Beruflich Dokumente
Kultur Dokumente
Final
Hochgeladen von
Sasa LiliCopyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Final
Hochgeladen von
Sasa LiliCopyright:
Verfügbare Formate
www.elsevier.
nl / locate / jinorgbio
Journal of Inorganic Biochemistry 81 (2000) 8187
Investigation of the transported heavy metal ions in xylem sap of
cucumber plants by size exclusion chromatography and atomic absorption
spectrometry
b , Boglarka
a , *, Edit Cseh d
Victor G. Mihucz a , Eniko0 Tatar
Kmethy c , Gyula Zaray
a
Research Group of Environmental and Macromolecular Chemistry of the Hungarian Academy of Sciences, P.O. Box 32, H-1518 Budapest 112,
Hungary
b
University, P.O. Box 32, H-1518 Budapest 112, Hungary
Department of Inorganic and Analytical Chemistry, L. Eotvos
c
University, P.O. Box 32, H-1518 Budapest 112, Hungary
Department of Chemical Technology and Environmental Chemistry, L. Eotvos
d
University, P.O. Box 330, H-1445 Budapest, Hungary
Department of Plant Physiology, L. Eotvos
Received 4 January 2000; received in revised form 28 March 2000; accepted 19 April 2000
Abstract
An off-line high performance liquid chromatographygraphite furnace atomic absorption spectrometry (HPLCGF-AAS) method
using a size exclusion chromatography (SEC) column was developed to investigate heavy metal ions in xylem sap samples of cucumber
plants grown in hydroponics containing iron as Fe(III)-ethylenediaminetetraacetate (Fe(III) EDTA), Fe(III) citrate or FeCl 3 and exposed
to lead, nickel or vanadium contamination. The SEC chromatogram of the samples contained the peak of nitrate ions (in significant
concentration 1400 mg / ml) and some small, unidentified compounds with molecular weight lower than 700 Da. The results indicate that
Cu and Mn which were added to the hydroponics as nutrient elements determined in the collected fractions during the
chromatographic runs are transported in the xylem vessels together with small inorganic ions like nitrate ions. In case of nickel other
low-molecular weight compounds eluting earlier than the nitrate ions may take part in its transport toward the shoots. Lead could not be
detected in the above mentioned fractions. Determination of vanadium in the fractions was not expected since it could not be detected in
the sap samples. 2000 Elsevier Science S.A. All rights reserved.
Keywords: Xylem; Size exclusion chromatography; Atomic absorption spectrometry; Heavy metals; Cucumber
1. Introduction
The nickel and vanadium emission from the burning of
fuels by power plants consists mainly of water-soluble
species like nickel- and vanadyl sulfate [1]. Though
nowadays the use of lead tetraethyl for the automotive has
been minimized or managed to be avoided, its impact on
the environment, e.g., on plants, cannot be neglected [2].
While the essentiality of nickel for plants and human
beings has not been completely cleared yet, and only a few
data are available about the toxicity of vanadium, the
adverse affects of lead are well known. Thus, lead damages plant cells and membranes while its main toxic effect
*Corresponding author. Department of Chemical Technology and
University, P.O. Box 32, H-1518
Environmental Chemistry, L. Eotvos
Budapest 112, Hungary. Tel.: 136-1-2090-555, int. 6047; fax: 136-13722-655.
E-mail address: zaray@ludens.elte.hu (G. Zaray).
for mankind and animals is the inhibition of the synthesis
of hemoglobin [3]. Compounds absorbed by the roots of
plants or synthesized in the roots move upward to the
shoots in the xylem vessels through the transpiration
stream. Being transported in the xylem, heavy metals may
have some influence on the plant metabolic processes. The
highest risk for human health is when plants develop
tolerance mechanisms against this kind of toxicity [4].
Xylem exudates contain low-molecular weight organic
acids, inorganic anions such as nitrate, sulfate, phosphate
and a limited number of amino acids, amides and other
solutes [5]. For the determination of the elements present
in concentration range of mg / ml and ng / ml in various
samples of biological origin, several efficient techniques
are used such as graphite furnace atomic absorption
spectrometry (GF-AAS) [6,7], inductively coupled plasma
atomic emission spectrometry (ICP-AES) [8,9] and total
reflection X-ray fluorescence spectrometry (TXRF)
[10,11]. A real challenge for the researcher is to be able to
0162-0134 / 00 / $ see front matter 2000 Elsevier Science S.A. All rights reserved.
PII: S0162-0134( 00 )00091-X
82
V.G. Mihucz et al. / Journal of Inorganic Biochemistry 81 (2000) 81 87
determine the right chemical form of the transported metal
that would help the elucidation of the many times
altered plant physiological processes.
