Beruflich Dokumente
Kultur Dokumente
Corona Discharge
Hochgeladen von
Zia Ateeq0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
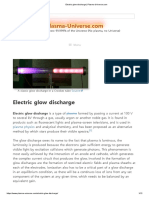
82 Ansichten2 SeitenA corona discharge is a non-thermal plasma that occurs near a thin, high potential electrode such as a wire or pin. It forms via an electron avalanche process where free electrons are accelerated by a strong electric field and collide with gas molecules, liberating more electrons. This builds an avalanche that ionizes the entire gas. Coronas can be positive or negative depending on electrode polarity. In positive coronas, electrons travel toward the electrode and ions away, while in negative coronas the directions are reversed. Secondary electrons needed to sustain the discharge come from photon impacts near the positive electrode or the negative electrode surface. A key feature of negative coronas is they require electronegative gases that can capture electrons and form negative ions
Originalbeschreibung:
corona discharge... HVE
Copyright
© © All Rights Reserved
Verfügbare Formate
DOC, PDF, TXT oder online auf Scribd lesen
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenA corona discharge is a non-thermal plasma that occurs near a thin, high potential electrode such as a wire or pin. It forms via an electron avalanche process where free electrons are accelerated by a strong electric field and collide with gas molecules, liberating more electrons. This builds an avalanche that ionizes the entire gas. Coronas can be positive or negative depending on electrode polarity. In positive coronas, electrons travel toward the electrode and ions away, while in negative coronas the directions are reversed. Secondary electrons needed to sustain the discharge come from photon impacts near the positive electrode or the negative electrode surface. A key feature of negative coronas is they require electronegative gases that can capture electrons and form negative ions
Copyright:
© All Rights Reserved
Verfügbare Formate
Als DOC, PDF, TXT herunterladen oder online auf Scribd lesen
0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
82 Ansichten2 SeitenCorona Discharge
Hochgeladen von
Zia AteeqA corona discharge is a non-thermal plasma that occurs near a thin, high potential electrode such as a wire or pin. It forms via an electron avalanche process where free electrons are accelerated by a strong electric field and collide with gas molecules, liberating more electrons. This builds an avalanche that ionizes the entire gas. Coronas can be positive or negative depending on electrode polarity. In positive coronas, electrons travel toward the electrode and ions away, while in negative coronas the directions are reversed. Secondary electrons needed to sustain the discharge come from photon impacts near the positive electrode or the negative electrode surface. A key feature of negative coronas is they require electronegative gases that can capture electrons and form negative ions
Copyright:
© All Rights Reserved
Verfügbare Formate
Als DOC, PDF, TXT herunterladen oder online auf Scribd lesen
Sie sind auf Seite 1von 2
What is a corona discharge and how does it work?
Prof Clive Beggs, Medical Biophysics Group, University of Bradford
(e-mail: c.b.beggs@bradford.ac.uk)
A Corona discharge is sustained non-thermal plasma which occurs in
close vicinity to a thin discharge electrode, such as a pin or a wire, at a
high potential. Coronas may be either positive or negative, depending on
the polarity of the electrode. Although both positive and negative coronas
share many similar characteristics, the physical processes involved differ
between the two.
One feature common to both positive and negative coronas is the
formation of an electron avalanche. Such an avalanche occurs when a
strong electric field acts on naturally occurring free electrons in the air.
The electric field accelerates these electrons so that they gain sufficient
kinetic energy to cause ionisation when they collide with neutral gas
molecules in their path. Additional electrons are liberated during these
collisions, which after acceleration are also able to ionise. As the process
continues more and more electrons are liberated and an avalanche
rapidly builds up. In this way a small number of seed electrons can cause
ionisation of an entire gas and turn it into plasma. In a positive corona
the avalanche electrons are drawn towards the electrode, while the
resultant positive ions are repelled. In a negative corona the avalanche is
in the opposite direction, with the electrons repelled and the positive ions
drawn to the electrode. In a positive corona the electrons accelerate as
the avalanche progresses, while in a negative corona they decelerate as
they travel away from the electrode.
