Beruflich Dokumente
Kultur Dokumente
Menjawab Dan Mengingat
Hochgeladen von
sensnaliquidOriginalbeschreibung:
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Menjawab Dan Mengingat
Hochgeladen von
sensnaliquidCopyright:
Verfügbare Formate
Menjawab dan Mengingat 1
(a)
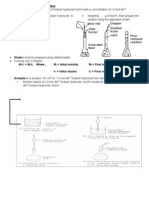
Diagram 7.1 shows an apparatus set-up to determine the empirical formula of magnesium oxide.
Rajah 7.1 menunjukkan susunan radas untuk menentukan formula empirik magnesium oksida.
(i) During the experiment, we need to raise the lid a little
at intervals. Why?
Ketika eksperimen dijalankan, kita perlu membuka penutup
sekali sekala. Mengapa?
[2 marks]
(ii)
(b)
Table 7 shows the results for the experiment to determine the empirical formula of
magnesium oxide.
Jadual 7 menunjukkan keputusan bagi satu eksperimen untuk menentukan formula
empirik bagi magnesium oksida.
Mass of crucible + lid
Jisim mangkuk pijar + penutup
28.24 g
Mass of crucible + lid + magnesium ribbon
Jisim mangkuk pijar + penutup + pita magnesium
30.64 g
Mass of crucible + lid + magnesium oxide
Jisim mangkuk pijar + penutup + magnesium oxide
32.24 g
Based on the results in Table 7, determine the empirical formula of magnesium oxide.
[ Relative atomic mass : Mg = 24 , O = 16 ]
Berdasarkan keputusan dalam Jadual 7, tentukan formula empirik bagi magnesium
oksida.
[ Jisim atom relatif : Mg = 24 , O = 16 ]
[5 marks]
Diagram 7.2 shows an apparatus set-up to determine the empirical formula of copper oxide.
Rajah 7.2 menunjukkan susunan radas untuk menentukan formula empirik kuprum oksida.
(i)
(ii)
(iii)
(iv)
Compare the method used in experiment in Diagram 7.2 with Diagram 7.1.
Bandingkan kaedah melakukan eksperimen dalam Rajah 7.2 dengan kaedah dalam
Rajah 7.1.
[4
marks]
State the reactants to produce hydrogen gas in Part A.
Nyatakan bahan-bahan untuk menghasilkan gas hidrogen di Bahagian A.
[2 marks]
Write the chemical equation for the reaction in Part A.
Tuliskan persamaan kimia untuk tindak balas di Bahagian A.
[1mark]
State three precautions that must be taken in Part B. Explain your answer.
Nyatakan tiga langkah berjaga-jaga yang mesti diambil dalam Bahagian B. Terangkan
jawapan anda.
[6 marks]
Element
Magnesium,
Oxygen, O
Mg
Mass(g)
30.64 28.24 =
32.24 30.64 =
Question
2.4
1.6 Explanation
No.
Number of
2.4
1.6
8 moles
(a)(i) of atoms 24
= 0.1
16 = 0.1
Simplest ratio
1
1
Diagram 7.1
Diagram 7.2
of(ii)
moles
It involves heating MgO It involves heating
Empirical
Similarity
The reaction is between The reaction is between
formula
a gas and a solid
a gas and a solid
Metal oxide is formed
Metal is formed
Metal is reacted with
Metal oxide is reacted
oxygen gas
with hydrogen gas
Difference The mass of the solid
The mass of the solid
increases
decreases
Mark
- To allow the
oxygen / air to
enter the crucible
- for the complete
combustion of
magnesium
1
1
2
1
1
1
(b)(i)
1
1
1
1
1
(ii)
(iii)
1
1
(iv)
- Dilute hydrochloric acid // or any dilute acid
- Zinc // or any reactive metal
2HCl + Zn ZnCl2 + H2
Precaution
Dry hydrogen gas is passed
through the combustion tube for
a few minutes / throughout the
experiment to remove all the air
in the tube.