A promising tool for speciation studies can be the size
exclusion chromatography (SEC) technique, commonly
referred to as gel filtration, which separate molecules by
size, or hydrodynamic volume. Recently, increased usage
of high-performance columns as compared to soft-gel
conventional packings has been observed [12]. High
performance columns are favored because of their speed
and high resolution [13]. For aqueous SEC the most
widespread columns are the silica based ones due to their
high efficiency (smaller particle size), shorter analysis time
and robustness [12]. The major advantage of SEC is that
the interaction between the sample molecules and the
stationary phase is not as significant as in other high
performance chromatography (HPLC) methods. The most
important consequence of this feature is that an employed
SEC method is the least likely HPLC method to denature a
sample [14]. Besides organic analysis, SEC is also suitable
for inorganic cation or anion analysis. Regarding the
inorganic analysis on modern gel chromatography, the
majority of the published articles are concerned with
questions of theoretical importance such as the partitioning
of acetylacetonates [15], dissociation of dithizonates [16],
etc. For both inorganic cation and anion analysis, Konchiyawa et al. developed an on-line SEC-AAS method for
the separation of magnesium monophosphate and polyphosphates and simultaneous determination of their magnesium content [17]. An on-line HPLCICP-MS method
served for the investigation of the binding properties of
heavy metalphytochelatin peptide complexes in Silene
vulgaris cell cultures [18] and tomato cell cultures [19].
The results of these investigations indicated that independently of the exposition time of these cultures, less than
5% of the total Cd and Cu content was incorporated into
the phytochelatins that have the following general structure: (g-Glu-Cys) n -Gly, where n5211. An off-line
conventional SEC-AAS method was developed to characterize cadmium binding peptides in pepper plants (Capsicum annuum) [20] whose Cd tolerance is high (up to 150
mM Cd). From the root extracts of pepper plants, a 10-kDa
protein could be isolated to which Cd content could be
associated.
The success of a hyphenated technique depends on the:
(i) concentration of the element of interest in the original
sample; (ii) multi-fold dilution of the sample during the
chromatographic separation; and (iii) the sample throughput and the detection limit of the applied spectrometric
technique. Very few data are known regarding the transport
of heavy metal species in xylem vessels. This fact is due to
the problem that usually the heavy metal tolerance of the
plants is low, it is in the mg / g range and only few plants
can accumulate heavy metals by detoxification for example
by forming chelate complexes with phytochelatins. On the
other hand, difficulties arise regarding the extraction of the
xylem saps, because the exudation rate of xylem sap varies
with the plant species. For plants with low exudation
ability vacuum or pressure chambers are employed, which
yield small volumes of fluid that may also contain compounds extracted from non-xylem tissues or non-functional
xylem vessels [21]. At the same time the most powerful
chromatographic techniques like HPLC and capillary
electrophoresis (CE) and techniques for element analysis
like GF-AAS and TXRF require small volumes of samples
for the investigations.
The objective of this study was the determination of the
chemical form of toxic (Pb, Ni) and nutrient (Cu, Mn)
heavy metal ions transported in xylem sap of cucumber
plants grown in toxic heavy metal containing hydroponics.
This work may help to understand possible mechanisms of
plant physiological processes when heavy metals are also
involved.
2. Experimental
2.1. Plant material
Cucumber plants (Cucumis sativus var. Budai korai)
were grown under controlled conditions in toxic heavy
metal-free and lead nitrate, nickel(II) sulfate or vanadyl
sulfate contaminated hydroponics as we reported earlier
[22]. In brief, the concentrations of these heavy metals and
micronutrient elements like Cu, Fe, Mn and Zn in the
nutrient solutions amounted to 10 and 0.0810 mM,
respectively. Cucumber was chosen because of its importance in food industry and its ability to exude relatively
large amounts of xylem fluid. Due to the possible hydrolysis of inorganic iron(III) salts present in the nutrient
solutions, in the experiments beside iron(III) chloride,
Fe(III) EDTA and Fe(III) citrate were also used in
concentration of 10 mM. The xylem sap samples were
collected after decapitation of 36 1-month-old plants for 1
h using micropipettes in polyethylene (PE) vials kept
immersed in beakers containing an ice-salt bath. The
analyzed samples were a mixture of the xylem sap
collected from three plants and frozen immediately.
2.2. Reagents and standard solutions
For performing the SEC measurements, ammonium or
sodium acetate of HPLC purity grade were used for the
preparation of the mobile phase and supplied by Scharlau
Chemie (Barcelona, Spain). Bidistilled water was used
throughout the HPLC analyses. The mobile phase was
filtered through a NALGENE nylon membrane filter of
0.45-mm pore size supplied by NALGE (Rochester, NY,
USA). Ovalbumin and phenylalanine were supplied by
SigmaAldrich (Budapest, Hungary).
In case of element analysis of the xylem saps, the
standard solutions were prepared diluting stock solutions
V.G. Mihucz et al. / Journal of Inorganic Biochemistry 81 (2000) 81 87
of the respective metals supplied by Merck (Darmstadt,
Germany). Bidistilled water was used for the preparation
of the standard solutions. For the determination of the
metal concentrations of the chromatographic fractions, the
solutions used for calibration were prepared in the mobile
phase used for the HPLC analyses. The final concentration
of Suprapur HNO 3 (Merck) in the solutions used for
calibration and in the samples was 0.1%. For the nickel
recovery study Ni(NO 3 ) 2 was supplied by REANAL
(Budapest, Hungary).
