch keine Bewertungen
- LMTD Vs AMTDDokument1 SeiteLMTD Vs AMTDMujahidNoch keine Bewertungen
- Shell and Tube Heat ExchangerDokument34 SeitenShell and Tube Heat ExchangerJimNoch keine Bewertungen
- Heat 4e Chap11 LectureDokument32 SeitenHeat 4e Chap11 LectureSalim ChohanNoch keine Bewertungen
- Everything You Need to Know About BHEL's Heat Exchanger and Condenser ManufacturingDokument37 SeitenEverything You Need to Know About BHEL's Heat Exchanger and Condenser ManufacturingShubham Dubey100% (2)
- Heat Exchanger Lab Report FinalDokument22 SeitenHeat Exchanger Lab Report FinalAliyu AbdulqadirNoch keine Bewertungen
- ME 2251 Heat Transfer Concepts and EquationsDokument11 SeitenME 2251 Heat Transfer Concepts and Equationsதியாகராஜன் அரிதாஸ்Noch keine Bewertungen
- Heat Exchangers LectureDokument37 SeitenHeat Exchangers LectureTerna Orlanda100% (2)
- General Interview Question For Chem-EDokument3 SeitenGeneral Interview Question For Chem-EDan LaNoch keine Bewertungen
- Heat Exchangers Review StudyDokument11 SeitenHeat Exchangers Review StudyDharmendra PrajapatiNoch keine Bewertungen
- Boiling and CondensationDokument12 SeitenBoiling and CondensationKarthik SadasivuniNoch keine Bewertungen
- Introduction To Microscale Heat TransferDokument14 SeitenIntroduction To Microscale Heat TransferManoj Kumar MoharanaNoch keine Bewertungen
- Solving Heat Transfer Problems Using Transient Conduction AnalysisDokument103 SeitenSolving Heat Transfer Problems Using Transient Conduction Analysismaria xNoch keine Bewertungen
- Heat Transfer Course Overview at NUSTDokument46 SeitenHeat Transfer Course Overview at NUSTabdullah1sNoch keine Bewertungen
- Assignment 2Dokument28 SeitenAssignment 2ahsan aliNoch keine Bewertungen
- Nitrile Rubber InsulationDokument2 SeitenNitrile Rubber Insulationavid_ankurNoch keine Bewertungen
- Geothermal HTE for Hydrogen ProductionDokument9 SeitenGeothermal HTE for Hydrogen ProductionAnonymous d2K8lZPRugNoch keine Bewertungen
- Heat Exchangers ReportDokument16 SeitenHeat Exchangers Report刘羿村Noch keine Bewertungen
- 1 History of Refrigeration: LessonDokument1.051 Seiten1 History of Refrigeration: LessonJonathanE.TambanNoch keine Bewertungen
- Vapor Compression Refrigration PDFDokument155 SeitenVapor Compression Refrigration PDFمحمد متوليNoch keine Bewertungen
- Cryogenic TurboexpandersDokument9 SeitenCryogenic TurboexpandersDwinaRahmayaniNoch keine Bewertungen
- RefrigerantDokument59 SeitenRefrigerantObula Reddy KNoch keine Bewertungen
- HEAT TRANSFER EQUIPMENT DESIGN AND TYPESDokument26 SeitenHEAT TRANSFER EQUIPMENT DESIGN AND TYPESJazer Mari CantosNoch keine Bewertungen
- Mass Transfer Part 8Dokument54 SeitenMass Transfer Part 8SreejithNoch keine Bewertungen
- Heat Exchanger PDFDokument9 SeitenHeat Exchanger PDFsunita45Noch keine Bewertungen
- Heat Transfer:: Differences Between Thermodynamics andDokument11 SeitenHeat Transfer:: Differences Between Thermodynamics andNitin KumarNoch keine Bewertungen
- Heat ExchangerDokument32 SeitenHeat ExchangerRaj Khasnobish100% (1)
- ME 8391 Engineering Thermodynamics Workbook - UNIT 1Dokument154 SeitenME 8391 Engineering Thermodynamics Workbook - UNIT 1BIBIN CHIDAMBARANATHANNoch keine Bewertungen
- PERFORMANCE ANALYSIS OF INTERNAL HEAT EXCHANGERDokument39 SeitenPERFORMANCE ANALYSIS OF INTERNAL HEAT EXCHANGERNeal Christian ParatoNoch keine Bewertungen
- Heat and Mass Transfer NotesDokument48 SeitenHeat and Mass Transfer Notessanthanam102Noch keine Bewertungen