During cooling, the flow of
hydrogen is continued.
The heating, cooling and
weighing processes are repeated
until a constant mass is obtained.
The combustion tube must be
slanted slightly towards the tiny
Explanation
A mixture of hydrogen and air
can cause an explosion when
lighted.
To ensure the oxygen from the
air does not oxidise the hot
copper to copper(II) oxide.
To ensure that all of the
copper(II) oxide has been
reduced into copper.
To prevent the water formed
during the reaction from flowing
hole.
towards the hot porcelain dish.
Total marks 20
9. (a) The statement below is about a reaction.
Pernyataan di bawah ialah tentang suatu tindak balas.
0.01 mol of hydrogen chloride gas reacts with 0.01 mol of ammonia gas produces 0.01 mol of
ammonium chloride solid
0.01 mol gas hydrogen klorida bertindak balas dengan 0.01 mol gas ammonia menghasilkan 0.01 mol
pepejal ammonium klorida
(i)
(ii)
(iii)
(b)
What is the meaning of a mole?
Apakah yang dimaksudkan dengan satu mol?
Calculate the numbers of particles in 0.01 mol of ammonium chloride.
[Avogadro constant = 6.02 X 1023 mol-1]
Hitungkan bilangan zarah-zarah dalam 0.01 mol ammonium klorida.
[Pemalar Avogadro = 6.02 X 1023 mol-1]
[1 mark]
[1 mark]
Write the molecular formulae of hydrogen chloride and ammonia. Calculate their relative
molecular mass.[Relative atomic mass: H = 1, N = 14, Cl = 35.5]
Tuliskan formula molekul bagi hydrogen klorida dan ammonia. Hitungkan jisim molekul mereka.
[Jisim atom relative: H = 1, N = 14, Cl = 35.5]
[4 marks]
Below is a chemical equation for a reaction.
Di bawah ialah persamaan kimia bagi suatu tindak balas.
2KI(aq) + Pb(NO3)2(aq)
2KNO3(aq) + PbI2(s)
(i)
Name the reaction and state one observation for the reaction.
Namakan tindak balas itu dan nyatakan satu permerhatian bagi tindak balas itu. [2 marks]
(ii) Based on the chemical equation, interpret the equation qualitatively and quantitatively.
Berdasarkan kepada persamaan kimia itu, tafsirkan persamaan itu seca kualitatif
dan kuantitatif.
[4 marks]
(c) The statement below describe an activity in laboratory.
Pernyataan di bawah menghuraikan satu aktiviti dalam makmal.
An activity is carried out as follows:
Copper (II) carbonate is heated in a test tube. Gas produced is passed in lime water through a delivery tube.
Suatu aktiviti dijalankan seperti berikut:
Kuprum(II) karbonat dipanaskan dalam sebuah tabung uji. Gas yang terhasil dihasilkan dilalukan ke
dalam air kapur melalui tiub penghantar.
(i) Draw the apparatus set-up for the activity.
Lukis susunan radas bagi aktiviti itu.
[2 marks]
(ii) Write the chemical equation for the reaction.
Tulis persamaan kimia bagi tindak balas itu.
(iii)
6.2 g of copper(II) carbonate is used in the reaction.Calculate the volume of carbon dioxide gas
produced at room condition. [Relative atomic mass: C = 12, O = 16, Cu = 64.
Molar volume at room condition = 24 dm3 mol-1]
6.2 g kuprum(II) karbonat digunakan dalam tindak balas ini.Hitungkan isipadu gas karbon
dioksida dihasilkan pada keadaan bilik.