In an avalanche the electron collisions excite the positive ions so that
photons of short wavelength light are emitted. It is this that gives a
corona discharge its characteristic glow. These photons play an
important part in producing the new seed electrons which are required to
sustain the corona.
In a positive corona the electron avalanche is initiated by an exogenous
ionisation event in a region of high potential gradient. The electrons
resulting from the ionisation collision are attracted toward the positive
electrode, and the positive ions are repelled from it. The secondary
electrons, required to seed further avalanches, are generated at the
boundary of ionisation region by photons of light released during the
ionisation process. When these photons strike neutral gas molecules
they liberate electrons (through to the photoelectric effect), which are
then drawn to the positive electrode. It is these electrons which seed
and sustain further avalanches. While the electrons travel to the positive
electrode the positive ions drift away from it towards the earthed
electrode.
As with the positive corona, the electron avalanche in a negative corona
is initiated by an exogenous ionisation event in a region of high potential
gradient. However, the electrons travel in the opposite direction, away
from the negative electrode, while the positive ions are drawn towards it.
Unlike the positive corona, electrons ionised from the neutral gas are of
relatively little use in sustaining a negative corona because the general
movement of electrons is away from the negative electrode. Instead, the
dominant process for generating secondary electrons is the photoelectric
action of photons striking the surface of the negative electrode. Indeed,
the energy required to liberate the electrons from the electrode surface
is considerably lower than that required to ionise air at standard
temperatures and pressures, making it a more liberal source of
secondary electrons. Consequently, the avalanche seed electrons are
generated on the surface of the electrode and are repelled from it. As
these electrons leave the ionisation region they attach themselves to
neutral gas molecules to form small negative air ions which drift towards
the earthed electrode.
A feature of negative coronas is that they can only be sustained in fluids
which contain electronegative molecules, such as O 2, H20 and CO2.
These gases have molecules which readily scavenge free electrons.
Without electronegative molecules to capture free electrons, small
negative ions cannot form, with the result that a simple path of electron
flow of ionised gas will form between the two electrodes and an arc will
develop.
Clive Beggs
24th October 2006
Das könnte Ihnen auch gefallen
- Physics for Kids : Electricity and Magnetism - Physics 7th Grade | Children's Physics BooksVon EverandPhysics for Kids : Electricity and Magnetism - Physics 7th Grade | Children's Physics BooksBewertung: 5 von 5 Sternen5/5 (1)
- Plasma Generation: Theory and Experiments in Electrical DischargesDokument25 SeitenPlasma Generation: Theory and Experiments in Electrical DischargesAnggunNoch keine Bewertungen
- Negative DC CoronaDokument3 SeitenNegative DC Coronasravan KNoch keine Bewertungen
- How Our Space Program Uses Ion Propulsion | Children's Physics of EnergyVon EverandHow Our Space Program Uses Ion Propulsion | Children's Physics of EnergyNoch keine Bewertungen
- Corona DischargeDokument10 SeitenCorona DischargeAmir Haider JaffriNoch keine Bewertungen