- Projeect PPT-1Dokument28 SeitenProjeect PPT-1Shubham PawarNoch keine Bewertungen
- Exp. 5 Heat Transfer Study On Plate Heat ExchangerDokument6 SeitenExp. 5 Heat Transfer Study On Plate Heat ExchangerElaine PuiNoch keine Bewertungen
- RAC - Unit 5 - Types of EvaporatorsDokument12 SeitenRAC - Unit 5 - Types of EvaporatorsSAATVIK JAINNoch keine Bewertungen
- Radiation Heat Transfer ExperimentDokument11 SeitenRadiation Heat Transfer ExperimentYunus Emre Güzelel0% (1)
- GATE Tutor (ME) - Heat&Mass Transfer 1Dokument30 SeitenGATE Tutor (ME) - Heat&Mass Transfer 1SabariMechy0% (1)
- Refrigeration & Air Conditioning Laboratory Manual Course Code: ME344 B.Tech 6 SemDokument53 SeitenRefrigeration & Air Conditioning Laboratory Manual Course Code: ME344 B.Tech 6 SemYour dadNoch keine Bewertungen
- HMT 2marksDokument12 SeitenHMT 2markssanthanam102Noch keine Bewertungen
- Heat 4e Chap09 LectureDokument33 SeitenHeat 4e Chap09 Lecturemsiembab17Noch keine Bewertungen
- Flow and Heat Transfer in A Mixing ElbowDokument5 SeitenFlow and Heat Transfer in A Mixing Elbowjose antonioNoch keine Bewertungen
- How Car Cooling Systems WorkDokument4 SeitenHow Car Cooling Systems WorkyagneshNoch keine Bewertungen
- Heat 4e Chap02 LectureDokument48 SeitenHeat 4e Chap02 LectureSubho Samanta100% (4)
- Chapter 7 Shell Tube Heat ExchangerDokument138 SeitenChapter 7 Shell Tube Heat ExchangerPHƯƠNG ĐẶNG YẾNNoch keine Bewertungen
- Chap5 Design Specification Column D-101Dokument10 SeitenChap5 Design Specification Column D-101Liew KahJiannNoch keine Bewertungen
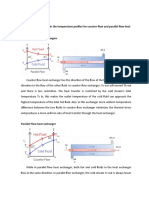
- Describe The Difference in The Temperature Profiles For Counter-Flow and Parallel Flow Heat Exchangers. Counter Flow Heat ExchangersDokument3 SeitenDescribe The Difference in The Temperature Profiles For Counter-Flow and Parallel Flow Heat Exchangers. Counter Flow Heat Exchangersbyun baekNoch keine Bewertungen
- Chilton and Colburn J-Factor Analogy: Sieder-Tate EquationDokument23 SeitenChilton and Colburn J-Factor Analogy: Sieder-Tate EquationAnkan ChaudhuryNoch keine Bewertungen
- Double Pipe Heat Exchanger - SsDokument72 SeitenDouble Pipe Heat Exchanger - SsNathanian100% (2)
- Thermodynamics: An Introduction to Basic Concepts and ApplicationsDokument140 SeitenThermodynamics: An Introduction to Basic Concepts and ApplicationsMuhammad Adib Haikal100% (1)
- Heat ExchangersDokument77 SeitenHeat ExchangersMervin PerezNoch keine Bewertungen
- Heat PipeDokument27 SeitenHeat Pipesaurabh maneNoch keine Bewertungen
- AVC Fume Hood Control System Product Sheet - 0216 PDFDokument4 SeitenAVC Fume Hood Control System Product Sheet - 0216 PDFTeo Pei SanNoch keine Bewertungen
- AVC Fume Hood Control System Product Sheet - 0216 PDFDokument4 SeitenAVC Fume Hood Control System Product Sheet - 0216 PDFTeo Pei SanNoch keine Bewertungen
- Accutrol Healthcare Brochure PDFDokument4 SeitenAccutrol Healthcare Brochure PDFTeo Pei SanNoch keine Bewertungen
- Air Change - ASHRAEDokument5 SeitenAir Change - ASHRAETeo Pei SanNoch keine Bewertungen
- M KC Product Sheet Flexible DuctDokument2 SeitenM KC Product Sheet Flexible DuctTeo Pei SanNoch keine Bewertungen
- Perforated Diffuser CatalogueDokument7 SeitenPerforated Diffuser CatalogueTeo Pei SanNoch keine Bewertungen
- Belimo LM230A-S Data Sheet EngDokument4 SeitenBelimo LM230A-S Data Sheet EngTeo Pei SanNoch keine Bewertungen
- EDGE Hospital User Guide Version 1Dokument140 SeitenEDGE Hospital User Guide Version 1mrccahmedNoch keine Bewertungen
- OT Canopies FFA 4Dokument12 SeitenOT Canopies FFA 4Teo Pei SanNoch keine Bewertungen