[4 marks]
No. of Q
9
[2 marks]
Explanation
Marks
Total
Mark
(a)(i)
Amount of substance that contain as many particle as the number
of atoms is exactly 12g of carbon-12 //
6 X 1023 of particles in a substance
(ii)
0.01 X 6.02 X 1023 / 6.02 X 1021
1+1
1+1
2
2
(iii)
Molecular formula
HCI
NH3
RMM
1+35.5 //36.5
14 +3(1) //17
Double decomposition // precipitation Yellow
precipitate
b(i)
(ii)
1 names of reactants
2 names of products
3 physical states
4 moles of reactants and products
No. of Q
c(i)
Explanation
Marks
2 mol / formula units of potassium iodide aqueous reacts with 1 mol /
formula units lead(II) nitrate aqueous produces 2 mol / formula units of
potassium nitrate aqueous and 1 mol / formula units lead(11) iodide
solid
Functional diagram:
Test tube with clamp, no leakage in stepper & delivery tube
inside lime water
Label
heat with arrow / /draw Bunsen burner, lime water and copper(II)
carbonate
(ii)
Total
Mark
CuCO3 CuO + CO2
Correct reactant
correct products
1
1
2
3
4
Number of mol of CuCO3 6.2 = 0.05
124
I mol of CuCO3 produces 1 mol of CO2 II 0.05 mol of
CuCO3
produces 0.05 mol of CO,
Volume of CO2 = 0.05 X 24
1.2 dm3 / 1200 cm3
1
1
4
1
20
Das könnte Ihnen auch gefallen
- Thermal Engineering I Full Book FinalDokument175 SeitenThermal Engineering I Full Book FinalKumar SubramanianNoch keine Bewertungen
- Auto Parts Industry MexicoDokument39 SeitenAuto Parts Industry MexicoAna Celene RojasNoch keine Bewertungen
- Abb Price ListDokument340 SeitenAbb Price ListyogeshNoch keine Bewertungen
- OLSS & EOLSS Valves - S&F - A3 SizeDokument4 SeitenOLSS & EOLSS Valves - S&F - A3 Sizeprem sagar100% (3)
- SPM 2022 Chemistry Paper3 Kerja AmaliDokument28 SeitenSPM 2022 Chemistry Paper3 Kerja Amali22 LEE KE YIN 李科莹Noch keine Bewertungen
- Latihan Empirical FormulaDokument11 SeitenLatihan Empirical FormulaRusdi Chodeng100% (1)
- GP 43-47-Pipeline Commissioning and Handover To OperationsDokument20 SeitenGP 43-47-Pipeline Commissioning and Handover To Operationsmengelito almonte0% (2)
- SPM Kimia Tingkatan 4,5 - Paper3 - 20170420112820Dokument4 SeitenSPM Kimia Tingkatan 4,5 - Paper3 - 20170420112820nurzatilNoch keine Bewertungen
- Rate of ReactionDokument20 SeitenRate of ReactionHAKIMIN_KHAIRUL3674Noch keine Bewertungen
- Module 62 Rate of Reaction Concentration Effect - DwiDokument2 SeitenModule 62 Rate of Reaction Concentration Effect - Dwirudi_zNoch keine Bewertungen
- Chemistry Form 4 Chapter 9 ExerciseDokument7 SeitenChemistry Form 4 Chapter 9 ExerciseAngie Kong Su MeiNoch keine Bewertungen
- Latihan Gabungan Alkana N AlkenaDokument6 SeitenLatihan Gabungan Alkana N AlkenaJuni FarhanaNoch keine Bewertungen
- Addmath Exercise Form 4Dokument2 SeitenAddmath Exercise Form 4Ainun SyakirahNoch keine Bewertungen
- Plant NutritionDokument37 SeitenPlant NutritionWen Shan ChuaNoch keine Bewertungen
- Module The MoleDokument44 SeitenModule The MoleChin Chin YipNoch keine Bewertungen
