Beruflich Dokumente
Kultur Dokumente
Effects of UV Radiation On Photosynthesis of Phytoplankton Exposed To Solar Simulator Light
Hochgeladen von
Alexsandro ClaudinoOriginaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Effects of UV Radiation On Photosynthesis of Phytoplankton Exposed To Solar Simulator Light
Hochgeladen von
Alexsandro ClaudinoCopyright:
Verfügbare Formate
Jomamlof
B :B I O LO G Y
ELSEVIER
Journal of Photochemistry and Photobiology B: Biology 34 (1996) 21-28
Effects of UV radiation on photosynthesis of phytoplankton exposed to
solar simulator light
H. Herrmann a, D.-P. H~ider a, M. K6fferlein b, H.K. Seidlitz b, F. Ghetti c,.
a institut3~r Botanik und Pharmazeutische Biologie, Erlangen, Germany
GSF-Forschungszentrum fiir Umwelt und Gesundheit GmbH, Expositionskammern, Neuherberg, Germany
c CNR Istituto di Biofisica, via San Lorenzo 26, 56127 Pisa, Italy
Received 2 May 1995; accepted 2 October 1995
~_bstract
A sunlight simulator was used to investigate the effects of short term irradiation on photosynthetic oxygen production and chlorophyll
fluorescence in the unicellular algae Dunaliella salina and Ochromonas danica. Oxygen evolution was measured with a Clark-type electrode
and initial (Fo) and maximal (Fro) fluorescence were determined by means of a pulse amplitude-modulated (PAM) fluorometer. Using
different long-pass filters, a UV exclusion study was performed comparing the effects of irradiation with full sun simulator light, with sun
fimulator light without UV-B and short wavelength UV-A radiation and without any UV component. In D. salina, exposure to full sun
simulator light caused a marked drop in photochemical efficiency indicated by a decline in the initial slope of the irradiance-response curve
tbr photosynthetic oxygen production and by a decrease in the ratio Fv/Fm, whereas only a slight inhibition of photosynthetic activity was
3bserved irradiating with PAR and UV-A radiation or with PAR alone. In the case of O. danica, a strong inhibition was observed under all
~rradiation conditions. The results are consistent with preliminary data obtained with outdoor experiments.
Keywords: Dunaliella salina; Oxygen evolution; Ochromonas danica; PAM fluorescence; Sunlight; Sunlight simulator; UV-B radiation; UV-A radiation
1. Introduction
In recent years much effort has centred on acquiring irradiation systems with an emission spectrum simulating solar
radiation, in order to meet the requirement of reproducible
light conditions for ecophysiological plant research and, more
generally, in the field of environmental photobiology [ 1-3 ].
Such simulators allow experiments to be carried out in a
controlled environment, where irradiation conditions and climatic factors can be reproduced at any time and disturbing
agents, often encountered in field experiments, can be
excluded.
The results of indoor experiments, extrapolated to the natural conditions after comparison with data from field experiments, will allow prediction of the consequences of possible
global changes in the natural light environment. In this
respect, sunlight simulators could be a useful tool to determine whether terrestrial plants and aquatic photosynthetic
organisms are sensitive to present levels of solar UV radiation
and to forecast the effects of increasing ultraviolet irradiance
* Corresponding author. Tel.: + 39 50 513214, fax: + 39 50 553 501, email: GHE'I~rI @IB.PI. CNR.IT
1011-1344/96/$15.00 1996 Elsevier Science S.A. All rights reserved
SSDI 101 l- 1 3 4 4 ( 9 5 ) 0 7 2 4 5 - 4
on the Earth' s surface, due to the depletion of the stratospheric
ozone layer.
For phytoplankton organisms many studies reported the
deleterious effects of UV radiation on growth, metabolism,
motility, photo- and gravi-orientation, pigmentation and photosynthetic capability (Refs. [ 4,5 ] ). The contribution of UVB (280-315 nm) and UV-A (315--400 nm) radiation in
inhibiting photosynthesis was described for various phytoplankton species and/or communities under artificial [6-9]
and solar [ 10-16] irradiation conditions. UV-B radiation, in
marine phytoplankton exposed to different spectral distribution of solar light, was shown to be almost as effective in
inhibiting photosynthesis as UV-A radiation, although total
energy in the UV-B was about 10% of that in UV-A [ 11 ].
Moreover, the substantial role of short wavelength ( ~<350
nm) UV-A radiation in reducing carbon uptake was demonstrated [ 15]. Larkum and Wood [9], using artificial irradiation, showed that the drop in photosynthetic oxygen
production caused by UV-B radiation in various species of
phytoplankton is stronger than inhibition due to PAR (photosynthetic active radiation) and that the observed decrease
in variable fluorescence is a fast and sensitive indicator of
UV-B effects.
H. Herrmann et al. / Journal of Photochemistry and Photobiology B: Biology 34 (1996) 21-28
22
10~
10
'E
,o. . . . . . . . . . .
.....................
~D
'~
102
10-3
. . . . I. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
I
J
104
]
300
I
400
i
500
I
600
I
700
Wavelength [nm]
Fig. 1. Spectral distributions in the three experimental conditions of irradiation: unfiltered sun simulator radiation, with quartz window covering the
samples (solid line) ; sun simulator radiation filtered with WG 360 (dashed
line) and GG 400 (dotted line) cut-off filters (-i.e. sun simulator radiation
deprived of UV-B and total UV components, respectively).
In this work, a sunlight simulator [3] was used in the
investigation of the effects of short term ( ~<30 min) irradiation on the photosynthetic activity of two unicellular flagellate algae: the halotolerant chlorophycean Dunaliella salina
and the freshwater chrysophycean Ochromonas danica. Preliminary UV exclusion studies in the field (see below) had
shown in these two organisms quite different effects of exposure to solar radiation and in this work the reproducibility of
these results in the controlled environment of the sun simulator was tested.
In order to estimate the inhibition of photosynthesis caused
by UV-B and short wavelength UV-A radiation, the sunlight
simulator was used for a UV exclusion study, in which the
effects of irradiation in the following three experimental conditions were compared: (a) full sun simulator light (A > 295
nm); (b) sun simulator light deprived of UV-B and short
UV-A wavelengths (A > 338 nm); (c) sun simulator light
deprived of almost all UV (A>385 nm). Photosynthetic
activity was probed by measuring oxygen production with a
Clark-type electrode and chlorophyll fluorescence by means
of a pulse amplitude-modulated fluorometer (PAM).
2. Materials and methods
Dunaliella salina was grown under white light (about 10
W m-2), in a light-dark cycle (15 h light-9 h dark), at 25
C in a medium described by Johnson et al. [ 17]. Ochromonas danica (SAG 933-7, G6ttingen) was grown as
described by Tippit et al. [ 18] under continuous white light
(about 5 W m - z ) at 23 C.
The experiments were performed in a climatized sun simulator especially designed for plant experiments [3]. The
light is emitted by an array of several lamp types. Metal halide
( Osram HQI-T5 400W / D), q uartz-halogen ( Osram Halostar 500W) and blue fluorescent (Philips TL36/18) lamps
provide the main lighting in the visible region. Excess IR
radiation is absorbed by a layer of circulating deionized water.