- Accutrol Healthcare Brochure PDFDokument4 SeitenAccutrol Healthcare Brochure PDFTeo Pei SanNoch keine Bewertungen
- (Global) StandardIE2IE3Motor AESV AESU 0711Dokument26 Seiten(Global) StandardIE2IE3Motor AESV AESU 0711AndriBagusNoch keine Bewertungen
- (Global) StandardIE2IE3Motor AESV AESU 0711Dokument26 Seiten(Global) StandardIE2IE3Motor AESV AESU 0711AndriBagusNoch keine Bewertungen
- KK-IGMH-M-2005-ACMV - OT SCH - Ra - 08.11.17Dokument1 SeiteKK-IGMH-M-2005-ACMV - OT SCH - Ra - 08.11.17Teo Pei SanNoch keine Bewertungen
- DYN-236 ACM 0413 Carbon MatrixDokument4 SeitenDYN-236 ACM 0413 Carbon MatrixTeo Pei SanNoch keine Bewertungen
- Leaflet UniCollarDokument4 SeitenLeaflet UniCollarTeo Pei SanNoch keine Bewertungen
- Duct Velocity D - 928 PDFDokument7 SeitenDuct Velocity D - 928 PDFTeo Pei SanNoch keine Bewertungen
- Ffa 29.29.4Dokument1 SeiteFfa 29.29.4Teo Pei SanNoch keine Bewertungen
- AAF CatalogueDokument14 SeitenAAF CatalogueTeo Pei SanNoch keine Bewertungen
- Green Mark Nonresi v4Dokument19 SeitenGreen Mark Nonresi v4Teo Pei SanNoch keine Bewertungen
- Duct Velocity D - 928 PDFDokument7 SeitenDuct Velocity D - 928 PDFTeo Pei SanNoch keine Bewertungen
- Microbiological Commissioning and Monitoring of Operating Theatre SuitesDokument29 SeitenMicrobiological Commissioning and Monitoring of Operating Theatre SuitesTeo Pei SanNoch keine Bewertungen
- RS 9 Hospital Air ChangesDokument4 SeitenRS 9 Hospital Air ChangesAlexiz Silva A.Noch keine Bewertungen
- Noise Calculation ChartDokument3 SeitenNoise Calculation ChartTeo Pei SanNoch keine Bewertungen
- Brochure - Industrial Dehumidification PDFDokument8 SeitenBrochure - Industrial Dehumidification PDFTeo Pei SanNoch keine Bewertungen
- Brochure - Industrial Dehumidification PDFDokument8 SeitenBrochure - Industrial Dehumidification PDFTeo Pei SanNoch keine Bewertungen
- Yamamoto Pressure & Compound GaugesDokument4 SeitenYamamoto Pressure & Compound GaugesTeo Pei SanNoch keine Bewertungen
- Catalogue - Nippon SteelDokument22 SeitenCatalogue - Nippon SteelTeo Pei SanNoch keine Bewertungen
- ZZ Chem 1202 Test 1 New Formula SheetDokument5 SeitenZZ Chem 1202 Test 1 New Formula Sheetavanit1Noch keine Bewertungen
- Property relations and equations for homogeneous phasesDokument20 SeitenProperty relations and equations for homogeneous phasesAbdur RehmanNoch keine Bewertungen
- BME Refrigeration and Air ConditioningDokument6 SeitenBME Refrigeration and Air ConditioningalysonmicheaalaNoch keine Bewertungen
- Air Conditioning (EDITED)Dokument6 SeitenAir Conditioning (EDITED)Justin MercadoNoch keine Bewertungen
- Sec01 - GroupE - (Unsteady State Heat Transfer)Dokument11 SeitenSec01 - GroupE - (Unsteady State Heat Transfer)Dzulfadhly ShaariNoch keine Bewertungen
- 8 Heat and TemperatureDokument3 Seiten8 Heat and TemperatureMelanie TrinidadNoch keine Bewertungen
- Equilibrium Between Phases of MatterDokument411 SeitenEquilibrium Between Phases of MatterJonatas LopesNoch keine Bewertungen
- Note 6 - Multicomponent DistillationDokument32 SeitenNote 6 - Multicomponent DistillationKaleeshNoch keine Bewertungen
- Determination of Heat of Solution by Solubility Method.: Experiment: 01Dokument3 SeitenDetermination of Heat of Solution by Solubility Method.: Experiment: 01javeria namoosNoch keine Bewertungen
- Chapter 10 Vapor Liquid Equilibrium - May20Dokument55 SeitenChapter 10 Vapor Liquid Equilibrium - May20Scorpion RoyalNoch keine Bewertungen