2.3. Instrumentation and analytical procedures
2.3.1. Conventional size exclusion chromatography
( SEC)
Prior to passing xylem samples through the HPLC
system, investigations were carried out on home-made
conventional dextrin gel columns of exclusion limits of
700 or 40 000 Da. These columns were prepared by
pouring, into a 50038-mm glass holder, the appropriate
MOLSELECT (REANAL) gel suspension that was previously swelled in water. Then the gel column was washed
with the sodium acetate buffer several hours and coupled
to the UV detector of the HPLC instrument. The uniform
flow rate of 0.5 ml / min was ensured by a VGA-76 (Varian,
Mulgrave, Victoria, Australia) peristaltic pump connected
between the column and the UV detector. Five hundred ml
of xylem sap samples were layered on top of the column
using a syringe and washed with the acetate buffer in
concentration of 50 mM of the same pH (5.6) as that of the
samples. The UV detector was set to scanning mode. The
chromatogram was registered using the WINCHROM
software (GBC, Australia) of the HPLC system.
2.3.2. SECHPLC analysis
Analysis of xylem sap by an HPLSEC method was
carried out on a GBC instrument (GBC LC 1440 system
organizer, GBC LC 1150 pump, GBC LC 1206 multiwavelength UV-Vis detector) suplied by GBC (Dandenong, Victoria, Australia). For the analysis, silica based
and diol layer containing 25034.6 mm 7 mm Macrosphere
GPC 60 PEEK-coated analytical column (ALLTECH,
Deerfield, IL, USA) connected with a 7.534.6-mm guard
cartridge of the same packaging was used. The frac-
83
tionation range of the column was between 250 and 28 000
Da. The columns were thermostated at 288C. The composition of the mobile phase was 50 mM ammonium
acetate (NH 4 Ac) at pH 5.6 adjusted with glacial acetic acid
(HAc). The employed flow rate was 0.5 ml / min. The
retention time of the compounds totally excluded from the
pores of the column and for the totally permeated neutral
compounds was determined by passing through the column
an ovalbumin and phenylalanine standard solution in
concentration of 0.2 and 1.0 mg / ml, respectively. The UV
detector was set to scanning mode for recording the spectra
of the peaks eluted from the column. 20 ml of the sap
samples was injected directly on the column after their
filtration through a Minisart RC 15 membrane filter of
0.2-mm pore size supplied by Sartorius (Gottingen,
Germany).
2.3.3. GF-AAS analysis
The determination of the heavy metal content of the
xylem saps and the collected fractions during the chromatographic runs was carried out using a SpectrAA-20
Varian Atomic Absorption Spectrometer and a Varian
GTA-96 graphite tube atomizer equipped with a Varian
PSC-56 programmable sample changer. For the measurements, partition tubes coated with pyrolytic graphite
supplied by VARIAN were used. The samples were
homogenized on a Vortex Genie-2 apparatus, supplied by
Scientific Industries (Bohemia, NY, USA) and analyzed
making one replicate. Twenty-five ml from the samples
were injected into the graphite furnace, where it was dried,
charred and atomized. The operating conditions for the
GF-AAS and the detection limits of the investigated
elements are listed in Table 1. Each completed determination was followed by a 10-s long tube clean at 27008C.
During the drying, charring and cleanup cycles argon gas
was passed through the furnace at a flow rate of 3000
ml / min. The analytical signal was measured using peak
height mode. The calibration curves were linear up to 20
ng / ml (r50.999) for Cu, 30 ng / ml (r50.997) for Ni, 20
ng / ml (r50.998) for Pb, 5.0 ng / ml (r50.999) for Mn and
160 ng / ml (r50.995) for vanadium. Iron and zinc could
not be determined in the collected fractions because of
their relatively high content in the mobile phase and in
water, respectively. Each chromatographic fraction was
Table 1
Operating conditions of the GF-AAS and detection limits of for the investigated elements
Element
Cu
Mn
Ni
Pb
V
Detection
wavelength
(nm)
Drying
temperature
(8C)
Charring
temperature
(8C)
Atomizing
temperature
(8C)
324.8
279.5
232.0
283.3
318.5
120
120
120
120
120
200
600
800
200
1000
1800
2300
2200
1800
2500
Detection limit (ng / ml)
Xylem sap
Collected
fractions
0.8
0.1
0.6
0.7
2.6
0.1
0.2
0.5
0.7
84
V.G. Mihucz et al. / Journal of Inorganic Biochemistry 81 (2000) 81 87
collected manually in PE vessels for 1 min from the
beginning of each chromatographic run. For these measurements in order to get blank samples, same volumes of
mobile phase were collected and analyzed for each metal
investigated. The dead time at the collection of chromatographic fractions was less than 10 s.
