Beruflich Dokumente
Kultur Dokumente
PDF 29
Hochgeladen von
Olvaria MisfaOriginaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
PDF 29
Hochgeladen von
Olvaria MisfaCopyright:
Verfügbare Formate
Clin Auton Res (2009) 19:88101
DOI 10.1007/s10286-009-0515-2
Fion Bremner, PhD, FRCOPhth
Received: 13 October 2008
Accepted: 11 December 2008
Published online: 3 February 2009
F. Bremner, PhD, FRCOPhth (&)
Dept. of Neuro-Ophthalmology
(Internal Box 142)
National Hospital for Neurology
and Neurosurgery
Queen Square
London WC1N 3BG, UK
Tel.: +44-20/7837-3611 ext. -3382
Fax: +44-20/7676-2041
E-Mail: fion.bremner@uclh.nhs.uk
REVIEW ARTICLE
Pupil evaluation as a test for autonomic
disorders
j Abstract Pupil tests provide a
convenient and simple method for
evaluation of autonomic function.
Most patients with autonomic
disorders show evidence of sympathetic or parasympathetic deficits in the pupil, and these can be
detected using a combination of
clinical signs, pupillometric tests
(measuring the responses to light,
or an accommodative effort, or a
sudden noise) and pharmacological tests (using topically applied
drugs both to confirm a deficit and
to localize the lesion). Caution is
needed in the interpretation of
CAR 515
Introduction
Examination of the pupil is a simple, convenient, noninvasive technique for evaluating function in the
autonomic nervous system. The size of the pupil is
determined by the tone in two opposing smooth
muscles; pupillary constriction or miosis is brought
about by the action of the sphincter muscle under
parasympathetic control, whereas pupillary dilation
or mydriasis is brought about by the action of the
dilator muscle under sympathetic control. At any
point in time the balance of activity in parasympathetic and sympathetic supplies depends on a number
of factors, including genetic influences, age, wakefulness, accommodative state and ambient lighting
conditions. When these are standardized or controlled for then measurement of pupil size can be used
to identify parasympathetic or sympathetic deficits.
these tests, particularly if the deficits are mixed (i.e. sympathetic
and parasympathetic) or bilateral.
The pattern of autonomic disturbance in the pupils often correlates poorly with autonomic
function elsewhere, but may have
diagnostic value in discriminating
between different underlying conditions.
j Key words pupils
tonic pupil Horner syndrome
dysautonomia
Sensitivity and specificity may be further improved by
measuring the dynamic pupillary responses to physiological stimuli such as light, sound or accommodation, or to pharmacological agents administered
topically.
Over the centuries a wide range of ingenious
instruments have been devised to measure the pupil.
Initially these could only provide static or steady
state estimations of pupil size with the lights on. The
modern era of pupillometry was ushered in by the
development of infra-red video techniques allowing
dynamic continuous recording of pupil size in complete darkness [20]. Most dedicated clinical pupil research laboratories now use their own custom-built
pupillometers. However the need for accurate estimates of pupil size when planning laser refractive
surgery in the eye has led to the recent development
of a number of simpler and relatively inexpensive
commercial devices [32]. All modern pupillometers
89
Tests of sympathetic integrity
Ach: M3
Ach: N
j Anatomy
EWN
III
CG
C8-T1
HYP
central
CSC
SPCN
SCG
SM
NA: 1
Ach: N
pre
NEJ
post
NEJ
DM
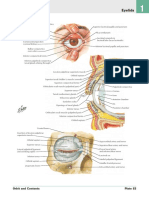
Fig. 1 Schematic diagram summarizing the nerve supply of the iris sphincter
muscle (SM) and dilator muscle (DM) by the parasympathetic and sympathetic
nervous systems respectively. EWN Edinger-Westphal nucleus; III: oculomotor
nerve, CG ciliary ganglion; SPCN short posterior ciliary nerves, NEJ neuro-effector
junction, HYP hypothalamus, CSC ciliospinal centre of Budge & Waller, SCG
superior cervical ganglion
are designed along the same principles: an infra-red
source illuminates the iris, a video camera records the
reflected light, computerized image analysis techniques are used to detect the pupil within each video
frame and provide continuous measurement of pupil
size, and a light stimulus can be presented to the eye
using a photostimulator. However such technology is
not always required: in some cases autonomic disturbances of the pupil may be reliably diagnosed
using little more than bedside examination with a pen
torch. In this article I will review the current approaches to diagnosing sympathetic or parasympathetic deficits in the pupil, and discuss interpretation
of the test results and their application in the wider
context of evaluating function in the autonomic nervous system.
Fig. 2 Clinical signs of sympathetic
deficit (Horners syndrome). Upper
panel shows right Horner following
birth injury. Lower panel shows
bilateral Horner in patient with
dopamine hydroxylase deficiency
The sympathetic innervation of the iris dilator muscle
arises from a long polysynaptic pathway originating
in the ipsilateral hypothalamus (see Figure 1). The
central neurone descends uncrossed through the
brainstem and upper spinal cord to terminate in
the ciliospinal center of Budge and Waller at the level
of C8-T1 (sometimes T2). Pre-ganglionic sympathetic
fibres then emerge from the spinal cord in the ventral
roots (mainly T1) and join the cervical sympathetic
chain before terminating in the superior cervical
ganglion. The post-ganglionic fibres ascend with the
internal carotid artery into the middle cranial fossa,
enter the orbit through the superior orbital fissure
and pass forwards as the long ciliary nerves to activate
noradrenergic (mainly a-1) adrenoceptors on the
dilator muscle fibres.
j Clinical signs
The clinical features of an oculo- sympathetic paresis
were first described in the cat by the French physiologist Claude Bernard, and soon after in man by his
American neurology student Silas Weir Mitchell.
However it is the later paper in 1869 by the Swiss
ophthalmologist Johann Friedrich Horner which
earned him the eponym [12], and sympathetic deficits
in the eye are now generally referred to as Horners
syndrome (for full history see Stanley Thompsons
editorial) [28]. A typical example is shown in Figure 2
(upper panel). This child had a difficult birth
requiring forceps delivery which injured the brachial
plexus and sympathetic chain on the right side of
the neck. The resulting oculo-sympathetic paresis is
90
associated with relative miosis of the pupil and anisocoria which is greater in dim light than in bright
light.
The sympathetic supply to the eye includes other
non-pupillary fibres which if also damaged may give
rise to a number of additional clinical signs. In the
first year of life sympathetic nerve fibres are important in supporting the normal melanisation of ocular
tissues, and so a congenital Horner may be associated with relative hypopigmentation of the iris
ipsilateral to the lesion (heterochromia iridis).
Sympathetic fibres also supply Muellers muscles,
slips of smooth muscle in both upper and lower lids
which assist in opening the eyes; damage to these
fibres causes ptosis of the upper lid and elevation
of the lower lid with narrowing of the palpebral
aperture, thus resulting in an apparent or pseudoenophthalmos. Damage to sympathetic vasoconstrictor fibres leads to mild injection (pinkness) of
the conjunctiva and a lower intraocular pressure,
whilst damage to sympathetic vasodilator fibres
interferes with the normal facial flushing reflex that
occurs with thermal stress (harlequin syndrome)
[18]. Damage to sympathetic sudomotor fibres leads
to anhydrosis and increased temperature of the affected area of skin; in theory a pre-ganglionic lesion
is expected to affect sweating over the whole ipsilateral side of the face whereas a post-ganglionic
lesion should only affect a small patch above the
brow, although in practice these distinctions are
difficult to make clinically and there are better ways
of localising the lesion (see below).