- 3 Chemical Formulae and EquationDokument43 Seiten3 Chemical Formulae and EquationmawarhanifNoch keine Bewertungen
- Dwibahasa - Modul Latihan Asas Persediaan Maths Tingkatan 3Dokument26 SeitenDwibahasa - Modul Latihan Asas Persediaan Maths Tingkatan 3Manik SobriNoch keine Bewertungen
- Modul Biologi 2016 t4 Soalan PDFDokument87 SeitenModul Biologi 2016 t4 Soalan PDFIsmaliza IshakNoch keine Bewertungen
- An Experiment Is Conducted To Determine The Rate of Reaction Between 25 CMDokument3 SeitenAn Experiment Is Conducted To Determine The Rate of Reaction Between 25 CMJuni FarhanaNoch keine Bewertungen
- Jawapan Bagi Bahan Bengkel Seminar Kimia SPM 2014 Oleh Cikgu AduraDokument63 SeitenJawapan Bagi Bahan Bengkel Seminar Kimia SPM 2014 Oleh Cikgu AduraCikgu FaizalNoch keine Bewertungen
- 8A Salts - AnswerDokument14 Seiten8A Salts - AnswerFrankieNgNoch keine Bewertungen
- Peraturan Pemarkahan Percubaan SBP Pt3 2015 - SainsDokument13 SeitenPeraturan Pemarkahan Percubaan SBP Pt3 2015 - Sainsfira100% (3)
- DIFFERENTIATIONDokument2 SeitenDIFFERENTIATIONBalkis Abdul ManafNoch keine Bewertungen
- Graphing spring constant and surface area pressure relationshipDokument6 SeitenGraphing spring constant and surface area pressure relationshipSeraMa JambuiNoch keine Bewertungen
- Effect of Temperature on Amylase ReactionDokument13 SeitenEffect of Temperature on Amylase ReactioncekminNoch keine Bewertungen
- Question Score A Chapter 1Dokument14 SeitenQuestion Score A Chapter 1Dee -AdilaNoch keine Bewertungen
- SOALAN STRUKTUR Form 5Dokument9 SeitenSOALAN STRUKTUR Form 5Willey TaluanNoch keine Bewertungen
- 7 Transfer of Electrons at A DistanceDokument15 Seiten7 Transfer of Electrons at A DistancenamikNoch keine Bewertungen
- Chemistry (The Mole)Dokument44 SeitenChemistry (The Mole)Aisya AnwarNoch keine Bewertungen
- Displacement Reactions of Metals in Salt SolutionsDokument4 SeitenDisplacement Reactions of Metals in Salt SolutionsAini HasshimNoch keine Bewertungen
- Johor-Answer P2-Trial SPM 2007Dokument8 SeitenJohor-Answer P2-Trial SPM 2007kamalharmozaNoch keine Bewertungen
- Form 4 - Salts (+experiment)Dokument4 SeitenForm 4 - Salts (+experiment)kanryu_zonasNoch keine Bewertungen
- Soluble and Insoluble Salts GuideDokument5 SeitenSoluble and Insoluble Salts GuideAzrel YusoffNoch keine Bewertungen
- Chemistry SkemaMara2009Dokument13 SeitenChemistry SkemaMara2009spm_victim2010Noch keine Bewertungen
- Kadar Tindak Balas.K 2 & K3Dokument16 SeitenKadar Tindak Balas.K 2 & K3Narah NasNoch keine Bewertungen
- ModulDokument39 SeitenModulThanabalan MunuswamyNoch keine Bewertungen
- Manufactured Substances in IndustryDokument13 SeitenManufactured Substances in IndustryNorsuriani AwangNoch keine Bewertungen
- Answer Gerak Gempur Chemistry 2013Dokument11 SeitenAnswer Gerak Gempur Chemistry 2013ryder1man6433Noch keine Bewertungen