3. Results and discussion
The knowledge of the chemical form of the transported
heavy metals especially in plants of economic interest may
be crucial because actions can be taken to reduce or
minimize the toxic effects of the environment pollutant
heavy metals. Passing through the conventional gel columns xylem exudates of cucumber plants previously
collected for 1 h, elution of compounds with a molar mass
higher than 700 g / mol could not be observed. The
chromatogram on these columns contained only the peak
of the compounds of low molecular weight with high UV
absorbance such as nitrate ions present in 3.75 mM
concentration in the nutrient solutions. Thus, it seemed to
be justified to inject the samples directly on the SEC
HPLC column without applying any previous sample
preparation in order to remove high-molecular weight
macromolecules that would have altered the separation
efficiency and consequently shorten the life of the column.
A typical chromatogram of a xylem sample recorded on
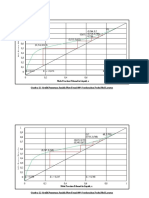
the SEC HPLC column can be seen in Fig. 1, which also
illustrates the exclusion and permeation range by overlaying chromatograms of ovalbumin and phenylalanine, respectively. The chosen integration wavelength for the
Fig. 1. Comparison of the SEC chromatograms of ovalbumin (1) and
phenylalanine standard (3) in concentration of 1 and 0.2 mg / ml,
respectively, with one of a xylem sap of cucumber (2) grown in
nickel-contaminated nutrient solutions containing Fe(III) EDTA. Chromatographic conditions: 25034.6 mm Macrosphere GPC 60 PEEKcoated column of 7-mm particle size; 7.534.6 mm Macrosphere GPC 60
metal-free guard cartridge of 7-mm particle size. Mobile phase, 50 mM
acetate buffer, pH 5.6; flow rate, 0.5 ml / min; column thermostation at
288C; injection volume, 20 ml; UV detection, 260 nm for xylem sap and
280 for ovalbumin and phenylalanine.
xylem sap chromatogram was 260 nm, because at this
wavelength beside one peak with high UV absorbance,
several peaks were revealed. Injecting on the column and
recording the chromatogram of potassium nitrate standard
in concentration of 100 mg / ml, the peak with high UV
absorbance turned out to be the peak of nitrate ions. Its raw
estimated concentration in the sap was 1400 mg / ml.
The explanation for the earlier-than-expected elution of
nitrate ions is in connection with the chemical structure of
the column support and with the pH of the mobile phase.
The pH of the mobile phase identical with the pH of the
samples and the ionic strength of the mobile phase was
chosen to maintain as much as possible the biological
activity of the sample, which is mandatory for metal
speciation studies. But at this pH (5.6) the silica support is
partially ionized and have negative electric charge like the
nitrate ions, which causes electrostatic repulsion between
them and thus, earlier elution of nitrate ions occurs. The
unwanted interaction between the solutes and the column
support is also responsible for the elution of some small
peaks after the elution of phenylalanine, which indicates
that the compounds corresponding to these peaks may have
an overall positive charge. In order to optimize the SEC
HPLC separation method we took into consideration the
eluent type, concentration of the buffer, flow rate and
temperature but in spite of them the unwanted interaction
mentioned above could not be eliminated.
Due to the low heavy metal concentration in the sap
samples and in the chromatographic fractions collected
during the SEC analysis, the latter being a consequence
of the multi-fold dilution that occurs during the chromatographic run, the determination of elements was carried
out using the GF-AAS technique. The results for the
determination of elements in the xylem sap samples and in
the fractions in which their presence could be detected are
listed in the Tables 24. It should be mentioned that lead
could not be detected in the chromatographic fractions.
The concentration (ng / ml) and the standard deviation of
lead in the xylem sap of plants contaminated with lead was
200.4612.2, 155.463.9 and 36.862.1 in case of Fe(III)
chloride, Fe(III) citrate and Fe(III) EDTA, respectively.
Vanadium could not be detected in the sap as we reported
earlier [22]. The concentration of Cu, among the heavy
metals determined, was the lowest both in the sap and the
fractions collected. The highest concentrations for heavy
metals were observed in the seventh fraction, which
corresponds in the chromatogram to the 67-min time
period. By superposing the results obtained at the determination of heavy metals and the chromatograms of the
exudates, it can be concluded that there is a strong
correlation between the peak of nitrate ions and the amount
of the transported copper, manganese and nickel. This fact
is supported by the results obtained by applying for
example this off-line HPLC-GF-AAS method for a
Ni(NO 3 ) 2 standard solution in concentration of 1.2 mg / ml
for nickel. The recovery of Ni in this case was 89.99%.
V.G. Mihucz et al. / Journal of Inorganic Biochemistry 81 (2000) 81 87
85
Table 2
Heavy metal concentrations (ng / ml6RSD (%)) in the xylem sap and in the chromatographic fractions and mass balance in case of plants growing in
Fe(III)-chloride containing nutrient solutions a
Sample
Fe(III)-chloride
Pb 21 addition
Control plants
Ni 21 addition
VO 21 addition
Cu
Mn
Cu
Mn
Cu
Mn
Ni
Cu
Mn
Xylem
5th fraction
6th fraction
7th fraction
8th fraction
9th fraction
10th fraction
11th fraction
27.064.3
n.d.
n.d.