Extra quartz-halogen lamps located below the water filter
provide long-wave irradiation otherwise eliminated by the
water filter. UV-B fluorescence lamps (Philips TL40W/12)
were mounted below the water filter on the walls of the lamp
house. Short wavelength UV-B and UV-C radiation (i.e.
wavelengths shorter than 290 nm) are rejected by two layers
of borosilicate glass (Schott, TEMPAX) each 6.5 mm thick.
Thus an almost optimal spectral match of natural global radiation is obtained. The spectral irradiance in the sun simulator
was determined by means of a radiometric calibrated double
monochromator system (Bentham M300, UK).
The organisms were exposed to simulator light in small
Petri dishes, kept in a water bath at a temperature of 23 _+2
C. They were irradiated with unfiltered and filtered sun simulator radiation: WG 360 or GG 400 long-pass filters (3 mm,
Schott & Gen., Mainz, Germany) were used to cut off UVB and short wavelength UV-A or almost all UV, respectively.
A quartz window was used to cover the samples irradiated
with unfiltered sun simulator light in order to compensate for
reflection losses of the long-pass filters. In Fig. 1 the spectral
distributions obtained in the three different irradiation conditions are shown. Table 1 shows the respective integrated
spectral irradiances.
Absolute photosynthetic oxygen production was determined at room temperature, before and after exposure, using
a Clark-type electrode inserted in a cuvette for liquid-phase
measurements (DW2/2, Hansatech Instruments, Norfolk,
Table 1
Integrated spectral irradiances for UV-B, UV-A and PAR ranges of the sun simulator and of the sun (in Tramariglio, about 40o36 ' N, 8 11' E, Alghero, Sardinia,
Italy, May 1993
Irradiation conditions
UV-B
( W m -2)
UV-A
( W m 2)
PAR
( W m -2)
Sun simulator
Unfiltered
Filtered with WG360
Filtered with WG400
1.6
0.0
0.0
47.6
22.0 ( h > 338 nm)
1.3
336
336
318
Sun
Unfiltered
Filtered with WG360
Filtered with WG400
1.1
0.0
0.0
45.2
18.5 (A > 338 rim)
2.0
390
367
364
Data are calculated from spectral irradiance measurements performed by means of a double monochromator spectroradiometer.
H. Herrmann et al. / Journal of Photochemistry and Photobiology B: Biology 34 (1996) 21-28
UK). In order to stimulate photosynthetic oxygen production,
samples were irradiated with actinic white light provided by
a quartz-halogen lamp filtered by an infrared absorbing filter.
Irradiance-response curves were determined varying the irradiance by means of neutral density filters. The photosynthetic
activity of the algal samples was calculated as femtomoles of
oxygen per cell per minute. Oxygen consumption due to
respiration was measured for each sample, kept in the dark.
As respiration was not significantly affected by the different
irradiation conditions, the absolute value of oxygen consumption determined in the dark was subtracted from the
absolute values of oxygen production under light irradiation,
m order to evaluate the net photosynthetic oxygen production
rate.
In vivo chlorophyll fluorescence was measured at room
temperature with a pulse amplitude modulation fluorometer
(PAM 101, Waltz, Effeltrich, Germany). Before and after
exposure to simulated solar radiation, F o (initial fluorescence,
all reaction centers are in an open state), Fm (maximal fluorescence, all reaction centers are closed), Fv (variable fluorescence, F v = F m - F 0 )
and the ratio F J F m were
:letermined. Before each measurement, the cells were dark
adapted for 5 rain. Fo was induced by low fluence rate red
light pulses (0.031 W m 2. emission peak at 650 nm) emitted
~y an LED (type USBR, Stanley) for 1 /~s at a frequency of
1.6 kHz. Fm was induced by the application of a saturating
!~ulse of white light (770 W m -2; 800 ms) using a fiber optic
-on nected to a halogen cold light lamp (KL 1500 electronic:
Schott, Mainz, Germany).
Analogous field experiments were performed at the Center
tbr Advanced Research in Photobiology (Tramariglio, about
-~0~36' N, 811' E, Alghero, Sardinia, Italy, May 1993). In
Fig. 2 the spectral irradiance of natural sunlight (measured
:luring experiments by means of a double monochromator
spectroradiometer, Optronic 752, USA) is plotted together
wilh the unfiltered spectral irradiance of the sun simulator.
101
I0o
'E
I0'
10 2
10 3
10-~
300
400
500
600
700
--
Wavelength [nm]
Fig. 2. Spectral irradiance of the sun simulator (dotted line) compared to
'.he spectral irradiance of natural sunlight (solid line) measured during field
,_~xperiments,performedat the Center for AdvancedResearchin Photobiology (Alghero, Sardinia) in Summer1993.
23
Table 1 shows the integrated spectral solar irradiances measured under the three experimental conditions.
3. Results and discussion
The data points of the irradiance-response curves of photosynthetic oxygen production were fitted with the hyperbolic
saturation function y = a . x~ ( b + x) and the calculated parameters a and b were used to estimate the light-saturated rate
of photosynthetic oxygen production and the optimal photosynthetic irradiance value, respectively [ 19]. An inhibitory
effect due to irradiation is indicated not only by a decrease in
the light-saturated rate of photosynthetic oxygen production,
but also by a decline in the maximum light utilization effciency, i.e. the initial slope of the curve [ 19]. This is given
by the ratio a/b, derivative of the hyperbolic function for
x = 0. Standard errors for parameters a and b were obtained
from the fitting procedure and by means of them the uncertainty in the ratio a / b was calculated.
Chlorophyll fluorescence parameters were used for estimating the degree of inhibition of photosynthetic activity
observed after exposure to the different irradiation conditions. Inhibition of photosynthesis, observed within minutes
to hours in response to excessive irradiation, is associated
with a decrease in Fv, whereas changes in Fo may vary [20].
The consequent decrease in the ratio (Fv/Fm) of variable to
maximal chlorophyll fluorescence (which in phytoplankton
organisms has a maximum value of approximately 0.65, independent of growth irradiation conditions) is correlated with
a loss of effective absorption cross-section of PSII and, hence,
with a decline in the maximum light utilization efficiency,
i.e. the initial slope of the irradiance-response curves of photosynthetic oxygen production [ 19,20]. Repetition of measurements on different samples exposed to the same irradiation
conditions allowed an estimate of errors in Fo and Fm of about
5%, from which the uncertainty in the ratio Fv/Fm was calculated to be about 7%.
In Dunaliella salina, exposed to unfiltered sun simulator
radiation, a marked decline in the initial slope of the irradiance-response curve of photosynthetic oxygen production
was observed, whereas in the samples exposed to radiation
without UV-B and short wavelength UV-A or without UV
the initial slope only slightly changed in comparison with the
unexposed control sample (Fig. 3 ( A ) ) . After 30 rain of
irradiation, in fact, the initial slope decreased to 30% of the
control value in the case of unfiltered samples, whereas it
decreased only to approximately 80% in filtered samples
(Fig. 3). This observed decline in the initial slope of the
curve seems due to an increase in the optimal photosynthetic
irradiance value. In fact, whereas the parameter b was significantly higher in the case of the unfiltered sample (control:
2 6 + 5 W m 2; unfiltered: 8 0 + 2 0 W m-2; WG360:30_+5
W m-2; GG400:37 _+5 W m - 2 ) , the saturation parameters
a did not significantly differ (control: 5.2 +_0.5; unfiltered:
5.0+_0.5; WG360: 5.7_+0.5; GG400:6.0+_0.5 W m 2).