- Nutrition 3Dokument2 SeitenNutrition 3azszahNoch keine Bewertungen
- Reading Part 2Dokument14 SeitenReading Part 2Nur HafezaNoch keine Bewertungen
- Sdar Pemantapan Pt3 2019Dokument27 SeitenSdar Pemantapan Pt3 2019norazily81100% (1)
- KimDokument104 SeitenKimBayby SiZzle'zNoch keine Bewertungen
- SPM PAST YEAR QUESTIONS: CHEMICAL BONDING PAPER 2Dokument11 SeitenSPM PAST YEAR QUESTIONS: CHEMICAL BONDING PAPER 2Luna LatisyaNoch keine Bewertungen
- Atomic structure and states of matter in Chemistry moduleDokument30 SeitenAtomic structure and states of matter in Chemistry moduleNur Irdina HaniNoch keine Bewertungen
- MATH MID YEAR F1 2023 (Ques & Ans)Dokument18 SeitenMATH MID YEAR F1 2023 (Ques & Ans)Maiza MizaNoch keine Bewertungen
- SPM State Trial Papers Form 5 Chapter 2: Carbon CompoundsDokument16 SeitenSPM State Trial Papers Form 5 Chapter 2: Carbon CompoundsLaw Jin YaoNoch keine Bewertungen
- Rajah 8.2 Menunjukkan Satu Cawan Bertutup. Cawan Ini Tidak Sesuai Untuk Mengekalkan Suhu Bagi Minuman Panas Dalam Masa Yang LamaDokument10 SeitenRajah 8.2 Menunjukkan Satu Cawan Bertutup. Cawan Ini Tidak Sesuai Untuk Mengekalkan Suhu Bagi Minuman Panas Dalam Masa Yang Lamajgd2080Noch keine Bewertungen
- PMTLDokument314 SeitenPMTLRahoul Chicharito RooneyNoch keine Bewertungen
- Bengkel Teknik Jawab kimia2016 Modul Smart1_Teknik Jawab Konsep Asas KimiaDokument24 SeitenBengkel Teknik Jawab kimia2016 Modul Smart1_Teknik Jawab Konsep Asas KimiaSiti Hajar Abd HamidNoch keine Bewertungen
- Final Form 4 2011 (Soalan Dan Skema), Peperiksaan Akhir Tahun Tingkatan 4 2011Dokument22 SeitenFinal Form 4 2011 (Soalan Dan Skema), Peperiksaan Akhir Tahun Tingkatan 4 2011Rohaya Morat60% (5)
- Matematik K1 Trial SPM SBP 2019Dokument28 SeitenMatematik K1 Trial SPM SBP 2019Fendi A. Bakar75% (4)
- 3A Chemical Formulae and Equations-AnswerDokument11 Seiten3A Chemical Formulae and Equations-AnswerWong Wai LunNoch keine Bewertungen
- Bio Form 4 Kertas 2 (2018) Set 2 (1) - CalonDokument15 SeitenBio Form 4 Kertas 2 (2018) Set 2 (1) - CalonHuda TahaNoch keine Bewertungen
- Ujian1 PHYSICS Form 4Dokument11 SeitenUjian1 PHYSICS Form 4Pauling ChiaNoch keine Bewertungen
- Skema Jawapan Kimia p2Dokument12 SeitenSkema Jawapan Kimia p2HenrySeow50% (8)
- Inertia Experiment Peka 2Dokument8 SeitenInertia Experiment Peka 2A. Suhaimi100% (8)
- Ujian Diagnostik Kimia t5Dokument5 SeitenUjian Diagnostik Kimia t5Kung Chui LingNoch keine Bewertungen
- PTN PPT Matematik Tambahan Tingkatan 4Dokument14 SeitenPTN PPT Matematik Tambahan Tingkatan 4Niceman NatiqiNoch keine Bewertungen
- Chemistry Module Form 4Dokument32 SeitenChemistry Module Form 4mohd faisol100% (3)
- Yearly Lesson Plan Mathematics Form 5Dokument10 SeitenYearly Lesson Plan Mathematics Form 5ryeNoch keine Bewertungen
- MSG456 Mathematical - Programming (May 2010)Dokument7 SeitenMSG456 Mathematical - Programming (May 2010)dikkanNoch keine Bewertungen