1.464.7
0.363.5
n.d.
n.d.
n.d.
1109.563.8
0.4621.5
0.3636.4
45.461.7
5.964.7
1.367.7
0.668.0
n.d.
37.465.3
n.d.
n.d.
1.765.1
n.d.
n.d.
n.d.
n.d.
1157.961.2
0.2644.9
0.3636.0
25.766.0
4.164.5
0.9611.6
0.369.5
0.3623.2
37.361.3
n.d.
n.d.
1.662.3
n.d.
n.d.
n.d.
n.d.
1437.160.3
0.3614.3
0.2616.7
51.064.6
7.164.1
1.7610.4
0.7614.3
0.3649.9
2017.265.9
n.d.
16.264.3
35.760.8
9.260.5
3.165.5
1.2612.1
0.568.3
32.665.3
n.d.
n.d.
1.062.5
0.363.1
n.d.
n.d.
n.d.
763.4.64.8
0.2622.9
n.d.
24.9617.2
4.864.4
1.168.3
0.6622.7
0.465.5
m (ng), xylem
m (ng), fracts.
Mass balance
0.54
22.19
0.85
26.95
0.75
0.85
23.16
15.90
0.75
0.80
28.74
30.65
40.34
32.95
0.65
0.65
15.27
16.00
The values were determined on the basis of two independent chromatographic measurements. n.d., not detectable.
Table 3
Heavy metal concentrations (ng / ml6RSD (%)) in the xylem sap and in the chromatographic fractions and mass balance in case of plants growing in
Fe(III)-citrate containing nutrient solutions a
Sample
Fe(III)-citrate
Pb 21 addition
Control plants
Ni 21 addition
VO 21 addition
Cu
Mn
Cu
Mn
Cu
Mn
Ni
Cu
Mn
Xylem
5th fraction
6th fraction
7th fraction
8th fraction
9th fraction
10th fraction
11th fraction
26.663.4
n.d.
n.d.
1.360.1
n.d.
n.d.
n.d.
n.d.
763.4610.4
0.268.8
0.3623.5
24.363.7
4.3623.0
1.0613.3
0.5612.1
0.464.1
54.368.2
n.d.
n.d.
2.563.0
n.d.
n.d.
n.d.
n.d.
1061.260.9
0.3654.2.
0.768.8
46.1618.6
5.667.9
1.360.1
0.5619.1
0.5620.5
30.067.2
n.d.
n.d.
0.863.8
n.d.
n.d.
n.d.
n.d.
774.560.6
0.3624.5
0.3629.6
28.263.4
7.260.9
1.5610.4
0.8616.7
0.5612.7
1452.760.6
n.d.
9.960.7
30.462.5
8.061.7
2.363.2
0.662.4
0.3646.4
20.163.5
n.d.
n.d.
0.966.2
n.d.
n.d.
n.d.
n.d.
666.664.4
n.d.
0.5619.6
30.767.0
8.067.4
1.7612.0
0.6610.8
0.5629.2
m (ng), xylem
m (ng), fracts.
Mass balance
0.53
15.27
0.65
13.35
1.09
1.25
21.22
27.50
0.60
0.40
15.49
19.40
29.05
25.75
0.40
0.45
13.33
21.00
The values were determined on the basis of two independent chromatographic measurements. n.d., not detectable.
Table 4
Heavy metal concentrations (ng / ml6RSD (%)) in the xylem sap and in the chromatographic fractions and mass balance in case of plants growing in
Fe(III)-EDTA containing nutrient solutions a
Sample
Fe(III)-EDTA
Pb 21 addition
Control plants
Ni 21 addition
VO 21 addition
Cu
Mn
Cu
Mn
Cu
Mn
Ni
Cu
Mn
Xylem
6th fraction
7th fraction
8th fraction
9th fraction
10th fraction
33.865.3
n.d.
1.763.0
0.3618.8
n.d.
n.d.
670.362.7
1.2614.3
20.961.9
4.065.4
1.460.8
0.4632.2
24.962.1
n.d.
1.262.3
n.d.
n.d.
n.d.
759.764.2
5.064.2
20.563.2
6.260.3
1.5613.7
n.d.
30.168.0
n.d.
1.260.1
n.d.
n.d.
n.d.
752.265.3
n.d.
20.564.4
5.166.1
1.263.1
n.d.
1076.364.6
6.664.2
21.065.9
5.866.2
2.361.6
0.269.1
23.560.5
n.d.
1.060.2
n.d.
n.d.
n.d.
495.560.7
n.d.
13.465.6
2.461.8
0.669.6
n.d.
m (ng), xylem
m (ng), fracts.
Mass balance
0.68
13.41
1.00
13.95
0.50
0.60
15.20
16.60
0.60
0.60
15.04
13.40
21.53
17.95
0.47
0.50
9.91
8.20
The values were determined on the basis of two independent chromatographic measurements. n.d., not detectable.