H. Herrmann et al. / Journal of Photochemistry and Photobiology B: Biology 34 (1996) 21-28
24
I0
15
20
25
0.18
100
'-'7
"U
&
75
~
>
0.16
0.14
50
0.12
t~
25
.............. ~
10
15
20
25
30
o.oo;
0.60
,..-y 0.50
Time [mini
0.40
-~
~D 4.0
0.30
"7
0.20
3.5
"~ 3.0
~
0.00 ~
2.5
0.70
/11
/ / ~ 1 1
,-- 2.0
o
.-.1
i..s
i~.1 ~ 1 i
I t l l i l
0.60
tLE 0.40
m
0.5
0.50
>
rr.
0.0
0.30
0.20
10
20
30
40
_~
50
60
70
lrradiance [W.m-2]
Fig. 3. (A) Relative maximum light utilization efficiency for photosynthetic
oxygen production at non-saturating irradiance in Dunaliella salina exposed
for different time intervals to unfiltered sun simulator radiation (circles) and
to sun simulator radiation filtered with WG 360 (squares) and GG 400
(triangles) cut-off filters; data are shown as percentage of the initial slope
of the control. (B) Irradiance-response curves of photosynthetic oxygen
production of DunalieUa salina before (diamonds, continuous line) and
after 30 min of exposure to unfiltered sun simulator radiation (circles, long
dashed) and to sun simulator radiation filtered with WG 360 (squares,
dashed) and GG 400 (triangles, short dashed) cut-off filters. The curves are
the result of fitting the data points with the hyperbolic saturation function
y=a.x/(b+x).
Under the same experimental conditions, F o for the irradiated samples decreased from 0.17 to about 0.15 within the
first 7-8 min of exposure, without further significant changes
after a prolonged exposure (Fig. 4). Fm showed a fast reduction from 0.58 to about 0.35-0.40 in the first 7 rain, but,
whereas in the filtered samples it slowly decreased to about
0.30-0.35 after 30 min exposure, in the unfiltered one it
diminished further to 0.19 (Fig. 4). Consequently, the ratio
Fv/Fm decreased from the control value (0.71) more in the
unfiltered samples (0.26, after 30 min) than in those irradiated with filtered light (0.52-0.57, after 30 rain) (Fig. 4).
In Ochromonas danica the initial slope of the irradianceresponse curves of photosynthetic oxygen production
decreased to 30-40% of the control value after 10 min of
irradiation under all experimental conditions (Fig. 5). In
contrast to what was observed in the case of D. salina, the
saturation parameter a decreased to about 65% in all irradi-
000;
30
Time [min]
Fig. 4. Values of Fo, Fm and of the ratio Fv/Fm in Dunaliella salina exposed
for different time intervals to unfiltered sun simulator radiation (circles) and
to sun simulator radiation filtered with WG 360 (squares) and GG 400
( triangles ) cut-off filters.
ated samples (control: 5.3+0.5; unfiltered: 3.8+0.5;
WG360: 3.2+0.5; GG400: 3.4+0.5), thus indicating a
decline in the light-saturated rate of photosynthetic oxygen
production. The parameter b increased under all irradiation
conditions and it was higher in the case of the unfiltered
sample (control: 30_+5 W m 2 ; unfiltered: 80+_5 W m - 2 ;
WG360:46_+6 W m - 2 ; GG400:59_+7 W m - 2 ) . After 5
min of irradiation, all samples showed an increase in Fu from
0.17 to about 0.21-0,23, followed by a saturation, whereas
Fm decreased slightly faster in the unfiltered samples than in
the filtered ones (Fig. 6). Consequently, after 10 min irradiation, the ratio Fv/Fm dropped from the control value
(0.57) to 0.20 in the unfiltered sample and to about 0.30 in
the other ones (Fig. 6).
According to the literature, exposure to an excess of white
light causes the formation of singlet oxygen mediated by the
triplet state of P680, with the consequent inhibition of electron transfer through PSII, the proteolytic degradation of the
Dl-polypeptide and, eventually, the disassembly of the PSII
complex [ 21,22 ]. The predominant effect of photoinhibition
is a decrease in photosynthetic efficiency at limiting light
levels and in the ratio of variable to maximal chlorophyll
fluorescence (F,,/Fm). Reductions in the light-saturated rate
H. Herrmann et al. / Journal of Photochemistry and Photobiology B: Biology 34 (1996) 21-28
i
25
I
i
I
i
I
i
0.24
0.22
'-'7.
"
75
50
25
0.20
0.18
0,16
A
0
0.00;
10
Time [minl
"7.
4.5
"-~
4.0
"7,=
3.5
"6
3.0
0.40
f'~
0.35
0.30
*
0.00;
0.60
2.5
2o
~zJ~~q
"-7. 0.50
~-
~i
0.40
1.5
~.
e-
1.0
0.30
0.5
0.20
o.o
-Q
/
25
50
75
]00
125
lrradiance [W.m2]
Fig. 5. ( A ) Relative maximum light utilization efficiency for photosynthetic
oxygen production at non-saturating irradiance in Ochromonas danica
exposed for different time intervals to unfiltered sun simulator radiation
(circles) and to sun simulator radiation filtered with W G 360 (squares) and
GG 400 (triangles) cut-off filters; data are shown as percentage of the initial
slope of the control. ( B ) h'radiance-response curves of photosynthetic oxygen production of Ochromonas danica before (diamonds, continuous line)
and after 10 min of exposure to unfiltered sun simulator radiation (circles,
long dashed) and to sun simulator radiation filtered with W G 360 ( squares,
dashed) and GG 400 (triangles, short dashed) cut-offfilters. The curves are
the result of fitting the data points with the hyperbolic saturation function
y=a.x/(b+x).
of photosynthetic oxygen production are also observed when
severe damage to PSII occurs [22].
In D. salina exposed to radiation of wavelength longer
than 385 nm or 338 nm (i.e., with the GG400 and the WG360
filters, respectively) the absence of decrease in photosynthetic efficiency and the slight reduction in FJFm indicated
a lack of pronounced photoinhibition (or, at least, a fast
recovery after irradiation treatment). This may partly be
explained by the capability of this organism to undergo State
1-State 2 transitions with a time course of a few minutes
[23]: at high irradiance levels the plastoquinone pool is
reduced and the light harvesting complex LHC-2 becomes
phosphorylated, with a consequent decrease in PSII crosssection [ 19,23 ]. This rapid and reversible mechanism (leading to a decrease or to an increase in PSII cross-section at
high or low irradiance levels, respectively) regulates adaptation to changes in irradiance in the natural environment
0.00 ~
10
Time [mini
Fig. 6. Values of Fo, Fm and of the ratio Fv/Fm in Ochromonas danica
exposed for different time intervals to unfiltered sun simulator radiation
(circles) and to sun simulator radiation filtered with W G 360 (squares) and
GG 400 (triangles) cut-off filters.
[ 19] and could account for the slight reduction in maximum
light utilization efficiency, observed in the samples irradiated
with filtered light, when transferred from the growth low
irradiance conditions to the sun simulator chamber and then
back to low irradiance conditions for oxygen and fluorescence
measurements.