Some caution is needed in interpreting these clinical signs. Firstly, most of these features of Horners
syndrome can only be appreciated if the lesion is
unilateral and comparison can be made with the
unaffected fellow eye. However, some patients, particularly those with generalised dysautonomias, have
bilateral sympathetic deficits. An example is shown in
Figure 2 (lower panel); this patient has inherited
dopamine b-hydroxylase deficiency with bilateral
Horner syndromes, but because her oculo-sympathetic deficits are symmetrical she displays few of the
clinical signs described above. Secondly, lesions of the
peripheral sympathetic pathway occasionally damage
some but not all fibres, giving rise to incomplete or
partial Horner syndromes (which might for example
spare the lids or the vasomotor or sudomotor fibres);
the lack of some of the typical clinical features may
lead to false-negatives and case under-ascertainment.
Thirdly and by contrast, some patients display falsepositive signs (pseudo-Horner) that may be misattributed as indicating oculo-sympathetic paresis
(discussed later). In conclusion, it is unwise to diagnose a sympathetic deficit on clinical grounds alone;
ideally the patient should be subjected to further
pupillometric or pharmacological tests to confirm the
diagnosis.
j Pupillometric tests
The relative weakness of the dilator muscle in patients
with a Horner is expected to cause dark miosis, i.e.
the pupil should remain small in complete darkness
and this can be measured straightforwardly using
infra-red techniques for pupillometry. However there
are a number of confounding influences that limit the
usefulness of this measure as a test of sympathetic
integrity. The dark diameter of the pupil depends
strongly on age (older people have smaller pupils): in
our cohort of 315 healthy subjects the expected dark
diameter can be calculated by the formula DDe =
8.283 ) (0.043 A) where A = age in years [6]. In
addition genetic background, wakefulness and
accommodative state also affect dark diameter measurements; as a result dark diameter varies across a
wide range even in healthy subjects of the same age
(the 95 and 99% limits to the normal range are given
by DDe 1.63 and 2.16 mm respectively). Many patients with Horners syndrome therefore show dark
diameters within this normal range (see Figure 3),
i.e. as a test for Horners syndrome the rate of falsenegatives is high. In our own consecutive case series
of 177 patients with unilateral Horner, dark diameter
measurements showed a sensitivity for diagnosing
sympathetic deficit of only 20.0% (but good specificity = 96.4%). The relative increase in anisocoria in
the dark compared with the light has more diagnostic
potential, but the most sensitive tests of sympathetic
integrity rely on dynamic recordings of the pupillary
responses to light or sound, or pharmacological responses to topically administered drugs.
The light response (LR) of the pupil follows a
characteristic waveform in healthy subjects. Figure 4a
shows an example of the response of a normal pupil
(solid line) to a 1-second square-wave pulse of light.
After a delay of approximately 250 ms the pupil
constricts rapidly, reaching maximal miosis about 1second later. The light-OFF signal then produces
redilatation of the pupil towards the starting diameter
in two phases: the initial redilatation is rapid and
reflects withdrawal of the parasympathetic drive to
the sphincter muscle, whereas the later phase is
slower and is thought to be actively driven by the
peripheral sympathetic supply to the dilator muscle
[19]. Damage to this peripheral sympathetic supply
therefore delays the later phase, giving rise to redilatation lag (see Figure 4a, dashed line).
In cases of unilateral Horner syndrome redilatation
lag may be readily detected both clinically (by directly
comparing the time taken for the pupils to dilate
91
Unaected side
Aected side
10
9
8
Dark diameter (mm)
Fig. 3 Graphs show the relationship
between size of the pupil in
darkness and age for 177 patients
with a unilateral Horner syndrome.
Measurements from the unaffected
eye are shown on the left, from the
affected eye on the right. The mean
and 95% upper and lower limits for
pupil size in healthy subjects
(N = 315) are indicated by the solid
and dashed lines respectively
7
6
5
4
3
2
0
20
40
60
80
100
20
40
60
80
100
6.5
6.0
4
T3/4 (seconds)
Fig. 4 Using measures of
redilatation to detect Horners
syndrome. a Responses of the right
pupil (solid line) and left pupil
(dashed line) to a 1-second flash of
light in a patient with a left Horner.
The right pupil response is normal
but the left pupil shows a reduced
rate of recovery to the starting
diameter from the point indicated
by the arrow (redilatation lag).
b Covariation of T3/4 measurements
with amplitude of the pupillary light
response in healthy subjects. Solid
and dashed lines indicate
mean 95% limits
Diameter (mm)
Age (years)
5.5
5.0
4.5
after the room lights are turned out) and photographically (anisocoria measurements are greater at
5 seconds compared with 15 seconds after onset of
darkness) [22]. However detection of bilateral sympathetic lesions requires an absolute measure of
redilatation. Studies with a-adrenoceptor antagonists
[25, 26] have shown that the time taken for threequarters redilatation (i.e. 75% recovery to starting
diameter, T3/4) most closely reflects the degree of
peripheral sympathetic drive; furthermore, this measure of redilatation appears not to be influenced by
age or starting pupil diameter, although it does
increase linearly with the amplitude of the light reflex
(Figure 4b). The expected value for T3/4 can be cal-
Time (seconds)
10
1
B
Amplitude (mm)
culated as [1.048 + (0.619 A)] where A is the
amplitude of the reflex in millimeters [6]. Values of
T3/4 greater than the 97.5 percentile indicate significant redilatation lag and may be used to detect unilateral or bilateral Horner syndrome; in our own
series of 177 consecutive cases of Horner (see above)
these T3/4 measurements were associated with a
diagnostic sensitivity of 79.2% and specificity of
94.3%.
An alternative means of stimulating the peripheral
sympathetic is by presentation of a sudden loud noise
such as a bang. The surprise evoked by a sudden
noise acts as a psychosensory stimulus causing
pupillary dilation with a latency of about one-second
92
5
noise
noise
Pupil diameter (mm)
noise
4
6
5
5
SR+
3
4
(SR-)
SR+
(SR-)
3
2
2
2
0
5
10
15
Time (seconds)
20
10
15
Time (seconds)
20
5
10
15
Time (seconds)
20
Fig. 5 The startle response (SR) in a healthy subject (left panel), a patient with
Horner syndrome caused by acute dissection of the right internal carotid artery
(middle panel) and a patient with generalized dysautonomia caused by diabetes
mellitus (right panel); right eye shown as solid lines, left eye as dashed lines.
Technique: the pupils are maximally constricted by a bright light for
10 seconds; the pupils then redilate and during this recovery phase a sudden
loud noise is presented (at approx. t = 15 seconds). Left panel shows the
symmetrical mydriatic responses of both pupils to this noise (SR+) in a healthy
subject (latency approximately 1 second). In the middle panel the startle
response is absent in the right eye (SR-) but intact in the left eye. No startle
response could be elicited from either pupil in the patient with bilateral
sympathetic deficits caused by diabetic autonomic neuropathy (right panel)
(see Figure 5). This startle response (SR) is generated
in part by central inhibition of Edinger Westphal
neurones and in part by activation of the peripheral
sympathetic nerves, but the latency is probably too
short for circulating catecholamines released from the
adrenal glands to contribute. We have found that the
optimum conditions for eliciting this reflex are to
maximally constrict the pupils with a bright light for
10 seconds then present the startling stimulus whilst
the pupil is actively re-dilating about 5 seconds after
the light stimulus is turned offthe autonomic
equilibrium in the pupil is tipped in favor of the
sympathetic supply at this point which reinforces the
SR. Patients with unilateral or bilateral Horner syndrome will often show no SR on the affected side in
contrast with healthy subjects (Figure 5). However in
my experience this test only has limited usefulness:
the sudden noise will often cause the anxious patient
to move their whole head or even body in fright,
preventing measurement of the pupil; the SR is not
easily quantified; moreover the test has a high falsenegative rate (i.e. some patients with known Horner
syndrome still show a preserved SR, presumably
generated centrally via inhibition of parasympathetic
tone) and therefore the sensitivity is rather low.