- Brønsted-Lowry Acids and BasesDokument39 SeitenBrønsted-Lowry Acids and BasesEr Bipin VermaNoch keine Bewertungen
- Thompson's CalorimeterDokument8 SeitenThompson's CalorimetergeethikaNoch keine Bewertungen
- Chapter 4 Vapor Liquid EquilibriumDokument61 SeitenChapter 4 Vapor Liquid Equilibriumprakash_krishnan_267% (3)
- Module 1-Thermal-ExpansionDokument14 SeitenModule 1-Thermal-ExpansionHazel A. BelloNoch keine Bewertungen
- Investigation of Effect of Orientation On Modified Annular Rectangular FinsDokument4 SeitenInvestigation of Effect of Orientation On Modified Annular Rectangular FinsInternational Journal of Application or Innovation in Engineering & ManagementNoch keine Bewertungen
- Extra Titration Practice Problems SolutionsDokument6 SeitenExtra Titration Practice Problems SolutionsAnna BoyajyanNoch keine Bewertungen
- Chemistry Grade 12 New TextbookDokument248 SeitenChemistry Grade 12 New Textbookdereje dawitNoch keine Bewertungen
- Chem Notes Chapter 6 - ThermochemistryDokument46 SeitenChem Notes Chapter 6 - ThermochemistryjohnNoch keine Bewertungen
- WFC-SC30 & -SH30 Water Chiller SpecsDokument2 SeitenWFC-SC30 & -SH30 Water Chiller SpecsKristina BožićNoch keine Bewertungen
- Indicators & PH CurvesDokument35 SeitenIndicators & PH CurvesSairam PrasathNoch keine Bewertungen
- Free Convection Manual: Heat & Mass Transfer LabDokument8 SeitenFree Convection Manual: Heat & Mass Transfer LabAnurag YadavNoch keine Bewertungen
- SCI 104 Lecture 3 ThermochemistryDokument50 SeitenSCI 104 Lecture 3 ThermochemistryYana100% (1)
- Chapter 3Dokument30 SeitenChapter 3Nashit AhmedNoch keine Bewertungen
- 1 3 3 Thermodynamics Doc PLTWDokument8 Seiten1 3 3 Thermodynamics Doc PLTWapi-30813196260% (5)
- Superheat Charging MethodDokument19 SeitenSuperheat Charging MethodambuenaflorNoch keine Bewertungen
- Psychrometric Chart Psychrometric Chart: Normal Temperature SI Units Sea Level Normal Temperature SI Units Sea LevelDokument1 SeitePsychrometric Chart Psychrometric Chart: Normal Temperature SI Units Sea Level Normal Temperature SI Units Sea LevelAnoop RastogiNoch keine Bewertungen
- Energy Efficient Buildings Design Heating & Cooling LoadsDokument5 SeitenEnergy Efficient Buildings Design Heating & Cooling Loadsaltamash thakurNoch keine Bewertungen
- Tinh Luong Nuoc Bay HoiDokument22 SeitenTinh Luong Nuoc Bay HoiVinh Do ThanhNoch keine Bewertungen
- Thermodynamics Laws SummaryDokument27 SeitenThermodynamics Laws SummaryMetalAnand ChelliahNoch keine Bewertungen
- TM-Editor 25.04.2016 Seite 1 050-Meteorology - LTMDokument308 SeitenTM-Editor 25.04.2016 Seite 1 050-Meteorology - LTMIbrahim Med100% (1)
- Power of Habit: The Ultimate Guide to Forming Positive Daily Habits, Learn How to Effectively Break Your Bad Habits For Good and Start Creating Good OnesVon EverandPower of Habit: The Ultimate Guide to Forming Positive Daily Habits, Learn How to Effectively Break Your Bad Habits For Good and Start Creating Good OnesBewertung: 4.5 von 5 Sternen4.5/5 (21)
- Introduction to Power System ProtectionVon EverandIntroduction to Power System ProtectionBewertung: 5 von 5 Sternen5/5 (1)
- Build Your Own Electric Vehicle, Third EditionVon EverandBuild Your Own Electric Vehicle, Third EditionBewertung: 4.5 von 5 Sternen4.5/5 (3)
- The Grid: The Fraying Wires Between Americans and Our Energy FutureVon EverandThe Grid: The Fraying Wires Between Americans and Our Energy FutureBewertung: 3.5 von 5 Sternen3.5/5 (48)