Moreover, the ability to rapidly adjust the Chl a/Chl b
ratio, altering the relative levels of PSII core complex and
light harvesting complex LHC-2, has been observed in D.
salina [ 23 ]. Even if it was shown that this reversible adaptation mechanism to changes in light intensity has a half time
of about 3 h [23], it may partly contribute to reduce the
effects of high irradiance levels on D. salina exposed to
radiation of wavelength longer than 385 nm or 338 nm.
D. salina is also well known to accumulate t-carotene
more than any other known alga [ 24,25 ] and, even though
the levels of salinity and irradiance of our growth conditions
are lower than those at which the maximum of B-carotene
content was observed [ 24 ], also a slightly higher concentration of carotenoids in the chloroplasts could increase the rate
of quenching of triplet states and singlet oxygen, thus preventing, at least for the relatively short times of these experiments, the occurrence of photodamage requiring long
26
H. Herrmann et al. /Journal of Photochemistry and Photobiology B: Biology 34 (1996) 21-28
recovery times. Actually, it was observed that even in carotenoid-poor cultures ofD. salina photon flux densities greater
than 1500/~Einstein m 2 s - 1 (value comparable to the irradiance in the sun simulator) were necessary for inhibiting
photosynthesis [ 24].
On the other hand, the data obtained for D. salina show
that short exposure to solar levels of UV-B and short wavelength UV-A radiation cause a marked drop in photochemical
efficiency, indicated by the decline in the initial slope of the
irradiance-response curve for photosynthetic oxygen production and by the pronounced decrease in the ratio Fv/Fm. The
absence of reduction in the light-saturated rate of photosynthetic oxygen production, however, does not indicate a severe
damage to PSII population [22]. According to our data no
inhibition seems to be caused by long wavelength UV-A
radiation.
These results are consistent with the outcome of previous
investigations showing that UV-B photons, at fluence rates
higher than those contained in sunlight at the Earth's surface,
are more effective than photons of the visible range in inhibiting PSII activity and in inducing D1 protein degradation
[26,27 ], through photosensitization pathways [ 28]. In particular, Greenberg et al. [28] showed, under artificial irradiation conditions, that the degradation rate of the D 1 protein
has a maximum in the UV-B range and it is significantly
enhanced under natural sunlight in comparison to sunlight
filtered with a GG400 long-pass filter, removing all UV light.
These authors suggested that this degradation process is photosensitized by plastoquinone, instead of chlorophyll [ 28 ].
Preliminary field experiments on D. salina showed results
analogous to those obtained in the sun simulator. After 30
min of exposure to natural sunlight, the initial slope of irradiance-response curves of photosynthetic oxygen production
was reduced to about 25% of the control (Fig. 7), a value
very close to that of the sun simulator experiments. In this
case, however, filtered sunlight also caused inhibition of photosynthetic activity, gradually reduced by cutting UV radiation (Fig. 7). This could be explained by the fact that PAR
was higher and UV-B lower in the field experiments in comparison with the simulated ones and the inhibitory effect of
UV-B could have been partly concealed by a general
photoinhibitory effect. In the field experiments too, the calculated saturation parameters (control: 1.6 + 0.1 ; unfiltered:
1.6 + 0.1 ; WG360:1.5 _ 0.1 ; GG400:1.6 ___0.1 ) do not indicate any significant reduction in the light-saturated rate of
photosynthetic oxygen production.
On the other hand, exposing D. salina to the light of a
Philips TL20W/12 UV lamp (with UV-B irradiance comparable to that of the previously described experiments and
with UV-A and PAR irradiances 10- and 100-times lower,
respectively) a similar decrease in the initial slope of irradiance-response curves of photosynthetic oxygen production
was observed (Fig. 8). In this case, however, the light-saturated rate of photosynthetic oxygen production decreased
from 1.5 + 0.1 in the control to about 0.9 + 0.1 and 0.6 + 0.1
after irradiation for 30 and 60 min, respectively, thus indi-
1.25
2..
"7
1.00
-~
O
0.75
"O
-~,
0.50
"0
0.25
e-L
0.00
2i"///
0
I
25
50
75
100
125
150
175
lrradiance [W.m2]
100
,-
'
75
50
-~
tz
25
0
Control
GG400
W G 3 6 0 Unfiltered
Fig. 7. (A) Irradiance-response curves of photosynthetic oxygen production
of Dunaliella salina before (diamonds, continuous line) and after 30 min
of exposure to unfiltered sun radiation (circles, long dashed) and to sun
radiation filtered with WG 360 (squares, dashed) and GG 400 (triangles,
short dashed) cut-off filters. The curves are the result of fitting the data
points with the hyperbolic saturation function y = a. x~ (b + x). (B) Relative
maximum light utilization efficiency for photosynthetic oxygen production
at non-saturating irradiance in Dunaliella salina exposed for 30 min to
unfiltered sun radiation and to sun radiation filtered with WG 360 and GG
400 cut-off filters; data are shown as percentage of the initial slope in the
control.
cating the occurrence of severe damage to PSII [22]. This
effect was probably due to the presence of UV-B wavelengths
shorter than 295 nm, which in the sun simulator are rejected
by the TEMPAX glass.
O. danica, even after short exposure times, underwent photoinhibition under all experimental conditions. As irradiation
without UV caused a strong decrease in photosynthetic activity, a possible UV-B or UV effect may hardly have been
detected taking into account the fact that UV-B radiation was
about 0.4% and UV about 12% of the irradiance in the range
280-700 nm. The decrease in the ratio Fv/Fm is due both to
an increase in Fo and to a decrease in Fro. It is assumed that
this increase in Fo reflects a photoinhibition of PSII [29] : in
fact, in red algae [30] as well as in Ulva rotundata [31] an
increase in Fo after photoinhibitory treatment was observed
and, in the case of U. rotundata, it was suggested that the
rapid decrease in Fv/Fm caused by an increase in Fo may
reflect damage to PSII [ 31 ]. In the case of O. danica, preliminary analogous experiments in the field had shown a com-
H. Herrmann et al. /Journal t~'Photochemistry and Photobiology B." Biology 34 (1996) 21-28
27
Acknowledgements
1.25
7i
1.00
0.75
0.50
~ / , ~ Q
o 0.25
e'~
t1~
_.11_.". . . .
_.ll--ll ~ -
The authors gratefully a c k n o w l e d g e financial support by a
grant from the E C Research Project E n v i r o n m e n t ( C o n t r a c t
E V 5 V - C T 9 1 - 0 0 2 6 ) and a grant from the State o f Bavaria
( B a y F O R K L I M , Project D I I I / l ). T h e y also are indebted to
S. Puntoni, L. Barsanti and V. Passarelli for kindly providing
Dunaliella
s a l i n a and O c h r o m o n a s
danica
for the
experiments.
O~ 0.00
0
25
50
75
100
125
150
175
Irradiance [W.m "2]
~\.\\
I
1oo
~a
e=
75
50
25
eD
\\
\\
30
6O
Time [rain]
Fig. 8. (A) Irradiance-response curves of photosynthetic oxygen production
of Dunaliella salina before (diamonds, continuous line) and after 30 (circles, long dashed) and 60 min (squares, short dashed) of exposure to the
radiation of an ultraviolet fluorescent lamp Philips TL20W/12. The curves
are the result of fitting the data points with the hyperbolic saturation function
v = a . x / ( b + x). (B) Relative maximum light utilization efficiency for photosynthetic oxygen production at non-saturating irradiance in Dunaliella
salina exposed for different time intervals to the radiation of an ultraviolet
fluorescent lamp Philips TL20W/12; data are shown as percentage of the
initial slope of the control.
parable d e c r e a s e in the ratio F v / F m f r o m 0.52 to 0.16 in the
unfiltered samples and to about 0.20 in the others.