cocaine. Among its many actions, this drug inhibits
the active re-uptake mechanism that terminates the
action of noradrenaline at post-ganglionic sympathetic neuro-effector junctions. In a healthy subject
the result is accumulation of higher concentrations of
noradrenaline and dilation of the pupil, whereas in a
patient with Horner syndrome cocaine has little or no
effect on pupil size (see Figure 6). The drug is usually
administered topically as eyedrops in either 4% (UK)
or 10% (USA) solutions, and measurements made of
the pupil diameter before and 3060 minutes after
exposure to the drug. In our laboratory we usually
measure resting pupil diameter with the room lights
on because making the drug work against the lightinduced miosis seems to increase the sensitivity of the
test. Kardon et al. [17] analysed photographs from a
large number of cocaine tests in Horner patients and
controls to establish the optimal diagnostic criteria;
they concluded that an increase of anisocoria of
0.8 mm or more gives a mean odds ratio of 1050:1
(95% lower limit 37:1) that the patient has a Horner.
An alternative approach is to apply weak solutions
of a1-adrenoceptor agonists. Denervation of the iris
dilator muscle leads to up-regulation of the number of
adrenoceptors expressed on the muscle membranes
and thus supersensitivity to exogenously administered sympathomimetics. This up-regulation occurs
irrespective of the site of the lesion and can be
demonstrated by observing a mydriatic response in
the denervated pupil to drugs that have little or no
effect on the normal pupil, either because the drug
j Pharmacological tests
Sympathetic integrity can be tested using a variety of
pharmacological agents. The commonest drug used is
93
Fig. 6 Using 4.0% cocaine to detect
sympathetic deficit. Photographs
show the pupils of an 84-year-old
man with an idiopathic right-sided
Horner syndrome before (upper
panel) and 30 minutes after (lower
panel) topical administration of
cocaine. Cocaine dilates the left
pupil but has no effect on the right
pupil; as a result the degree of
anisocoria increases, particularly
when observed in bright lighting
conditions
concentration is too low (e.g. 1% phenylephrine) [23]
or because the drug has relatively poor efficacy for a1adrenoceptors (e.g. 0.51.0% apraclonidine) [21]. The
practical advantages of apraclonidine (which is
readily available and dilates a Horner pupil, an active
sign which is easier to interpret) over cocaine
(unavailable in many hospitals and detects Horner by
failure to dilate the pupil, a negative sign which is
harder to interpret) have encouraged its widespread
use in recent years. There has been a small number of
case series published where the results of testing patients with both drugs were compared [8], but no
large-scale studies that would allow a more systematic
comparison of the sensitivity and specificity of these
two drug tests for diagnosing Horner syndrome [16].
Given the length of the peripheral sympathetic
pathway and the frequency with which Horner syndrome presents as an isolated neurological deficit (i.e.
with no other clinical signs to localize the lesion) the
clinician often needs a test which can distinguish
between pre-and post-ganglionic lesions. The drug
that is most commonly used to localize the lesion is
hydroxyamphetamine (OHA, usually as 1% solution).
This drug displaces noradrenaline from its storage
sites within the post-ganglionic sympathetic nerve
endings. In a healthy subject OHA will therefore dilate
the pupil; in a patient with a post-ganglionic Horner
the pupil shows little or no response to OHA because
there is no stored noradrenaline in the atrophied
sympathetic nerve endings; in contrast, there is an
exaggerated mydriatic response from the pupil of a
patient with a pre-ganglionic Horner because the intact sympathetic nerve endings in the eye have
abundant stores of noradrenaline and supersensitive
post-junctional membranes. Examples are shown in
Figure 7. OHA testing is reported to localize the lesion
in Horner syndrome with a sensitivity of 93% and
specificity of 83% [9].
Unfortunately hydroxyamphetamine has become
notoriously difficult to obtain now that it is no longer
manufactured commercially for use in the eye.
Alternatives include tyramine (2.55%) or pholedrine
(0.5%), both of which have similar mechanisms of
action and appear equally efficacious as a test to
localize oculo-sympathetic lesions [3]. It has also been
claimed that 1% phenylephrine can localize the lesion
in Horner syndrome because there is probably more
denervation supersensitivity following a post-ganglionic lesion than with a pre-ganglionic lesion. DaneshMeyer et al. [10] have performed the only head-tohead comparison of pupillary responses to OHA and
1% phenylephrine in 3 pre- and 11 post-ganglionic
Horner patients and found good concordance in the
results between these two drugs for all but one of the
patients in their series. However there is a wide range
in pupillary responses to weak agonists in the healthy
population [15] so the phenylephrine test should be
interpreted with caution and, where available, OHA
used instead.
Tests of parasympathetic integrity
j Anatomy
The parasympathetic innervation of the iris sphincter
muscle arises from pre-ganglionic neurones originating in the ipsilateral Edinger-Westphal nucleus of
the upper midbrain (see Figure 1). These join the
oculomotor nerve, traveling superficially in the subarachnoid space where they are susceptible to compressive injury from, for example, a posterior
communicating artery aneurysm. They terminate in
the ciliary ganglion which lies about 10 mm in front
of the superior orbital fissure in the loose fatty tissue
of the orbital cavity. This ganglion contains cell
94
Fig. 7 Using 1.0%
hydroxyamphetamine (OHA) to
localize the lesion in Horner
syndrome. Photographs show the
pupils before (upper) and
30 minutes after (lower) topical
administration of OHA. A: 66-yearold woman with an idiopathic
Horner in the right eyeboth pupils
have dilated in response to OHA
implying that the lesion is preganglionic. b 23-year-old man with
a traumatic Horner in the left
eyeOHA dilated the normal right
pupil but had no effect on the left
pupil implying that the lesion is
post-ganglionic
bodies of the postganglionic parasympathetic fibres
whose axons pierce the sclera and travel forwards in
the suprachoroidal space to activate muscarinic
(mainly M3) cholinoceptors on the sphincter muscle
fibres. The ciliary ganglion also contains the cell
bodies of the parasympathetic post-ganglionic motoneurones supplying the ciliary muscle; fibres subserving pupil constriction comprise only about 3% of
the parasympathetic outflow from the ganglion, the
remainder supplying the much larger ciliary muscle
and controlling accommodation. In addition there are
a small number of somatosensory (trigeminal afferent) and sympathetic nerve fibres that pass through
the ciliary ganglion without synapse.
j Clinical signs
The clinical signs of damage to this parasympathetic
supply depend on whether the lesion is pre- or postganglionic. Pre-ganglionic lesions usually cause similar degrees of paresis in both ciliary and sphincter
muscles, leading to weak or absent accommodation
and a large unreactive pupil. The pupil remains round
with no sector palsy (see below) and fails to constrict
to light or an accommodative effort. Cases of
incomplete oculomotor nerve palsy causing isolated
pupil and ciliary muscle signs are extremely rare [31];
most patients with signs of damage to the pre-ganglionic parasympathetic fibres have additional ophthalmoplegia or ptosis that localize the lesion to the
third cranial nerve.
In contrast, lesions of the ciliary ganglion or short
posterior ciliary nerves affect only the pupil and
accommodation sparing levator and the extraocular
muscles. The clinical signs associated with these lesions were first described by Holmes [11] and later
Adie [1]. In the acute phase the internal ophthalmoplegia is indistinguishable from that seen with preganglionic lesionsthe pupil is large and round and
will not constrict to light or an accommodative effort.