86
V.G. Mihucz et al. / Journal of Inorganic Biochemistry 81 (2000) 81 87
However, in case of the nickel determination of the
fractions, the sixth fraction had significant Ni concentration compared to the values obtained for the same
fractions for Mn. Thus, the compounds that elute earlier
than the nitrate ions may participate in the transport of
nickel (Fig. 2). However, it should be mentioned that the
other inorganic anions of the nutrient solutions, namely
sulfate and phosphate may co-elute with the nitrate ions,
but their presence could not be detected at the used
detection wavelengths. In the nutrient solutions the mol
ratio
of
the
main
inorganic
anions
is:
22
2
NO 2
:SO
:H
PO
515:2:1.
Also,
it
has
been
proved
that
3
4
2
4
among these ions the relative uptake of nitrate ions is the
highest [23].
For each heavy metal, its content in the chromatographic
fractions was summed and the sums were related to the
amount of the given metal in the sap injected on the
column. The deviations from the calculated mass balance
calculated were between 6.7 and 57.8% for Cu, 31.3 and
57.5% for Mn, 11.4 and 18.3% for Ni (Tables 24).
Regarding the chemical form of iron(III) used for the
preparation of the nutrient solutions, it could be concluded
that there were no significant changes in the chromatograms recorded in case of plant saps grown in each
iron(III) salt (not shown) and in the distribution of Cu, Mn
and Ni in the collected fractions.
4. Conclusions
Using a SEC method for analyzing xylem exudates of
cucumber exposed to lead, nickel or vanadium contamination collected for 1 h, besides nitrate ions only compounds
with lower molecular weight than 700 Da could be
observed.
The developed off-line HPLCGF-AAS analytical
method was suitable for determination of copper, manganese and nickel concentrations of the collected fractions
Fig. 2. Correlation between the heavy metal concentrations of the collected fractions and the peak of nitrate ions at 220 and 260 nm wavelengths in the
chromatogram of xylem sap of cucumber plants grown in control, as well as vanadium-, lead- or nickel-contaminated nutrient solutions in presence of
Fe(III) citrate. Chromatographic conditions: 25034.6 mm Macrosphere GPC 60 PEEK-coated column of 7-mm particle size; 7.534.6 mm Macrosphere
GPC 60 metal-free guard cartridge of 7-mm particle size. Mobile phase, 50 mM acetate buffer, pH 5.6; flow rate, 0.5 ml / min; Column thermostation at
288C.
V.G. Mihucz et al. / Journal of Inorganic Biochemistry 81 (2000) 81 87
during the chromatographic analysis of xylem sap samples
of cucumber. As the result of our study it seems that
inorganic ions like the nitrate ions, Cu, Mn and Ni ions
may be transported together through the transpiration
stream. However, in case of the nickel transport, the
possibility that the compounds eluting earlier than the
nitrate ions can take part in the transport processes can not
be excluded. The presence of lead could not be detected in
the collected fractions, although it was present in the sap in
significant concentration. Determination of vanadium in
the fractions was not expected since it could not be
detected in the sap samples.
The high concentration of nitrate ions found in the saps
may indicate a saturated nitrate reductase activity in the
roots of cucumber plants and this has the consequence of
nitrate ion transport to the shoots. This is important
because applying high levels of nitrate fertilizer in case of
plants of economic importance may increase the risk of
heavy metal transport in different plant parts.
Acknowledgements
This research was supported by the Hungarian National
Research Foundation and the Hungarian Academy of
Sciences through Grants T030845 and AKP-98-91 / 2, 4,
respectively.
References
Gy. Zaray,
[1] M. Ovari,
K. Danzer, G. Thiel, Microchem. J. (in press).
[2] M.E. Hilburn, Chem. Soc. Rev. 8 (1979) 63.
87
[3] S.E. Manahan, in: Environmental Chemistry, 6th Edition, Lewis
Publishers, Boca Raton, FL, 1994.
[4] A. Kabata-Pendias, H. Pendias, in: Trace Elements in Soils and
Plants, 1st Edition, CRC Press, Boca Raton, FL, 1984.
[5] D. Prima-Putra, B.J. Botton, Plant Physiol. 153 (1998) 670.
[6] L.H. Siertsema, Clin. Chim. Acta 69 (1976) 533.
[7] R. Boriello, G. Sciaudone, At. Spectrosc. 1 (1980) 131.
[8] M. Zerezghi, K.C. Ng, J.A. Caruso, Analyst 109 (1984) 589.
[9] M.H. Hahn, K.A. Wolnick, F.L. Fricke, J.A. Caruso, Anal. Chem. 54
(1982) 1048.
[10] A. Prange, H. Schwenke, Adv. X-ray Anal. 35 (1992) 899.
[11] R. Klockenkamper,
A. von Bohlen, R. Otto, G. Tolg,
Int. Arch.
Occup. Environ. Health 59 (1987) 403.
[12] H.G. Barth, B.E. Boyes, C. Jackson, Anal. Chem. 66 (1994) 595R.
[13] R.D. Ricker, L.A. Sandoval, J. Chromatogr. A 743 (1996) 43.
[14] L.R. Snyder, J.J. Kirkland, J.L. Glajch, in: Practical HPLC Method
Development, 2nd Edition, Wiley, New York, 1997.