- Ujian PBD Penggal 1 2022Dokument7 SeitenUjian PBD Penggal 1 2022FARID ARIFIN BIN MD ARIFIN MoeNoch keine Bewertungen
- Determining empirical formula of magnesium oxideDokument3 SeitenDetermining empirical formula of magnesium oxideLhogeswaran RaviNoch keine Bewertungen
- Revision Questions For Long TestDokument10 SeitenRevision Questions For Long Testzainab792009Noch keine Bewertungen
- Modul Kimia Set 1 Latest (1-4)Dokument23 SeitenModul Kimia Set 1 Latest (1-4)Afiq AtiqullahNoch keine Bewertungen
- Test For Cation and Anion Revise 2011Dokument2 SeitenTest For Cation and Anion Revise 2011sensnaliquidNoch keine Bewertungen
- Note Salt BMDokument5 SeitenNote Salt BMsensnaliquidNoch keine Bewertungen
- Menjawab Esei KimiaDokument2 SeitenMenjawab Esei KimiasensnaliquidNoch keine Bewertungen
- Chapter 9 Manufacture NotesDokument6 SeitenChapter 9 Manufacture NotessensnaliquidNoch keine Bewertungen
- NoteelectricityDokument1 SeiteNoteelectricitysensnaliquidNoch keine Bewertungen
- Chapter 2 (Structure of Atom) : ParticleDokument1 SeiteChapter 2 (Structure of Atom) : ParticlesensnaliquidNoch keine Bewertungen
- Borang Gred Induk Pentaksiran Kerja Amali KimiaDokument8 SeitenBorang Gred Induk Pentaksiran Kerja Amali KimiasensnaliquidNoch keine Bewertungen
- Chapter 9 Manufacture NotesDokument6 SeitenChapter 9 Manufacture NotessensnaliquidNoch keine Bewertungen
- Jaba Tan Pelajaran Negeri Johor Peperiksaan Percubaan Penilaian Menengah RendahDokument37 SeitenJaba Tan Pelajaran Negeri Johor Peperiksaan Percubaan Penilaian Menengah RendahsensnaliquidNoch keine Bewertungen
- Flow chart salt Y ions testDokument4 SeitenFlow chart salt Y ions testsensnaliquidNoch keine Bewertungen
- Note Preparation of Solution and DilutionDokument2 SeitenNote Preparation of Solution and DilutionsensnaliquidNoch keine Bewertungen
- PMR Sebenar Paper1 2008Dokument12 SeitenPMR Sebenar Paper1 2008sensnaliquidNoch keine Bewertungen
- Chem Paper 2f4Dokument12 SeitenChem Paper 2f4sensnaliquidNoch keine Bewertungen
- 2010 PSPM Kedah Chemistry 123 W AnsDokument88 Seiten2010 PSPM Kedah Chemistry 123 W Ansjee2kk100% (1)
- PMR Sebenar Paper1 2007Dokument15 SeitenPMR Sebenar Paper1 2007sensnaliquidNoch keine Bewertungen
- Calculation Mol and EmpericDokument1 SeiteCalculation Mol and EmpericsensnaliquidNoch keine Bewertungen
- PMR Sebenar Paper1 2006Dokument10 SeitenPMR Sebenar Paper1 2006sensnaliquidNoch keine Bewertungen
- ElectrochemistryDokument1 SeiteElectrochemistrysensnaliquidNoch keine Bewertungen
- Ionic and Covalent BondDokument1 SeiteIonic and Covalent BondsensnaliquidNoch keine Bewertungen
- Top 20 CSR InitiativesDokument16 SeitenTop 20 CSR InitiativesMylene Sunga AbergasNoch keine Bewertungen
- 4U - Physics Equations Formula SheetDokument2 Seiten4U - Physics Equations Formula Sheettrini_gangstaNoch keine Bewertungen