However as more time passes there is a typically
aberrant attempt to re-innervate both sphincter and
ciliary muscles and the signs change. The pupil becomes irregular in shape and progressively smaller in
size (although it takes many years before it becomes
smaller than the fellow pupil under normal lighting
conditions). A light reflex can sometimes be elicited
95
Fig. 8 Sector palsy is a sign of focal damage to the post-ganglionic
parasympathetic nerve fibres supplying the iris sphincter muscle. In those
meridians where the iris sphincter muscle is relatively paretic (at 7 and 11
oclock; black arrows) the pupil margin bows outwards under the tonic
influence of the intact dilator muscle. At other meridians the sphincter muscle
has a more intact innervation and constricts in response to light (white arrows).
The result is an irregularly shaped pupil (compare with a perfect circle shown
by the white dashed line) which shows vermiform movements when a beam
of light is shone into the eye due to the sectoral bunching or widening of the
iris crypts within the collarette. It is easiest to detect this clinical sign using slitlamp biomicroscopy
but it is grossly attenuated and slow, and when viewed
using slitlamp biomicroscopy the pupil margin is seen
to display vermiform movements to edge-light
stimuli indicating sector palsy (patchy re-innervation
of the sphincter muscle such that some meridians but
not others will constrict in response to light: see
Figure 8). Redilatation following light-OFF is similarly sluggish and so T3/4 measurements cannot be
used in these pupils as an indicator of peripheral
sympathetic integrity.
In contrast to the poor light reflex, the pupil shows
a preserved or even exaggerated miosis in response to
an accommodative effort. In healthy subjects the light
response (LR) of the pupil almost always exceeds the
near response (NR), i.e. LR > NR. The reversal of this
pattern in patients with post-ganglionic parasympathetic lesions, i.e. NR > LR, presumably reflects disproportionately more mistakes in re-innervating the
sphincter muscle compared with the ciliary muscle
(most of the parasympathetic outflow from the ciliary
ganglion is normally directed to the ciliary muscle as
discussed above, so misdirection of these accommodation fibres towards the sphincter muscle will occur
far more often than errors in the reverse direction).
The resulting light-near dissociation (LND) is an
important clinical sign, although it is not exclusive to
damage at this site (it is also seen with lesions of the
afferent limb of the light reflex and of the dorsal
midbrain). More specifically, but for reasons that are
poorly understood, this near response of the pupil is
grossly slowed or tonicit can take over 10 seconds
for peak miosis to be reached, and even longer for the
pupil to return to its starting diameter when the patient transfers their gaze back into the distance. Psychophysical measurements of the time taken for the
patient to transfer their focus from far-to-near or vice
versa (normally around 1 second) are also grossly
abnormal indicating that similar tonic changes are
occurring in the re-innervation pattern of the ciliary
muscle.
These clinical pupil signs (attenuated and slow
light reflex, irregular shape with sector palsy, and
exaggerated but tonic near response with LND) are
collectively described as the features of a tonic pupil.
They are never observed with pre-ganglionic lesions
however longstanding, and may be regarded as
pathognomonic of damage to the ciliary ganglion and/
or short posterior ciliary nerves.
j Pupillometric tests
Parasympathetic deficit is expected to increase the
resting diameter of the pupil in the light, with the
result that there is little change in pupil size according
to the lighting conditions and any anisocoria consequently becomes greater in the light than in the dark
if the lesion is unilateral (see Figure 9). As with
Horner syndrome, however, there are a number of
confounders that limit the usefulness of a static
diameter measurement for diagnosing parasympathetic lesions. Firstly, there is an even wider normal
range for resting pupil diameters in the light than in
darkness in the healthy population, and so the false
negative rate is high (i.e. the test has low sensitivity).
Secondly, tonic pupils progressively miose over time
and eventually become smaller than normalmeasurements of pupil diameter can therefore only be
interpreted in suspected cases if the duration of the
damage is short or the rate of subsequent change is
small. Thirdly, bilateral tonic pupils are relatively
common and so there is often no control eye for
comparison. In practice, measurement of resting pupil diameter on its own is only of very limited value in
diagnosing parasympathetic lesions.
It is more useful to examine the dynamic pupillary
responses to light and to an accommodative effort. A
typical case is shown in Figure 9. The affected pupil
clearly shows an attenuated, slow light response and
an exaggerated tonic near response. Measurements of
the amplitude (Amp) and maximum constriction
96
Fig. 9 Pupil responses in a patient
with unilateral pupillotonia
associated with HolmesAdie
syndrome. a Light reflex
presentation of a 1 second light
stimulus (background = darkness)
causes brisk constriction of the
normal pupil (dashed line) but only a
slow and attenuated response from
the tonic pupil (solid line). b Near
responsewhen the patient
focused on a near target (room
lights on) the normal pupil
constricted briskly but less
extensively than after the light
stimulus; in contrast, the tonic pupil
showed a slower but more extensive
constriction which exceeded the
amplitude of the light response
(light-near dissociation)
7.5
7
Light
Light
7.0
6
Near
Near
6.5
5
6.0
4
5.5
5.0
3
12
14
16
18
Time (seconds)
velocity (CVmax) for both the LR and the NR can either be compared with the fellow eye if the suspected
lesion is known to be unilateral, or with previously
established normal ranges in potentially bilateral
cases. It is worth pointing out that the normal ranges
for measurements such as Amp and CVmax depend
critically on parameters of the recordings such as the
duration and intensity of the light stimulus, background luminance and retinal adaptive state, distance
and nature of the accommodative target etc. which are
not standardized between researchers. As such, and in
contrast with the resting dark diameter and T3/4
measurements discussed above, absolute determinations of abnormality (defined as values lying below
the 97.5% lower limits) require a lab-specific normative database to be first established. A further caveat is
that whereas the strength of the light stimulus can be
precisely defined when recording the LR, it is not
straightforward to quantify the degree of accommodative effort exerted by the patient when recording
their NR; a poor NR in both eyes may merely reflect
poor accommodative effort, especially in myopic or
presbyopic patients. In my experience clinical examination (especially at the slitlamp) can be just as
sensitive as pupillometric tests for detecting LND and
the tonicity of the NR (although no studies have been
published that allow a formal comparison of the
sensitivities of these two approaches).
20
10
12
14
16
18
20
Time (seconds)
0.1% pilocarpinethis weak muscarinic agonist has
little effect on the normal pupil but will produce an
exaggerated miosis (and myopic shift in refraction) in
any patient with a parasympathetic lesion (see Figure 10). Alternative agents are methacholine (2.5%)
and arecoline (0.1%), although there is usually no
difficulty in obtaining pilocarpine because of its
continued use by ophthalmologists for treating some
kinds of glaucoma.
Cholinergic supersensitivity has classically been
regarded as indicating that the lesion is post-ganglionic (cf. 1% phenylephrine in Horner syndrome
discussed above), i.e. the test was thought to have
localising value [27]. However a number of recent
publications show that supersensitivity can arise with
all cases of sphincter muscle denervation regardless of
whether the parasympathetic lesion is pre or postganglionic [13, 14]. The important clinical point is
that, although rare, internal ophthalmoplegia and
cholinergic supersensitivity can be observed as isolated neurological signs in patients later found to have
a pre-ganglionic mass lesion [2]. In my opinion, the
only reliable signs localising a parasympathetic lesion
as post-ganglionic are those indicating aberrant
regeneration (sector palsy and tonic NR) which is
never seen in third nerve palsies.
Discussion
j Pharmacological tests
Parasympathetic integrity may be tested by looking
for evidence of denervation supersensitivity of the iris
sphincter muscle fibres. The commonest drug used is
Sympathetic or parasympathetic denervation of the
pupil is frequently present in patients with autonomic
disorders. In a recent systematic study of 150 consecutive patients with various types of dysautonomia
97
Fig. 10 Using 0.1% pilocarpine to
detect parasympathetic deficit.