W o r k is in progress on D . s a l i n a , both in the field and with
simulated sun light, in order to better define the role of different solar U V spectral intervals in inhibiting photosynthesis
and to d e t e r m i n e an action spectrum o f U V effects under
p o l y c h r o m a t i c irradiation.
The results o f this U V exclusion study indicate that the use
of sun simulators, because o f the accurate reproducibility of
the irradiation conditions and the possibility o f excluding
disturbing agents, s e e m s v e r y p r o m i s i n g for the study of light
stress p h e n o m e n a in phytoplankton organisms and for acquiring useful data to s u p p l e m e n t the field observations. In particular, in the e x p o s u r e c h a m b e r s it seems possible to soundly
simulate, in intensity and spectral quality, not only the present
levels o f solar U V - B radiation, but also those resulting from
the reduction o f the stratospheric o z o n e layer.
References
[1] H.-D. Payer, T. Pfirrmann and P. Mathy (eds.), Environmental
research with plants in closed chambers, Air Pollution Research
Report, 26, CEC Brussels, 1990.
[2] L.O. Bj6m and A.H. Teramura, Simulation of daylight ultraviolet
radiation and effects of ozone depletion, in A.R. Young, L.O. Bjt~m,
J.Moan and W. Nultsch (eds.), Environmental UV Photobiology,
Plenum Press, New York, 1993, pp. 41-71.
[ 3 ] G. Seckmeyer and H.-D. Payer, A new sunlight simulator for ecological
research on plants, J. Photochem. Photobiol. B: Biol., 21 (1993) 175181.
[4] O. Holm-Hansen, D. Lubin and E.W. Helbling, Ultraviolet radiation
and its effects on organisms in aquatic environments, in A.R. Young,
L.O. Bj6m, J. Moan and W. Nultsch (eds.), Environmental UV
Photobiology, Plenum, New York, 1993, pp. 379-425, and references
cited therein.
[5] D.-P. H~der, Risks of enhanced solar ultraviolet radiation for aquatic
ecosystems, in F.E. Round and D.J. Chapman (eds.), Progress in
Phycological Research, Vol. 9, Biopress Ltd., 1993, pp. 1-45, and
references cited therein.
16] J.J. Cullen and M.P. Lesser, Inhibition of photosynthesis by ultraviolet
radiation as a function of dose and dosage rate: results for a marine
diatom, Mar. Biol., 111 ( 1991 ) 183-190.
[7] J.J. Cullen, P.J. Neale and M.P. Lesser, Biological weighting function
for the inhibition of phytoplankton photosynthesis by ultraviolet
radiation, Science, 258 (1992) 646-650.
[8] K.G Ryan, UV radiation and photosynthetic production in Antarctic
sea ice microalgae, J. Photochem. Photobiol. B: Biol., 13 (1992) 235240.
[9] A.W.D. Larkum and W.F. Wood, The effect of UV-B radiation on
photosynthesis and respiration of phytoplankton, benthic macroalgae
and seagrass, Photosynth. Res., 36 (1993) 17-23.
[ 10] R.C. Smith, B.B. Pr6zelin, K.S. Baker, R.R. Bidigare, N.P. Boucher,
T. Coley, D. Karentz, S. Maclntyre, H.A. Matlick, D. Menzies, M.
Ondrusek, Z. Wan and K.J. Waters, Ozone depletion: ultraviolet
radiation and phytoplankton biology in antarctic waters, Science, 255
(1992) 952-959.
[ 11] E.W. Helbling, V. Villafafie, M. Ferrario and O. Holm-Hansen, Impact
of natural ultraviolet radiation on rates of photosynthesis and on
specific marine phytoplankton species, Mar. Ecol. Prog. Ser., 80
(1992) 89-100.
[ 12] M.L. Bothwell, D. Sherbot, A.C. Roberge and R.J. Daley, Influence of
natural ultraviolet radiation on lotic periphytic diatom community
growth, biomass accrual, and species composition: short-term versus
long-term effects, J. Phycol., 29 (1993) 24-35.
[ 13] P.J. Neale, M.P. Lesser and J.J. Cullen, Effects of ultraviolet radiation
on the photosynthesis of phytoplankton in the vicinity of McMurdo
Station, Antarctica, in C.S. Weiler and P.A. Penhale (eds.), Ultraviolet
Radiation in Antarctica: Measurements and Biological Effects
(Antarctic Research Series, Vol. 62), American Geophysical Union,
Washington DC, 1994, pp. 125-142.
28
H. Herrmann et al. / Journal of Photochemistry and Photobiology B: Biology 34 (1996) 21-28
[ 14] M. Vernet, E.A. Brody, O. Holm-Hansen and B.G. Mitchell, The
response of antarctic phytoplankton to ultraviolet radiation: absorption,
photosynthesis, and taxonomic composition, in C.S. Weiler and P.A.
Penhale (eds.), Ultraviolet Radiation in Antarctica: Measurements
and Biological Effects (Antarctic Research Series, Vol. 62 ), American
Geophysical Union, Washington DC, 1994, pp. 143-158.
[15] E.W. Helbling, V. Villafafie and O. Holm-Hansen, Effects of
ultraviolet radiation on antarctic marine phytoplankton photosynthesis
with particular attention to the influence of mixing, in C.S. Weiler and
P.A. Penhale (eds.), Ultraviolet Radiation in Antarctica:
Measurements and Biological Effects (Antarctic Research Series, Vol.
62), American Geophysical Union, Washington DC, 1994, pp. 207227.
[ 16] S. Gerber and D.-P. H~ider, Effects of enhanced solar irradiation on
chlorophyll fluorescence and photosynthetic oxygen production of five
species of phytoplankton, FEMS MicrobioL Ecol., 16 (1995) 33-42.
[ 17] M.K. Johnson, E.J. Johnson, R.D. MacElroy, H.L. Speer and B.S.
Bruff, Effects of salts on the halophilic alga Dunaliella viridis, J.
Bacteriol., 95 (1968) 1461-1468.
[ 18] D.H. Tippit, L. Pillus and J. Pickett-Heaps, Organization of spindle
microtubules in Ochromonas danica, J. Cell Biol., 8 (1980) 531-545.
[19] P.G. Falkowski, R. Greene and Z. Kolber, Light utilization and
photoinhibition of photosynthesis in marine phytoplankton, in N.R.
Baker and J.R. Bowyer (eds.), Photoinhibition of Photosynthesis,
BIOS Scientific Publishers, Oxford, 1994, pp. 407-432.
[20] G.H. Krause and E. Weis, Chlorophyll fluorescence and
photosynthesis: the basics, Annu. Rev. Plant Physiol. Plant Mol. Biol.,
42 ( 1991 ) 313-349.
[21] J. Barber and B. Andersson, Too much of a good thing: light can be
bad for photosynthesis, TIBS, 17 (1992) 61~66.
[22] N.R. Baker, Photoinhibition of photosynthesis, in R. Jennings, G.