- Contactor FinderDokument10 SeitenContactor FinderEfrain Valiente RiveraNoch keine Bewertungen
- Datasheet For OT FIT 40 220-240 1A0 CSDokument4 SeitenDatasheet For OT FIT 40 220-240 1A0 CSpeter hansenNoch keine Bewertungen
- Rotavapor Buchi R-300Dokument102 SeitenRotavapor Buchi R-300ViridianaGarciaNoch keine Bewertungen
- Current Issues in Environmental Management in Australia: What Do People Think?Dokument21 SeitenCurrent Issues in Environmental Management in Australia: What Do People Think?Mahwestie PwarnasoekmaNoch keine Bewertungen
- Trickling Filter: Aerobic Biological Wastewater Treatment ProcessDokument10 SeitenTrickling Filter: Aerobic Biological Wastewater Treatment Processpriodeep chowdhuryNoch keine Bewertungen
- So No FusionDokument188 SeitenSo No FusionVincent J. CataldiNoch keine Bewertungen
- dcP-9055CDN DCP-9270CDN MFC-9460CDN MFC-9465CDN MFC-9560CDW MFC-9970CDW PDFDokument42 SeitendcP-9055CDN DCP-9270CDN MFC-9460CDN MFC-9465CDN MFC-9560CDW MFC-9970CDW PDFStefanGarnetNoch keine Bewertungen
- StressDokument13 SeitenStressLenielle AmatosaNoch keine Bewertungen
- Area StatementDokument15 SeitenArea StatementNeha GhatageNoch keine Bewertungen
- Flowserve Series 59Dokument4 SeitenFlowserve Series 59Sidney RiveraNoch keine Bewertungen
- Biosynthetic Pathways - GPDokument46 SeitenBiosynthetic Pathways - GPGhanshyam R ParmarNoch keine Bewertungen
- J Ijleo 2021 166787Dokument12 SeitenJ Ijleo 2021 166787Hammad AslamNoch keine Bewertungen
- Color Kinetics Inc b2bDokument8 SeitenColor Kinetics Inc b2bKaushal RaiNoch keine Bewertungen
- 0620:62:O:N 2016 Paper 6Dokument12 Seiten0620:62:O:N 2016 Paper 6CHANDREN ARUMUGAM100% (1)
- Sentra Aircon PDFDokument98 SeitenSentra Aircon PDFMed KevlarNoch keine Bewertungen
- The Physical Chemistry of Cytoplasm and Its Influence On Cell Function: An UpdateDokument4 SeitenThe Physical Chemistry of Cytoplasm and Its Influence On Cell Function: An UpdateIvan Jason SalyanoNoch keine Bewertungen
- Icl7660 PDFDokument11 SeitenIcl7660 PDFfran01334Noch keine Bewertungen
- Waste Management Study of FoundriesDokument64 SeitenWaste Management Study of FoundriesSumit GusainNoch keine Bewertungen
- Wet-Ic L&T Construction LTD., Water & Effluent Treatment IcDokument1 SeiteWet-Ic L&T Construction LTD., Water & Effluent Treatment IcRahesh MNoch keine Bewertungen
- Dam Outlet Works: 3.1 Introduction To Dam Out LetsDokument17 SeitenDam Outlet Works: 3.1 Introduction To Dam Out LetsNatty Tesfaye100% (1)
- KYOCERA km2540 SERVICE MANUALDokument364 SeitenKYOCERA km2540 SERVICE MANUALshaj100% (1)
- Case Study Plant Harris Non Chemical DechlorinationDokument1 SeiteCase Study Plant Harris Non Chemical DechlorinationIrfan OmercausevicNoch keine Bewertungen
- GEAR PUMPS: COMPACT AND HIGH-PERFORMANCE LIQUID TRANSFERDokument5 SeitenGEAR PUMPS: COMPACT AND HIGH-PERFORMANCE LIQUID TRANSFERGeorge Cobra100% (1)