Upper panel shows the pupils of a
patient with Holmes-Adie syndrome:
the left pupil is normal, the right
pupil is tonic. Lower panel shows
the same pupils 30 minutes after
topical administration of 0.1%
pilocarpine: the left pupil shows no
response to this weak muscarinic
agonist but the right pupil has
constricted demonstrating
denervation supersensitivity
referred for pupillometry [6] the overall prevalence of
pupil abnormalities was found to be 66%. However in
most patients these pupil changes are asymptomatic,
especially if bilateral and symmetric. Furthermore,
they are often missed by the referring clinicians. The
result is that involvement of the pupil tends to be
under-reported in the literature on autonomic disorders. In this article so far I have described the current
approaches to diagnosing autonomic disturbances in
the pupil. The limitations and caveats to these tests
will now be discussed.
j Patient confounders
Before embarking on tests of pupil function it is
important to consider any co-morbidity or medications taken by the patient. Ophthalmic problems are
the commonest confounders. There are many conditions within the eye that can affect the pupil, in some
cases permanently. The pupil may also be disturbed
by ophthalmic treatments, including intraocular or
orbital surgery, laser procedures and topical medications (particularly those given to treat glaucoma). In
addition to taking a full ophthalmic history from the
patient it is wise to perform a slit-lamp examination
of the anterior segment of the eye to exclude local
causes before attributing pupil signs to autonomic
failure. Many systemic disorders also influence the
outcome of pupil testing, either through direct effects
on the iris (e.g. diabetes mellitus), or by causing dry
eyes (which affects the penetration of test drugs
through the cornea) (e.g. rheumatoid arthritis, Sjogrens syndrome). A long list of medications (and
non-prescribed drugs) affects the pupil through their
influence on adrenergic, muscarinic or opiate receptors. Care is needed to exclude all such patient-specific confounders before interpreting pupil signs.
j Detecting bilateral autonomic deficits
The particular difficulty detecting bilateral sympathetic or parasympathetic deficits needs special
mention. If a lesion is unilateral there is a control eye
for comparison which greatly improves the sensitivity
of most of the tests described above. However many
autonomic disorders cause diffuse rather than focal
damage (e.g. diabetic autonomic neuropathy, pure
autonomic failure), and are therefore likely to affect
both eyes symmetrically. Under these circumstances
bilateral sympathetic failure is best diagnosed using
absolute measurements such as redilatation lag (T3/4)
the wide normal range in responses to drug tests,
including cocaine and weak agonists such as 1%
phenylephrine, renders these tests relatively insensitive if there is no control eye for comparison. Bilateral
parasympathetic failure is best diagnosed by a combination of the clinical signs (weak or absent LR with
sector palsy, tonic NR) and demonstration of denervation supersensitivity using 0.1% pilocarpine.
In our laboratory we routinely assess sympathetic
and parasympathetic integrity in any given patient
using a number of different tests, seven of which
(dark diameter, light diameter, dark anisocoria, light
reflex ratio, T3/4, near reflex ratio, light-near reflex
ratio) are determined using pupillometry and yield
quantitative measures with abnormality being defined
as a value lying more than 2 standard deviations
outside the mean (one-tailed). In this scenario of
multiple testing, for any given patient the probability
P of observing a single abnormal result by chance
alone is 0.15, and so we dismiss such isolated outlying
values as insignificant. We accept pupil measures as
indicating autonomic disturbance only if two or more
values lie outside the normal range (giving P < 0.012)
or a single measurement lies outside the 99.5% confidence interval (P < 0.034).
98
j Detecting combined sympathetic and
parasympathetic deficits in the pupil
It is to be expected that patients with generalised
dysautonomias affecting both sympathetic and parasympathetic supplies to end organs such as the
bladder, intestines and cardiovascular system would
show signs of both deficit in the pupils. In practice
evidence of combined sympathetic and parasympathetic failure is rarely seen. On our database of 688
cases with Horner syndrome and/or tonic pupils only
20 patients (i.e. 3%) had evidence of both sympathetic
and parasympathetic denervation. The most common
condition causing both types of pupil abnormality is
auto-immune autonomic neuropathy (AAN). An
example is shown in Figure 11. This patient presented
with sub-acute generalised dysautonomia and had a
high titre of antibodies directed against ganglionic
(nicotinic) acetylcholine receptors [30]. As might be
expected, his pupils were small and round but showed
no response to light, accommodative effort or startle;
pharmacological testing confirmed denervation
supersensitivity to weak muscarinic and adrenergic
agonists, but no response to cocaine or hydroxyamphetamine.
In most cases however, detection of combined
sympathetic and parasympathetic deficits is not so
Fig. 11 Use of pharmacological tests to diagnose combined sympathetic and
parasympathetic pupil deficits in a 57-year-old man with auto-immune
autonomic neuropathy (AAN) associated with ganglionic acetylcholine receptor
antibodies. Upper panel: under normal room lighting both pupils appear small
but round and show no response to light or to accommodative effort. Middle
panel: appearance of the pupils 30 minutes after topical administration of 0.1%
pilocarpine to the right eye and 4% cocaine to the left eyethe right pupil
shows denervation supersensitivity to this weak muscarinic agonist confirming
straightforward because each type of deficit tends to
mask the signs of the other. For example, tonic pupils
may have large or small resting diameters depending
on duration since onset, and their attenuated light
responses are slow to dilate so T3/4 measurements
cannot be used to diagnose additional sympathetic
dysfunction: only pharmacological tests can be used
reliably to investigate sympathetic integrity under
these circumstances. Conversely, the presence of a
subtle parasympathetic deficit in a patient with overt
Horner syndrome can sometimes be detected in unilateral cases if the indicators of parasympathetic
integrity are sufficiently asymmetric between the two
eyes, but in bilateral cases where pupil measurements
can only be compared with the healthy population the
wide normal range in responses markedly lowers
diagnostic sensitivity. It is perhaps not surprising
therefore that most patients with generalised dysautonomia appear to have either sympathetic or parasympathetic denervation of the pupil, the diagnosis
depending on which deficit predominates.
j Interpreting pharmacological tests
The use of topically applied drugs greatly improves
detection of autonomic pupillary disturbances but
parasympathetic blockade, whilst the left pupil shows no response to cocaine
confirming sympathetic blockade. Lower panel: on a separate occasion, the
pupils were examined 30 minutes after administration of 1% phenylephrine to
the left eye and 1% hydroxyamphetamine (OHA) to the right eyethe left
pupil shows denervation supersensitivity to this weak adrenergic agonist but
the right pupil shows no response to OHA indicating that the sympathetic
lesion is post-ganglionic
99
care is needed when interpreting the results. Firstly,
there is a wide range in the response of the pupil in
healthy subjects to all of the drugs described in this
review. In part this is due to variable binding of the
drug to melanin pigment in the irisdarker eyes bind
more drug with the result that the drug has less peak
effect but a longer duration of action. Secondly, most
of any topically applied drug enters the eye through
the cornea, whose permeability depends on the
integrity of the tear film and corneal epithelium. Patients with dry eyes (either caused by lacrimal gland
dysfunction or by local conditions affecting the eyelids and ocular surface) will show an enhanced drug
response due to increased drug penetration into the
eye [24]. Thirdly, tests using weak agonists to detect
denervation supersensitivity may show false-negative
results if performed very soon after onset of the lesion
because receptor up-regulation takes time to develop.