Zucchelli, F. Ghetti and G. Colombetti (eds.), Light as Energy Source
and Information Carrier in Plant Photophysiology, Plenum Press, New
York, in press.
[23] U. Pick, K. Gounaris and J. Barber, Dynamics of photosystem II and
its light harvesting system in response to light changes in the
halotolerant alga Dunaliella salina, Plant. Physiol., 85 (1987) 194198.
[24] M.A. Borowitzka and L.J. Borowitzka, Dunaliella, in M.A.
Borowitzka and L.J. Borowitzka (eds.), Micro-algal Biotechnology,
Cambridge University Press, Cambridge, 1988, pp. 27-58.
[25 ] J.L. G6mez-Pinchetti, Z. Ramazanov, A. Fontes and G. Garcfa-Reina,
Photosynthetic characteristics of Dunaliella salina (Chlorophyceae,
Dunaliellales) in relation to /3-carotene content, ,L Appl. Phycol., 4
(1992) 11-15.
[26] G. Renger, M. V~lker, H.J. Eckert, R. Fromme, S. Hohm-Veit and
P.Griiber, On the mechanism of photosystem II deterioration by UVB irradiation, Photochem. Photobiol., 49 (1989) 97-105.
[27] G.Friso, C. Spetea, G.M. Giacometti, I. Vass and R. Barbato,
Degradation of Photosystem II reaction center Dl-protein induced by
UV-B radiation in isolated thylakoids. Identification and
characterization of C- and N-terminal breakdown products, Biochim.
Biophys. Acta, 1184 (1994) 78-84.
[28] BM. Greenberg, V. Gaba, O. Canaani, S. Malkin, A.K. Mattoo and
M. Edelman, Separate photosensitizers mediate degradation of the 32kDa photosystem II reaction center protein in the visible and UV
spectral regions, Proc. Natl. Acad. Sci. USA, 86 (1989) 6617~620.
[29] B. Demmig-Adams, Carotenoids and photoprotection in plants: A role
for xanthophyll zeaxanthin, Biochim. Biophys. Acta, 1020 (1990) 124.
[30] D. Hanelt, K. Huppertz and W. Nultsch, Photoinhibition of
photosynthesis and its recovery in red algae, Bot. Acta, 105 (1992)
278-284.
[31] L.A. Franklin, G. Levavasseur, C.B. Osmond, W.J. Henley and J.
Rasmus, Two components of onset and recovery during
photoinhibition of Ulva rotundata, Planta, 186 (1992) 399-408.
Das könnte Ihnen auch gefallen
- MacArthur - Introduction To Field SpectrosDokument63 SeitenMacArthur - Introduction To Field SpectrosRute DanielaNoch keine Bewertungen
- CIELABDokument6 SeitenCIELABatdhepeNoch keine Bewertungen
- Astm G 138 2006 PDFDokument8 SeitenAstm G 138 2006 PDFJORGE ARTURO TORIBIO HUERTANoch keine Bewertungen
- ELCOMA Diractory 2021 2022 1 1 1Dokument237 SeitenELCOMA Diractory 2021 2022 1 1 1dipaksingh saudNoch keine Bewertungen
- Abstract:: Key Words: Antarctica, Cyanobacteria, Mcmurdo Sound, Mycosporine-Like-AmiDokument14 SeitenAbstract:: Key Words: Antarctica, Cyanobacteria, Mcmurdo Sound, Mycosporine-Like-AmiDaniel KurbielNoch keine Bewertungen
- How Can Physicists Study Photosynthesis ? History and Applications of The Photoacoustic TechniqueDokument7 SeitenHow Can Physicists Study Photosynthesis ? History and Applications of The Photoacoustic TechniqueGustavo BrasilNoch keine Bewertungen
- Jmse 08 00888 v3Dokument15 SeitenJmse 08 00888 v3López Espinoza Livia FernandaNoch keine Bewertungen
- Comparative Investigation of The UV Stabilization of PC-ABS With Different UV AbsorbersDokument8 SeitenComparative Investigation of The UV Stabilization of PC-ABS With Different UV AbsorbersVictor CastrejonNoch keine Bewertungen
- Artikel Prosiding IOP Sunscreen Red Lettuce - Rohmah - 2020 - IOP - Conf. - Ser.: - Earth - Environ. - Sci. - 519 - 012009Dokument8 SeitenArtikel Prosiding IOP Sunscreen Red Lettuce - Rohmah - 2020 - IOP - Conf. - Ser.: - Earth - Environ. - Sci. - 519 - 012009Jmltr RhmhNoch keine Bewertungen
- Groff 2010Dokument7 SeitenGroff 2010Emilene NunesNoch keine Bewertungen
- P 11Dokument9 SeitenP 11aliNoch keine Bewertungen
- PAS Dalam FotosintesisDokument18 SeitenPAS Dalam FotosintesisFRANCISCA HTNoch keine Bewertungen
- Lecture 5 Solar Radiation Part 1 Principles Notes PDFDokument21 SeitenLecture 5 Solar Radiation Part 1 Principles Notes PDF॰अचूक॰ Milan SubediNoch keine Bewertungen
- 1999 ArticleDokument8 Seiten1999 Articleelder diazNoch keine Bewertungen
- Solar Radiation of The High Alps: Mario BlumthalerDokument11 SeitenSolar Radiation of The High Alps: Mario BlumthalerDale Francis ClapanoNoch keine Bewertungen
- Degradation of 2,4,6-Trichlorphenol by Producing Hydrogen Using Ultrasonic Mist Generated From Photocatalysts SuspensionDokument6 SeitenDegradation of 2,4,6-Trichlorphenol by Producing Hydrogen Using Ultrasonic Mist Generated From Photocatalysts SuspensionNur IzzatieNoch keine Bewertungen
- 61302-Article Text-114824-1-10-20101022Dokument4 Seiten61302-Article Text-114824-1-10-20101022Oscar MacíasNoch keine Bewertungen
- Radioactivity and Its ApplicationDokument58 SeitenRadioactivity and Its ApplicationBipin SunarNoch keine Bewertungen
- Photolysis Study by FtirDokument8 SeitenPhotolysis Study by FtirrakibhossainNoch keine Bewertungen
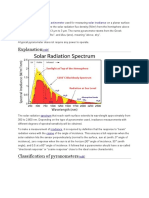
- Solar Radiation Measurement: Res/ Principles of Solar Radiation/ Goldvin Sugirtha Dhas.B, Ap/Eee 1Dokument4 SeitenSolar Radiation Measurement: Res/ Principles of Solar Radiation/ Goldvin Sugirtha Dhas.B, Ap/Eee 1shanuNoch keine Bewertungen
- Optical Lithography - Extreme Ultraviolet Lithography5Dokument6 SeitenOptical Lithography - Extreme Ultraviolet Lithography5Sonia KNoch keine Bewertungen
- Uv-B Effects O N Terrestrial: PlantsDokument9 SeitenUv-B Effects O N Terrestrial: PlantsXimena CáceresNoch keine Bewertungen
- Camacho 1999Dokument9 SeitenCamacho 1999Linggar T. GupitaNoch keine Bewertungen
- Ultraviolet Radiation Effects On Fruit Surface Respiration and Chlorophyll FluorescenceDokument8 SeitenUltraviolet Radiation Effects On Fruit Surface Respiration and Chlorophyll Fluorescenceavita rukmanaNoch keine Bewertungen