- Idaho Falls: The Untold Story of America's First Nuclear AccidentVon EverandIdaho Falls: The Untold Story of America's First Nuclear AccidentBewertung: 4.5 von 5 Sternen4.5/5 (21)
- The Cyanide Canary: A True Story of InjusticeVon EverandThe Cyanide Canary: A True Story of InjusticeBewertung: 4 von 5 Sternen4/5 (51)
- Formulas and Calculations for Drilling, Production, and Workover: All the Formulas You Need to Solve Drilling and Production ProblemsVon EverandFormulas and Calculations for Drilling, Production, and Workover: All the Formulas You Need to Solve Drilling and Production ProblemsNoch keine Bewertungen
- Asset Integrity Management for Offshore and Onshore StructuresVon EverandAsset Integrity Management for Offshore and Onshore StructuresNoch keine Bewertungen
- Renewable Energy: A Very Short IntroductionVon EverandRenewable Energy: A Very Short IntroductionBewertung: 4.5 von 5 Sternen4.5/5 (12)
- The Way Home: Tales from a life without technologyVon EverandThe Way Home: Tales from a life without technologyBewertung: 4 von 5 Sternen4/5 (45)
- Shorting the Grid: The Hidden Fragility of Our Electric GridVon EverandShorting the Grid: The Hidden Fragility of Our Electric GridBewertung: 4.5 von 5 Sternen4.5/5 (2)
- OFF-GRID PROJECTS: A Comprehensive Beginner's Guide to Learn All about OffGrid Living from A-Z and Live a Life of Self-SufficiencyVon EverandOFF-GRID PROJECTS: A Comprehensive Beginner's Guide to Learn All about OffGrid Living from A-Z and Live a Life of Self-SufficiencyNoch keine Bewertungen
- VSC-FACTS-HVDC: Analysis, Modelling and Simulation in Power GridsVon EverandVSC-FACTS-HVDC: Analysis, Modelling and Simulation in Power GridsNoch keine Bewertungen
- Industrial Piping and Equipment Estimating ManualVon EverandIndustrial Piping and Equipment Estimating ManualBewertung: 5 von 5 Sternen5/5 (7)
- Handbook on Battery Energy Storage SystemVon EverandHandbook on Battery Energy Storage SystemBewertung: 4.5 von 5 Sternen4.5/5 (2)
- Nuclear Energy in the 21st Century: World Nuclear University PressVon EverandNuclear Energy in the 21st Century: World Nuclear University PressBewertung: 4.5 von 5 Sternen4.5/5 (3)
- ISO 50001: A strategic guide to establishing an energy management systemVon EverandISO 50001: A strategic guide to establishing an energy management systemNoch keine Bewertungen
- The Rare Metals War: the dark side of clean energy and digital technologiesVon EverandThe Rare Metals War: the dark side of clean energy and digital technologiesBewertung: 5 von 5 Sternen5/5 (2)
- Well Control for Completions and InterventionsVon EverandWell Control for Completions and InterventionsBewertung: 4 von 5 Sternen4/5 (10)
- Art of Commenting: How to Influence Environmental Decisionmaking With Effective Comments, The, 2d EditionVon EverandArt of Commenting: How to Influence Environmental Decisionmaking With Effective Comments, The, 2d EditionBewertung: 3 von 5 Sternen3/5 (1)
- Operational Amplifier Circuits: Analysis and DesignVon EverandOperational Amplifier Circuits: Analysis and DesignBewertung: 4.5 von 5 Sternen4.5/5 (2)
- Energy, Light and Electricity - Introduction to Physics - Physics Book for 12 Year Old | Children's Physics BooksVon EverandEnergy, Light and Electricity - Introduction to Physics - Physics Book for 12 Year Old | Children's Physics BooksNoch keine Bewertungen
- Air-Cooled Condenser Fundamentals: Design, Operations, Troubleshooting, Maintenance, and Q&AVon EverandAir-Cooled Condenser Fundamentals: Design, Operations, Troubleshooting, Maintenance, and Q&ABewertung: 5 von 5 Sternen5/5 (1)