In the iris dilator muscle a positive apraclonidine test
has been reported only 2 weeks after onset of a
Horner syndrome caused by carotid dissection [4],
but little is known about the latency between lesion
onset and the test turning positive for these other
drugs. Finally, because of variable melanin-binding
the duration of action of some drugs may be 24 hours
or more; it is generally recommended that a wash-out
period of at least 48 hours is allowed between
sequential pupil drug tests.
j False positive results
So far I have mainly considered the limitations to
detection of autonomic disturbance in the pupils.
However, false-positive results are also occasionally
seen, that is patients with normal pupillary innervation who present with signs that seem to indicate a
sympathetic or a parasympathetic lesion. Two examples are shown in Figure 12. The upper panel shows a
Fig. 12 Masquerades of neurogenic
autonomic disturbance in the pupils.
Upper photograph shows a large
and unreactive left pupil caused by
inadvertent exposure to an antimuscarinic agent (atrovent
inhaler)the pupil had returned to
normal by the next day. Lower
photograph shows relative miosis
and ptosis in the left eye of another
patient, but pupillometric and
pharmacologic investigations failed
to show any evidence of
sympathetic deficitit was
concluded that the anisocoria was
physiological, and the ptosis was
caused by partial dehiscence of the
levator aponeurosis
patient admitted as an emergency for urgent investigation of their fixed dilated pupil. The patient was asthmatic and had been using an Atrovent inhaler
inadvertent administration of this anti-muscarinic drug
had caused a post-junctional (receptor) blockade of the
sphincter muscle in his left eye. The important distinction between a neurogenic etiology (e.g. oculomotor
nerve compression by a posterior communicating artery
aneurysm) and a local aetiology (e.g. exposure to an
antimuscarinic drug or pathology within the iris itself)
can be simply made by administration of a normal
concentration of a cholinergic agonist such as 2% pilocarpine: the pupil will constrict if the cause is neurogenic
but not if the cause is local within the eye [5].
The lower panel in Figure 12 shows an example of
a false-positive sympathetic lesion. This patient with
ptosis and miosis was referred to me for localisation
of her Horner. The dark diameters were normal in
both eyes, there was no redilatation lag and the startle
response was present and of equal amplitudes in both
eyes; moreover, both pupils showed a normal and
symmetrical mydriatic response to 4% cocaine. We
concluded that her longstanding anisocoria (seen in
previous photographs from years earlier) was physiological, and that her more recently acquired ptosis
was due to partial dehiscence of her levator aponeurosis (i.e. a local rather than neurogenic cause). There
are many reports of similar pseudo-Horner cases in
the literature [29] reinforcing the point made earlier
that it is unwise to diagnose a Horner syndrome on
the basis of the clinical signs alone.
j Diagnostic value of pupil testing
Sympathetic or parasympathetic deficits are common
in the pupils of patients with autonomic disorders. In
our series of 150 consecutive cases, some conditions
were only associated with pupil signs of sympathetic
100
loss (e.g. dopamine b-hydroxylase deficiency, MSA,
HSAN type III), others only with pupil signs of
parasympathetic loss (Triple A syndrome, Sjogrens
syndrome); however most types of dysautonomia
could present with either type of pupillary disturbance [6]. Moreover, the predominant deficit in the
pupils of any individual patient showed poor correlation with the pattern of autonomic disturbance seen
elsewhere in the bodysome patients with bilateral
tonic pupils showed predominantly sympathetic disturbance elsewhere and vice versa. Pupil tests cannot
therefore be used to predict the outcome of other
autonomic function tests.
Pupil evaluation can however assist in distinguishing between diagnoses. For example, in the
above case series [6] bilateral Horner syndrome or
tonic pupils was a common finding in patients with
pure autonomic failure (PAF) but was never seen in
multiple system atrophy (MSA)detection of these
bilateral deficits could therefore discriminate between
these diagnoses with a sensitivity of 54.5% and a
specificity of 100%. Moreover, careful attention to the
particular features of the pupillary autonomic disturbance may permit discrimination between diagnoses even when the same overall pattern of deficit is
being observed. For example, patients with bilateral
tonic pupils may have Holmes-Adie syndrome (a
relatively benign diagnosis) or a more generalized
neuropathy (which potentially carries a worse prognosis). Both diagnostic groups show evidence of
parasympathetic loss, but anisocoria >1 mm and
sector palsy are seen much more commonly in HAS;
the presence of these particular features can be used
to distinguish between these two groups with a sensitivity of 58% and a specificity of 90% [7].
A proposed algorithm for evaluating the autonomic innervation of the pupil is shown in Figure 13.
The pupils should first be examined clinically to exclude local pathology and to evaluate not only the
pupil signs (size, anisocoria, shape, heterochromia,
responses to light or accommodative effort) but also
other features such as disturbances of lid position, eye
movements, vasomotor and sudomotor function in
the face and tendon reflexes. If pupillometry is
available then the next step should be to measure the
resting size of the pupils in the light and dark and the
pupillary responses to light, accommodative effort
and a sudden sound. In some cases no further testing
is required to establish the pupil diagnosis, but in
others it is appropriate to proceed to pharmacologic
tests, particularly if a combination of parasympathetic
and sympathetic deficits is suspected. If pupillometry
is not available then it is usually advisable to proceed
pupils
if pupillometry
not available
Autonomic disturbance?
clinical
examination
1. Exclude local pathology
2. Check size, shape, LR & NR of pupils
3. Check lids, OM, face, tendon reexes
pupillometric
tests
1. Resting DD & LD (& anisocoria)
2. LR & NR (amplitude, CVmax, T3/4,)
3. SR (present / absent)
pharmacologic
tests
1. Symp (phe, apracl, coc to conrm)
2. Symp (1% OHA to localise)
3. P/S (0.1% pilo)
diagnosis
1.
2.
3.
4.
Normal innervation
Sympathetic decit (UL or BL)
P/S decit (UL or BL)
Combined symp + P/S decits
Fig. 13 Algorithm for evaluating the pupil in autonomic disorders. Key: LR
light response, NR accommodative (near) response, OM ocular movements, DD
dark diameter, LD light diameter, CVmax maximum constriction velocity, T3/4
time to redilate by 75%, SR startle response, phe = 1% phenylephrine; apracl
= 0.5% apraclonidine; coc = 4% cocaine; OHA = 1% hydroxyamphetamine;
symp sympathetic, P/S parasympathetic, UL unilateral, BL bilateral
directly to pharmacologic tests to confirm sympathetic or parasympathetic deficits (and, in the case of
Horners syndrome, to localize the lesion).
It is likely that the recent development of cheap,
user-friendly pupillometers for the refractive surgery
market will lead to more widespread use of pupillometric testing in autonomic laboratories. Many of the
required pupil measures are routinely provided by
these instruments (e.g. resting pupil size in the dark
or light, amplitude and maximum constriction
velocity of the light reflex response), but others can
only be derived by direct liaison with the manufacturer (e.g. T3/4). Inevitably there is a lack of standardization between these different commercially
manufactured devices regarding light levels (background and stimulus): this will not affect the interpretation of some measures (e.g. resting dark
diameter, T3/4), but for others a normal database will
need to be established by each lab using a cohort of
age-matched healthy controls.
Compared with many other available tests it is
relatively straightforward to measure the pupil and
diagnose autonomic disturbances. Moreover, it is
likely that systematic studies of pupil involvement in
different autonomic disorders will reveal more
examples of the diagnostic potential of pupil testing in
the future. For this reason I am sure pupil evaluation
will play an increasingly important role in the laboratory work-up of the patient with disease of the
autonomic nervous system.