- 1987 - Agrapidis-Paloympis and Nash - The Effect of Solvents On The Ultraviolet Absorbance of SunscreensDokument13 Seiten1987 - Agrapidis-Paloympis and Nash - The Effect of Solvents On The Ultraviolet Absorbance of SunscreensymiyazyNoch keine Bewertungen
- Jurnal HarismanDokument8 SeitenJurnal HarismanHarisman ErickNoch keine Bewertungen
- Activity Imaging Photosynthetic of Populus X Canadensis Moench Plants in Air PollutionDokument6 SeitenActivity Imaging Photosynthetic of Populus X Canadensis Moench Plants in Air PollutionInternational Journal of Engineering Inventions (IJEI)Noch keine Bewertungen
- A Practical Review of Shorter Than Excitation Wavelength Light Emission ProcessesDokument24 SeitenA Practical Review of Shorter Than Excitation Wavelength Light Emission ProcessesRobertas ŽilinskasNoch keine Bewertungen
- RRLDokument10 SeitenRRLHazzel AdraNoch keine Bewertungen
- Free Radical and Antioxidant Protocols - Chapter 3Dokument10 SeitenFree Radical and Antioxidant Protocols - Chapter 3Newocean NguyenNoch keine Bewertungen
- Hospital Management AppDokument12 SeitenHospital Management AppAnu VinodNoch keine Bewertungen
- Full Report Uv-Vis and FtirDokument25 SeitenFull Report Uv-Vis and FtirAmirHakimRusli100% (6)
- Reduction and Analysis Methods of IndigoDokument72 SeitenReduction and Analysis Methods of IndigoBilal MasoodNoch keine Bewertungen
- Solar Radiation Resource Assessment by Means of Silicon CellsDokument9 SeitenSolar Radiation Resource Assessment by Means of Silicon CellsalexanderritschelNoch keine Bewertungen
- Ass 1 SDFGHJK DFGHJK DFGHJK Ertyuiop SDFGHJKLDokument10 SeitenAss 1 SDFGHJK DFGHJK DFGHJK Ertyuiop SDFGHJKLriniz92Noch keine Bewertungen
- Explanation: ῦ (pyr), meaning "fire", and νω ἄDokument6 SeitenExplanation: ῦ (pyr), meaning "fire", and νω ἄMonika KshetriNoch keine Bewertungen
- Radical Status Factor j.1468-2494.2012.00718.xDokument6 SeitenRadical Status Factor j.1468-2494.2012.00718.xGalina TodorovaNoch keine Bewertungen
- 835 2310Dokument21 Seiten835 2310Addin AkbarNoch keine Bewertungen
- Desalination: Kumiko Oguma, Ryo Kita, Hiroshi Sakai, Michio Murakami, Satoshi TakizawaDokument7 SeitenDesalination: Kumiko Oguma, Ryo Kita, Hiroshi Sakai, Michio Murakami, Satoshi TakizawaADITHYA PAINoch keine Bewertungen
- 12 (629 633) IcgseeDokument5 Seiten12 (629 633) Icgseememo_gh89Noch keine Bewertungen
- Photoacoustic Spectroscopy in Trace Gas MonitoringDokument25 SeitenPhotoacoustic Spectroscopy in Trace Gas MonitoringRIzwanaNoch keine Bewertungen
- Uv Spectroscopy 5Dokument11 SeitenUv Spectroscopy 5Emmanuella OffiongNoch keine Bewertungen
- Lec5 Handout GapsDokument26 SeitenLec5 Handout GapsnatsdorfNoch keine Bewertungen
- CRPSCI 1100-Module7Dokument27 SeitenCRPSCI 1100-Module7carbonel.carlaNoch keine Bewertungen
- FT IR Analysis of The Photooxidation of Styrene Acrylonitrile Copolymers 1993 Polymer Degradation and StabilityDokument12 SeitenFT IR Analysis of The Photooxidation of Styrene Acrylonitrile Copolymers 1993 Polymer Degradation and StabilityBaraNoch keine Bewertungen
- Spectroscopy: Ass Spectrometry Agnetic Spin ResonanceDokument30 SeitenSpectroscopy: Ass Spectrometry Agnetic Spin ResonanceLisbeth Roos RoosNoch keine Bewertungen
- Ultraviolet and Visible (UV-Vis) Absorption Spectroscopy: A Ecl /I)Dokument17 SeitenUltraviolet and Visible (UV-Vis) Absorption Spectroscopy: A Ecl /I)Des MamNoch keine Bewertungen
- WILLMER E UNWIN, 1981. As Análises de Campo de Orçamentos Insect Calor Reflectance, Tamanho e Taxas de AquecimentoDokument6 SeitenWILLMER E UNWIN, 1981. As Análises de Campo de Orçamentos Insect Calor Reflectance, Tamanho e Taxas de AquecimentoGeovan SáNoch keine Bewertungen
- Solar Radiation MetersDokument14 SeitenSolar Radiation MetersObada Ar-ruzziNoch keine Bewertungen
- Pilobolus Articulo2 PDFDokument6 SeitenPilobolus Articulo2 PDFRoberto RuizNoch keine Bewertungen
- IR Sampling TechniquesDokument11 SeitenIR Sampling TechniquesMuhammad Hussnain100% (2)
- 4-Available Solar RadiationDokument16 Seiten4-Available Solar RadiationMustafa Abd-ElfattahNoch keine Bewertungen
- Module-6 Unit-4 UV-Vis Spectroscopy SpectrosDokument11 SeitenModule-6 Unit-4 UV-Vis Spectroscopy SpectrosManikandan KKNoch keine Bewertungen
- Performance of Composite Insulators With and Without Bio ContaminationDokument4 SeitenPerformance of Composite Insulators With and Without Bio ContaminationSiva KumarNoch keine Bewertungen
- Agro-Meteorology For BSC Agriculture StudentsDokument54 SeitenAgro-Meteorology For BSC Agriculture StudentsMadan ThapaNoch keine Bewertungen
- Ltraviolet Adiation: 8.1 UV Chemistry (Photochemical)Dokument25 SeitenLtraviolet Adiation: 8.1 UV Chemistry (Photochemical)Amit KumarNoch keine Bewertungen
- Phototropic TO Length: SensitivityDokument22 SeitenPhototropic TO Length: SensitivityN S Arun KumarNoch keine Bewertungen
- Gamma-Induced Modification On Optical Band Gap of CR-39 SSNTDDokument5 SeitenGamma-Induced Modification On Optical Band Gap of CR-39 SSNTDhamza_qayyumNoch keine Bewertungen
- Physica Project 27.03.2024Dokument33 SeitenPhysica Project 27.03.2024janaki computersNoch keine Bewertungen
- Biophoton Emission: New Evidence For Coherence and DNA As SourceDokument20 SeitenBiophoton Emission: New Evidence For Coherence and DNA As SourceValentina Carbini100% (1)
- The Global Carbon Cycle and Climate Change: Scaling Ecological Energetics from Organism to the BiosphereVon EverandThe Global Carbon Cycle and Climate Change: Scaling Ecological Energetics from Organism to the BiosphereNoch keine Bewertungen
- Interpretation of the Ultraviolet Spectra of Natural Products: International Series of Monographs on Organic ChemistryVon EverandInterpretation of the Ultraviolet Spectra of Natural Products: International Series of Monographs on Organic ChemistryNoch keine Bewertungen