[15] K. Saitoh, N. Suzuki, J. Chromatogr. 109 (1975) 333.
[16] T. Deguchi, A. Hisanaga, H. Naga, J. Chromatogr. 133 (1977) 173.
[17] K. Konchiyawa, N. Yoza, S. Ohashi, J. Chromatogr. 147 (1978) 271.
[18] I. Leopold, D. Gunther,
Fres. J. Anal. Chem. 359 (1997) 364.
[19] I. Leopold, D. Gunther,
D. Neumann, Analusis Mag. 26 (1998)
M28.
[20] F. Jemal, L. Didierjean, R. Ghrir, M.H. Ghorbal, G. Burkard, Plant
Sci. 137 (1998) 143.
[21] A.R. Fergusson, Ann. Bot. 46 (1980) 791.
V.G. Mihucz, A. Varga, Gy. Zaray,
[22] E. Tatar,
E. Cseh, J. Inorg.
Biochem. 75 (1999) 219.
[23] H. Marschner, in: Mineral Nutrition of Higher Plants, Academic
Press, London, 1995.
Das könnte Ihnen auch gefallen
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryVon EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryBewertung: 3.5 von 5 Sternen3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Von EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Bewertung: 4.5 von 5 Sternen4.5/5 (121)
- Grit: The Power of Passion and PerseveranceVon EverandGrit: The Power of Passion and PerseveranceBewertung: 4 von 5 Sternen4/5 (588)
- Never Split the Difference: Negotiating As If Your Life Depended On ItVon EverandNever Split the Difference: Negotiating As If Your Life Depended On ItBewertung: 4.5 von 5 Sternen4.5/5 (838)
- The Little Book of Hygge: Danish Secrets to Happy LivingVon EverandThe Little Book of Hygge: Danish Secrets to Happy LivingBewertung: 3.5 von 5 Sternen3.5/5 (400)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaVon EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaBewertung: 4.5 von 5 Sternen4.5/5 (266)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeVon EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeBewertung: 4 von 5 Sternen4/5 (5794)
- Her Body and Other Parties: StoriesVon EverandHer Body and Other Parties: StoriesBewertung: 4 von 5 Sternen4/5 (821)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreVon EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreBewertung: 4 von 5 Sternen4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyVon EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyBewertung: 3.5 von 5 Sternen3.5/5 (2259)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersVon EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersBewertung: 4.5 von 5 Sternen4.5/5 (345)
- Shoe Dog: A Memoir by the Creator of NikeVon EverandShoe Dog: A Memoir by the Creator of NikeBewertung: 4.5 von 5 Sternen4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerVon EverandThe Emperor of All Maladies: A Biography of CancerBewertung: 4.5 von 5 Sternen4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnVon EverandTeam of Rivals: The Political Genius of Abraham LincolnBewertung: 4.5 von 5 Sternen4.5/5 (234)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceVon EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceBewertung: 4 von 5 Sternen4/5 (895)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureVon EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureBewertung: 4.5 von 5 Sternen4.5/5 (474)
- On Fire: The (Burning) Case for a Green New DealVon EverandOn Fire: The (Burning) Case for a Green New DealBewertung: 4 von 5 Sternen4/5 (74)
- The Yellow House: A Memoir (2019 National Book Award Winner)Von EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Bewertung: 4 von 5 Sternen4/5 (98)
- The Unwinding: An Inner History of the New AmericaVon EverandThe Unwinding: An Inner History of the New AmericaBewertung: 4 von 5 Sternen4/5 (45)
- Unit Operation Iii: Vapor-Liquid Separation ProcessesDokument160 SeitenUnit Operation Iii: Vapor-Liquid Separation ProcessesDeny AlsanNoch keine Bewertungen
- Jsa Jis G 0321Dokument16 SeitenJsa Jis G 0321farhad pashaeimehrNoch keine Bewertungen
- Cloud Storage VS Local StorageDokument4 SeitenCloud Storage VS Local StorageSasa LiliNoch keine Bewertungen
- SEPARATION METHODS - AnimDokument58 SeitenSEPARATION METHODS - AnimSandi Mahesa0% (1)
- X-Ray DiffractionDokument6 SeitenX-Ray DiffractionAhmed Al-AwamiNoch keine Bewertungen
- Determining The Concentration of A Solution - Beer's LawDokument4 SeitenDetermining The Concentration of A Solution - Beer's LawDina Elyamany67% (3)