101
References
1. Adie WJ (1932) Tonic pupils and absent tendon reflexes: a benign disorder
sui generis: its complete and incomplete forms. Brain 55:98113
2. Ashker L, Weinstein JM, Dias M et al
(2008) Arachnoid cyst causing third
cranial nerve palsy manifesting as isolated internal ophthalmoplegia and iris
cholinergic supersensitivity. J Neuro
Ophthalmol 28(3):192197
3. Bates A, Chamberlain S, Champion M
et al (1995) Pholedrine: a substitute for
hydroxyamphetamine as diagnostic
eyedrop test in Horners syndrome. J
Neurol Neurosurg Psychiatry 58:215
217
4. Bohnsack BL, Parker JW (2008) Positive apraclonidine test within 2 weeks
of onset of Horner syndrome caused by
carotid artery dissection. J Neuro
Ophthalmol 28(3):235236
5. Bremner FD (2008) Pilocarpine: better
than a scan. Br Med J 336:171
6. Bremner FD, Smith SE (2006) Pupil
findings in a consecutive series of 150
cases of generalized autonomic neuropathy. J Neurol Neurosurg Psychiatry
77:11631168
7. Bremner FD, Smith SE (2007) Bilateral
tonic pupils: Holmes-Adie syndrome or
generalized neuropathy? Br J Ophthalmol 91:16201623
8. Chen P-L, Chen J-T, Lu D-W et al
(2006) Comparing Efficacies of 0.5%
Apraclonidine with 4% cocaine in the
diagnosis of Horner syndrome in
pediatric patients. J Ocular Pharm Ther
22(3):182187
9. Cremer SA, Thompson S, Digree KB
et al (1990) Hydroxyamphetamine
mydriasis in Horners syndrome. Am J
Ophthalmol 110:7176
10. Danesh-Meyer HV, Savino P, Sergott R
(2004) The correlation of phenylephrine 1% and hydroxyamphetamine 1%
in Horners syndrome. Br J Ophthalmol
88(4):592593
11. Holmes G (1931) Partial iridoplegia
associated with symptoms of other
diseases of the nervous system. Trans
Ophthalmol Soc UK 51:209228
12. Horner JF (1869) Ueber eine Form von
Ptosis. Klin Monatsbl Augenheilkd
7:193198
13. Jacobson DM (1990) Pupillary response
to dilute pilocarpine in pre-ganglionic
3rd nerve disorders. Neurology 40:804
808
14. Jacobson DM, Vierkant RA (1998)
Comparison of cholinergic supersensitivity in third nerve palsy and Adies
syndrome. J Neuro Ophthalmol 18:171
175
15. Kardon R (1998) Drop the Alzheimers
drop test. Neurology 50:588591
16. Kardon R (2005) Are we ready to replace cocaine with apraclonidine in the
pharmacologic diagnosis of Horner
syndrome? J Neuro Ophthalmol
25(2):6970
17. Kardon RH, Denison CE, Brown CK
et al (1990) Critical evaluation of the
cocaine test in the diagnosis of Horners syndrome. Arch Ophthalmol
108:384387
18. Lance JW, Drummond PD, Gandevia
SC et al (1988) Harlequin syndrome:
the sudden onset of unilateral flushing
and sweating. J Neurol Neurosurg
Psychiatry 51:635642
19. Lowenstein O, Loewenfeld IE (1950)
Mutual role of sympathetic and parasympathetic in shaping of the pupillary
reflex to light: pupillographic studies.
Arch Neurol Psychiatry 64:341377
20. Lowenstein O, Loewenfeld IE (1958)
Electronic pupillography: a new
instrument and some clinical applications. Arch Ophthalmol 59:352363
21. Morales J, Brown SM, Abdul-Rahim
et al. (2000) Ocular effects of apraclonidine in Horners syndrome. Arch
Ophthalmol 118:951954
22. Pilley S, Thompson HS (1975) Pupillary
dilatation lag in Horners syndrome.
Br J Ophthalmol 59:731735
23. Ramsay DA (1986) Dilute solutions of
phenylephrine and pilocarpine in the
diagnosis of disordered autonomic
innervation of the iris. J Neurol Sci
73:125134
24. Sakakibara R, Hirano S, Asahina M
et al (2004) Primary Sjogrens syndrome presenting with generalized
autonomic failure. Eur J Neurol 11:635
638
25. Smith SA, Smith SE (1990) The quantitative estimation of pupillary dilatation in Horners syndrome. In A Huber
(ed). Sympathicus und Auge; Proc. JF
Horner centenary symposium. pp 152
65. Ferdinand Enke Verlag, Stuttgart
26. Smith SA, Smith SE (1999) Bilateral
Horners syndrome: detection and
occurrence. J Neurol Neurosurg Psychiatry 66:4851
27. Thompson HS (1977) Adies syndrome:
some new observations. Trans Am
Ophthalmol Soc 75:587626
28. Thompson HS (18311886) Johann
Friedrich Horner. Am J Ophthalmol
1986; 102:792795
29. Thompson BM, Corbett JJ, Kline LB
et al (1982) PseudoHorners syndrome. Arch Neurol 39(2):108111
30. Vernino S, Low PA, Fealey RD et al
(2000) Autoantibodies to ganglionic
receptors in autoimmune autonomic
neuropathies. N Engl J Med 343:847
855
31. Wilhelm H, Klier R, Toth B et al (1995)
Oculomotor nerve paresis starting as
isolated internal ophthalmoplegia.
Neuro Ophthalmol 15(4):211215
32. Wilhelm H, Wilhelm B (2003) Clinical
applications of pupillography. J Neuro
Ophthalmol 23:4249
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
Das könnte Ihnen auch gefallen
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeVon EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeBewertung: 4 von 5 Sternen4/5 (5794)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreVon EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreBewertung: 4 von 5 Sternen4/5 (1090)
- Never Split the Difference: Negotiating As If Your Life Depended On ItVon EverandNever Split the Difference: Negotiating As If Your Life Depended On ItBewertung: 4.5 von 5 Sternen4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceVon EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceBewertung: 4 von 5 Sternen4/5 (895)
- Grit: The Power of Passion and PerseveranceVon EverandGrit: The Power of Passion and PerseveranceBewertung: 4 von 5 Sternen4/5 (588)
- Shoe Dog: A Memoir by the Creator of NikeVon EverandShoe Dog: A Memoir by the Creator of NikeBewertung: 4.5 von 5 Sternen4.5/5 (537)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersVon EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersBewertung: 4.5 von 5 Sternen4.5/5 (345)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureVon EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureBewertung: 4.5 von 5 Sternen4.5/5 (474)
- Her Body and Other Parties: StoriesVon EverandHer Body and Other Parties: StoriesBewertung: 4 von 5 Sternen4/5 (821)
- The Emperor of All Maladies: A Biography of CancerVon EverandThe Emperor of All Maladies: A Biography of CancerBewertung: 4.5 von 5 Sternen4.5/5 (271)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Von EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Bewertung: 4.5 von 5 Sternen4.5/5 (121)