- PW,,H,, DL+ LG.: Scorazanone, A Laza-Anthraqutnone From GonzothalamusDokument3 SeitenPW,,H,, DL+ LG.: Scorazanone, A Laza-Anthraqutnone From GonzothalamusAlexsandro ClaudinoNoch keine Bewertungen
- 08 - Primer Ecology - (Gotelli, 1995) PDFDokument6 Seiten08 - Primer Ecology - (Gotelli, 1995) PDFAlexsandro ClaudinoNoch keine Bewertungen
- Alkaloids From The Stem Bark of Orophea Hexandra (Annonaceae) PDFDokument2 SeitenAlkaloids From The Stem Bark of Orophea Hexandra (Annonaceae) PDFAlexsandro ClaudinoNoch keine Bewertungen
- Lowering Chlorophyll Fluorescence After Saturating Light: Cold-Induced Sudden Reversible VivoDokument3 SeitenLowering Chlorophyll Fluorescence After Saturating Light: Cold-Induced Sudden Reversible VivoAlexsandro ClaudinoNoch keine Bewertungen
- Plant Natural Products Active Against SnakeDokument16 SeitenPlant Natural Products Active Against SnakeAlexsandro ClaudinoNoch keine Bewertungen
- Electrospray Characterization of Selected Medicinal PlantDokument2 SeitenElectrospray Characterization of Selected Medicinal PlantAlexsandro ClaudinoNoch keine Bewertungen
- Workshop Advanced Analytical Techniques in Phytochemistry''Dokument2 SeitenWorkshop Advanced Analytical Techniques in Phytochemistry''Alexsandro ClaudinoNoch keine Bewertungen
- Farmacia Verde LivroDokument1 SeiteFarmacia Verde LivroAlexsandro ClaudinoNoch keine Bewertungen
- Phytochemistry 06Dokument2 SeitenPhytochemistry 06Alexsandro ClaudinoNoch keine Bewertungen
- GT Maria SileneDokument8 SeitenGT Maria SileneAlexsandro ClaudinoNoch keine Bewertungen
- Bem-Aventurados Os Simples (Psicografia Waldo Vieira - Espírito Valérium)Dokument673 SeitenBem-Aventurados Os Simples (Psicografia Waldo Vieira - Espírito Valérium)Alexsandro ClaudinoNoch keine Bewertungen
- Biotecnologia de Microalgas (1988 R) - (De La Noue and de Pauw) - The Potential of Microalgal Biotechnology A Review of Production and Uses of MicroalgaeDokument46 SeitenBiotecnologia de Microalgas (1988 R) - (De La Noue and de Pauw) - The Potential of Microalgal Biotechnology A Review of Production and Uses of MicroalgaeAlexsandro ClaudinoNoch keine Bewertungen
- Herculanum (Psicografia Wera Krijanowskaia - Espirito J. W. Rochester)Dokument52 SeitenHerculanum (Psicografia Wera Krijanowskaia - Espirito J. W. Rochester)Alexsandro ClaudinoNoch keine Bewertungen
- Worksheet 1Dokument8 SeitenWorksheet 1darshanpandaNoch keine Bewertungen
- Mil STD 3009Dokument95 SeitenMil STD 3009premNoch keine Bewertungen
- NiacinamidaDokument6 SeitenNiacinamidasofimm9804Noch keine Bewertungen
- ISO 24443 2012 (En)Dokument32 SeitenISO 24443 2012 (En)DUVAN DROIDNoch keine Bewertungen
- Vol 30 Issue 61Dokument18 SeitenVol 30 Issue 61Martha MalancoNoch keine Bewertungen
- Iso 13655 2017Dokument15 SeitenIso 13655 2017Cường Ngô MinhNoch keine Bewertungen
- Spectraval 1511Dokument2 SeitenSpectraval 1511Steffen Stengård VilladsenNoch keine Bewertungen
- Evaluating Blue-Light HazardDokument11 SeitenEvaluating Blue-Light HazardSilvioGlasgow100% (1)
- Dcna Fixed Prosthodontics April 2004 PDFDokument236 SeitenDcna Fixed Prosthodontics April 2004 PDFMiki FloreaNoch keine Bewertungen
- Ideal Lighting Design Software: Ian Ashdown, P. Eng. (Ret.) Senior Research Scientist Suntracker Technologies LTDDokument12 SeitenIdeal Lighting Design Software: Ian Ashdown, P. Eng. (Ret.) Senior Research Scientist Suntracker Technologies LTDinzanerNoch keine Bewertungen
- Technical SpecificationDokument17 SeitenTechnical SpecificationPMSi Cal6Noch keine Bewertungen
- RADTECH - Glossary of TermsDokument6 SeitenRADTECH - Glossary of TermsjlbbcoelhoNoch keine Bewertungen
- SQ 515 ManualDokument22 SeitenSQ 515 ManualDispositivos PerinaNoch keine Bewertungen
- Measurement of Emission Characteristics and Requirements For LED UV-A Lamps Used in Fluorescent Penetrant and Magnetic Particle TestingDokument8 SeitenMeasurement of Emission Characteristics and Requirements For LED UV-A Lamps Used in Fluorescent Penetrant and Magnetic Particle TestingXAVIER AUCAYNoch keine Bewertungen
- Remote Sensing of Suspended Sediments in Surface Waters - Ritchie - 1976Dokument7 SeitenRemote Sensing of Suspended Sediments in Surface Waters - Ritchie - 1976FLávio ViníciusNoch keine Bewertungen
- Fogra Softproof HandbookDokument43 SeitenFogra Softproof HandbooktruearslanNoch keine Bewertungen
- Portable CCD SpectroradiometerDokument5 SeitenPortable CCD SpectroradiometerLISUN GROUPNoch keine Bewertungen
- Astm G207 - 11Dokument7 SeitenAstm G207 - 11Sofia YuliNoch keine Bewertungen
- Neonatal Phototherapy Radiometer Current Performance Characteristics and Future Requirements.Dokument26 SeitenNeonatal Phototherapy Radiometer Current Performance Characteristics and Future Requirements.Metrologia BiomedicaNoch keine Bewertungen
- Metrología FotometríaDokument4 SeitenMetrología FotometríaBellbrujaNoch keine Bewertungen
- Mil PRF 53134Dokument38 SeitenMil PRF 53134SagarNoch keine Bewertungen
- Coverage Error of Three Conceptually Different Shade Guide Systems To Vital Unrestored DentitionDokument21 SeitenCoverage Error of Three Conceptually Different Shade Guide Systems To Vital Unrestored Dentitionjanicesusanto2000Noch keine Bewertungen
- Annex I To CE Recommendation No 26 Double Plate Method ProtocolDokument32 SeitenAnnex I To CE Recommendation No 26 Double Plate Method ProtocolKirk BorromeoNoch keine Bewertungen
- Integrating Sphere Theory Apps Tech GuideDokument22 SeitenIntegrating Sphere Theory Apps Tech GuideAnil KumarNoch keine Bewertungen
- Calibration of Broadband UV Radiometers - MethodolDokument5 SeitenCalibration of Broadband UV Radiometers - MethodolIsrael AyllonNoch keine Bewertungen
- E 824 Â " 94 - RTGYNC05NADokument5 SeitenE 824 Â " 94 - RTGYNC05NAhans ccNoch keine Bewertungen