- Acetyl Coenzyme A - AssignmentDokument2 SeitenAcetyl Coenzyme A - AssignmentSasa LiliNoch keine Bewertungen
- Aqua Mini ProjectDokument7 SeitenAqua Mini ProjectSasa LiliNoch keine Bewertungen
- PhytoalexinDokument3 SeitenPhytoalexinSasa LiliNoch keine Bewertungen
- KNJ1231 Mechanical and Manufacturing Engineering Laboratory 1 Faculty of Engineering Universiti Malaysia SarawakDokument6 SeitenKNJ1231 Mechanical and Manufacturing Engineering Laboratory 1 Faculty of Engineering Universiti Malaysia SarawakSasa LiliNoch keine Bewertungen
- Crowe, F. W., Et Al. A Clinical, Pathological, and Genetic Study of Multiple Neurofibromatosis (Springfield, Illinois, Charles C. Thomas, 1956)Dokument1 SeiteCrowe, F. W., Et Al. A Clinical, Pathological, and Genetic Study of Multiple Neurofibromatosis (Springfield, Illinois, Charles C. Thomas, 1956)Sasa LiliNoch keine Bewertungen
- Assignment 2 ThermodynamicsDokument1 SeiteAssignment 2 ThermodynamicsSasa LiliNoch keine Bewertungen
- Answer 589Dokument3 SeitenAnswer 589Sasa LiliNoch keine Bewertungen
- Courses in English 2017-2018 01Dokument25 SeitenCourses in English 2017-2018 01Milovan NikolicNoch keine Bewertungen
- Report On NeemDokument16 SeitenReport On Neemadityaksrivastava100% (1)
- CefprozilDokument2 SeitenCefprozilJuan PerezNoch keine Bewertungen
- Group 1 - Chemical EngineeringDokument4 SeitenGroup 1 - Chemical EngineeringNikma AmeliaNoch keine Bewertungen
- Assignment Chapter 4-pH-Acidity-Alkalinity-VFA HKTMTDokument9 SeitenAssignment Chapter 4-pH-Acidity-Alkalinity-VFA HKTMTThành Lợi0% (1)
- Membrane Test UnitDokument14 SeitenMembrane Test UnitAzzian AriffinNoch keine Bewertungen
- Chapter 1. Introduction: 1. Generation of X-RayDokument127 SeitenChapter 1. Introduction: 1. Generation of X-Raynirmal_phyNoch keine Bewertungen
- Silica Proteins: Principles of Gel ElectrophoresisDokument5 SeitenSilica Proteins: Principles of Gel ElectrophoresisSai SridharNoch keine Bewertungen
- Chapter 428 Reactionsin Aqueous Equilibria 29Dokument99 SeitenChapter 428 Reactionsin Aqueous Equilibria 29Kent NguyenNoch keine Bewertungen
- Grafik Bab IDokument3 SeitenGrafik Bab IPulbiNoch keine Bewertungen
- Gravimetric Analysis WorksheetDokument2 SeitenGravimetric Analysis WorksheetShurlandJamesJr.100% (2)
- A. L. Burlingame, Steven A. Carr Biological Mass Spectrometry 1996Dokument512 SeitenA. L. Burlingame, Steven A. Carr Biological Mass Spectrometry 1996Sonik AlexNoch keine Bewertungen
- 21-22 Buffer PreparationDokument2 Seiten21-22 Buffer Preparationbf4810Noch keine Bewertungen
- Chapter 3 - Solutions and Solution PreparationDokument35 SeitenChapter 3 - Solutions and Solution Preparationbahru demekeNoch keine Bewertungen
- Chapter 4.0 ACID-BASE EQUILIBRIUMDokument54 SeitenChapter 4.0 ACID-BASE EQUILIBRIUMMuhd Mirza HizamiNoch keine Bewertungen
- CHEMISTRY PROJECT - MergedDokument14 SeitenCHEMISTRY PROJECT - Mergedarpitarathore024Noch keine Bewertungen
- ICP Stanndard Solution IV 1113550100 - HC90682355 - SU - ENDokument2 SeitenICP Stanndard Solution IV 1113550100 - HC90682355 - SU - ENZulfahmi Al UsuiNoch keine Bewertungen
- ChromatographyDokument2 SeitenChromatographyEaEamNoch keine Bewertungen
- Data Sheet: Experiment 1: Chemical ReactionsDokument17 SeitenData Sheet: Experiment 1: Chemical ReactionsLinh NguyễnNoch keine Bewertungen
- Chapter 4: Imperfections in SolidsDokument46 SeitenChapter 4: Imperfections in SolidsJesse De LeonNoch keine Bewertungen
- Introduction To Molarity: High Concentration Indicates A Low Concentration Indicates ADokument10 SeitenIntroduction To Molarity: High Concentration Indicates A Low Concentration Indicates AGrace BatlleNoch keine Bewertungen
- 188-Article Text-4754-1-10-20220921Dokument7 Seiten188-Article Text-4754-1-10-20220921Firkatun ZiahNoch keine Bewertungen
- Full Report - Reverse Osmosis FIXDokument39 SeitenFull Report - Reverse Osmosis FIXendang dian lestariNoch keine Bewertungen
- EXP 3 CHM213 UiTMDokument5 SeitenEXP 3 CHM213 UiTMnureenNoch keine Bewertungen
- Titrimetric Determination of Free Boric Acid and Tetrafluoroboric Acid in Nickel Plating BathsDokument3 SeitenTitrimetric Determination of Free Boric Acid and Tetrafluoroboric Acid in Nickel Plating BathsDang ThinhNoch keine Bewertungen