- The Little Book of Hygge: Danish Secrets to Happy LivingVon EverandThe Little Book of Hygge: Danish Secrets to Happy LivingBewertung: 3.5 von 5 Sternen3.5/5 (400)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyVon EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyBewertung: 3.5 von 5 Sternen3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)Von EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Bewertung: 4 von 5 Sternen4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaVon EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaBewertung: 4.5 von 5 Sternen4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryVon EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryBewertung: 3.5 von 5 Sternen3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnVon EverandTeam of Rivals: The Political Genius of Abraham LincolnBewertung: 4.5 von 5 Sternen4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealVon EverandOn Fire: The (Burning) Case for a Green New DealBewertung: 4 von 5 Sternen4/5 (74)
- Osama - S Ophthalmology Notes FEBRUARY 2019 EDITIONDokument42 SeitenOsama - S Ophthalmology Notes FEBRUARY 2019 EDITIONNazish Saghir67% (3)
- The Unwinding: An Inner History of the New AmericaVon EverandThe Unwinding: An Inner History of the New AmericaBewertung: 4 von 5 Sternen4/5 (45)
- Anatomy of Trigeminal NerveDokument39 SeitenAnatomy of Trigeminal NerveBharath Kumar Uppuluri100% (1)
- 2023.neuroscience - Trans10.posterior Fossa Level Brainstem Cranial Nuclei and NervesDokument7 Seiten2023.neuroscience - Trans10.posterior Fossa Level Brainstem Cranial Nuclei and NervesstellaNoch keine Bewertungen
- Neurology pdf-1Dokument142 SeitenNeurology pdf-1Ahmed Adel SaadNoch keine Bewertungen
- Benedict SyndromeDokument5 SeitenBenedict SyndromeMarthin Fernandes PasaribuNoch keine Bewertungen
- Conduction Block and Tonic Pupils in Charcot-Marie-Tooth Disease Caused by A Myelin Protein Zero p.Ile112Thr MutationDokument4 SeitenConduction Block and Tonic Pupils in Charcot-Marie-Tooth Disease Caused by A Myelin Protein Zero p.Ile112Thr MutationOlvaria MisfaNoch keine Bewertungen
- Neurosyphilis Masquerading As An Acute Adie's Tonic Pupil: Report of A CaseDokument6 SeitenNeurosyphilis Masquerading As An Acute Adie's Tonic Pupil: Report of A CaseOlvaria MisfaNoch keine Bewertungen
- Tonic Pupil and Corneal Anesthesia After Vitrectomy and Encircling Band For Retinal Detachment in An Ex-Premature ChildDokument5 SeitenTonic Pupil and Corneal Anesthesia After Vitrectomy and Encircling Band For Retinal Detachment in An Ex-Premature ChildOlvaria MisfaNoch keine Bewertungen
- Effects of Some Ophthalmic Medications On Pupil Size: A Literature ReviewDokument5 SeitenEffects of Some Ophthalmic Medications On Pupil Size: A Literature ReviewOlvaria MisfaNoch keine Bewertungen
- Review Article: Neurological Disorders in Primary SJ Ogren's SyndromeDokument11 SeitenReview Article: Neurological Disorders in Primary SJ Ogren's SyndromeOlvaria MisfaNoch keine Bewertungen
- Goldinger Papesh CDPS 2012Dokument6 SeitenGoldinger Papesh CDPS 2012Olvaria MisfaNoch keine Bewertungen
- Tonic Pupils: Andrew S. Gurwood, Od, Faao and Laura E. Lehn, OdDokument3 SeitenTonic Pupils: Andrew S. Gurwood, Od, Faao and Laura E. Lehn, OdOlvaria MisfaNoch keine Bewertungen
- Case Report: Paul A Ford, Peter J Barnes, Omar S UsmaniDokument1 SeiteCase Report: Paul A Ford, Peter J Barnes, Omar S UsmaniOlvaria MisfaNoch keine Bewertungen
- EScholarship UC Item 7293c32tDokument1 SeiteEScholarship UC Item 7293c32tOlvaria MisfaNoch keine Bewertungen
- Resident and Fellow Section: Teaching Case: Migraine and Pupil DilationDokument3 SeitenResident and Fellow Section: Teaching Case: Migraine and Pupil DilationOlvaria MisfaNoch keine Bewertungen
- Surgical Management of Third Nerve PalsyDokument7 SeitenSurgical Management of Third Nerve Palsyhanaddul100% (1)
- Zanki Neuro BoldedDokument25 SeitenZanki Neuro Boldedsmian08Noch keine Bewertungen
- BRAINSTEM II Clinical CorrelatesDokument6 SeitenBRAINSTEM II Clinical CorrelatesrizaNoch keine Bewertungen
- Cranial Nerves Summary PDFDokument9 SeitenCranial Nerves Summary PDFMudassar SattarNoch keine Bewertungen
- Physical AssessmentDokument8 SeitenPhysical Assessmentjanelee2824Noch keine Bewertungen
- Rovielyn P. Ogayre Pt-11 Anaphy: Scalp Muscles Scalp MuscleDokument45 SeitenRovielyn P. Ogayre Pt-11 Anaphy: Scalp Muscles Scalp MuscleRovielyn P OgayreNoch keine Bewertungen
- Eye Movements GuytonDokument10 SeitenEye Movements GuytonDavis KallanNoch keine Bewertungen
- Atlas of Human Anatomy - Including Student Consult Interactive Ancillaries and GuidesDokument11 SeitenAtlas of Human Anatomy - Including Student Consult Interactive Ancillaries and GuidesAndrijana StevancevicNoch keine Bewertungen
- Navy Medical AssessementDokument36 SeitenNavy Medical AssessementstreetdoccanedoNoch keine Bewertungen
- Cranial Nerve Examination 231109 191528Dokument43 SeitenCranial Nerve Examination 231109 191528najlaxd2002Noch keine Bewertungen
- R.jogi Basic Opthalmology PDFDokument512 SeitenR.jogi Basic Opthalmology PDFLuhurulAmriNoch keine Bewertungen
- Anatomy Cranial Nerves LEII LorincziDokument36 SeitenAnatomy Cranial Nerves LEII LorincziBianca IlieNoch keine Bewertungen
- Sensory Receptors Science Presentation in Red Cream Playful Illustrative StyleDokument15 SeitenSensory Receptors Science Presentation in Red Cream Playful Illustrative StylePrincess MapanaoNoch keine Bewertungen
- Wa0003. 1Dokument3 SeitenWa0003. 1Afnankh698Noch keine Bewertungen
- Anatomy and Physiology of The EyeDokument55 SeitenAnatomy and Physiology of The EyeWahyu Caesar Ramdani100% (1)
- Term 2 Fall 2018Dokument90 SeitenTerm 2 Fall 2018Artemio ZavalaNoch keine Bewertungen
- FAQ: How To Examine The Cranial Nerves? PLAB 2 - Part 2 of Professional and Linguistic Assessments Board Exam Conducted by GMC, UK ForumsDokument12 SeitenFAQ: How To Examine The Cranial Nerves? PLAB 2 - Part 2 of Professional and Linguistic Assessments Board Exam Conducted by GMC, UK ForumsjahangirealamNoch keine Bewertungen
- CHAPTER17 Anatomy and Physiology of OcularMotor SystemsDokument78 SeitenCHAPTER17 Anatomy and Physiology of OcularMotor Systemsbenjumear_719607598Noch keine Bewertungen
- Adults MidbrainDokument2 SeitenAdults MidbrainRightBrain PatelNoch keine Bewertungen
- Trigeminal NerveDokument131 SeitenTrigeminal NerveLavanya KalapalaNoch keine Bewertungen
- Sensory and Motor Cranial NervesDokument13 SeitenSensory and Motor Cranial NervesAnn HeerahNoch keine Bewertungen
- Abducent Nerve: DR. Nirmal Jayadev Final Year PG Student (MS Ophthalmology) MKCG Medical College Berhampur OdishaDokument58 SeitenAbducent Nerve: DR. Nirmal Jayadev Final Year PG Student (MS Ophthalmology) MKCG Medical College Berhampur OdishaZeptalanNoch keine Bewertungen
- Management of DiplopiaDokument5 SeitenManagement of Diplopiacahyati syhrilNoch keine Bewertungen
- FMGE Old QuestionsDokument156 SeitenFMGE Old QuestionsAnonymous S0H8cqgnfi100% (11)
- 22 - Brain Stem (Neu)Dokument64 Seiten22 - Brain Stem (Neu)checkmate100% (3)