- Corona Discharge: From Wikipedia, The Free EncyclopediaDokument5 SeitenCorona Discharge: From Wikipedia, The Free EncyclopediaAliq FazliNoch keine Bewertungen
- Corona Discharge - Wikipedia, The Free EncyclopediaDokument8 SeitenCorona Discharge - Wikipedia, The Free EncyclopediavagoliyoNoch keine Bewertungen
- Speech To PhysicsDokument2 SeitenSpeech To PhysicsMaoNoch keine Bewertungen
- Module 2 - Conduction and Breakdown in GasesDokument58 SeitenModule 2 - Conduction and Breakdown in GasesFah RukhNoch keine Bewertungen
- Electrostatics SS1Dokument6 SeitenElectrostatics SS1OlusolaNoch keine Bewertungen
- Corona DischargeDokument16 SeitenCorona DischargejuarozNoch keine Bewertungen
- Aether and Structure of ElectronDokument7 SeitenAether and Structure of ElectrondllabarreNoch keine Bewertungen
- What is a Mass SpectrometerDokument11 SeitenWhat is a Mass SpectrometerFiliz Kocayazgan ShanablehNoch keine Bewertungen
- Corona DischargeDokument9 SeitenCorona DischargewassimNoch keine Bewertungen
- Physics-Static and Current ElectricityDokument10 SeitenPhysics-Static and Current ElectricityCodeen WhiteNoch keine Bewertungen
- Radiation & Radioactivity: Department of Medical Biophysics and Informatics 3rd Medical Faculty of Charles UniversityDokument34 SeitenRadiation & Radioactivity: Department of Medical Biophysics and Informatics 3rd Medical Faculty of Charles UniversityahkiaenaaaaNoch keine Bewertungen
- Emission of ElectronsDokument8 SeitenEmission of ElectronsNivashini VindhyaNoch keine Bewertungen
- Gases and PlasmasDokument1 SeiteGases and PlasmasracNoch keine Bewertungen
- Electric CurrentDokument20 SeitenElectric CurrentBik WodeNoch keine Bewertungen
- CHPPT 3Dokument28 SeitenCHPPT 3Mekonnen AyalNoch keine Bewertungen
- Simple Lemon Battery Generates Electricity Using Zinc & Copper ElectrodesDokument2 SeitenSimple Lemon Battery Generates Electricity Using Zinc & Copper ElectrodesSt. Anthony of PaduaNoch keine Bewertungen
- Chapter Five (Interaction of Radiation With Matter)Dokument8 SeitenChapter Five (Interaction of Radiation With Matter)White HeartNoch keine Bewertungen
- Gases Breakdown MechanismsDokument20 SeitenGases Breakdown Mechanismsamrit403Noch keine Bewertungen
- EEC 124 Week OneDokument18 SeitenEEC 124 Week OneOlisaemeka EmmanuelNoch keine Bewertungen
- Corona PDFDokument31 SeitenCorona PDFShawn Aryan Arora67% (3)
- The Photoelectric Effect ExplainedDokument16 SeitenThe Photoelectric Effect ExplainedRushita LingiahNoch keine Bewertungen
- Group 5Dokument19 SeitenGroup 5KISAAKYE SIMON PETER KASOZINoch keine Bewertungen
- Chapter 3 NotesDokument7 SeitenChapter 3 NotesNickNoch keine Bewertungen
- Physics ProjectDokument10 SeitenPhysics ProjectAkash NarayanNoch keine Bewertungen
- US Iran ConflictDokument6 SeitenUS Iran ConflictIhsaan GulzarNoch keine Bewertungen
- Photoelectric Effect: Jump To Navigation Jump To SearchDokument10 SeitenPhotoelectric Effect: Jump To Navigation Jump To SearchSrynnENoch keine Bewertungen
- What Is Corona?: Power Systems, IncDokument8 SeitenWhat Is Corona?: Power Systems, IncLakshmi NarayanNoch keine Bewertungen
- Tugas Bahasa InggrisDokument6 SeitenTugas Bahasa InggrisSuci CahyantiNoch keine Bewertungen
- Stimulated Emission: Classical View Nucleus Atom OrbitalsDokument2 SeitenStimulated Emission: Classical View Nucleus Atom OrbitalsMatthew CrawfordNoch keine Bewertungen
- Lec# 03 Ionization and TypesDokument20 SeitenLec# 03 Ionization and TypesMuhammad Umer AliNoch keine Bewertungen
- Chapter 1 (Semicon) FLOYDDokument2 SeitenChapter 1 (Semicon) FLOYDanon_337840562Noch keine Bewertungen
- LASER07022023Dokument39 SeitenLASER07022023Akhyaya PattanaikNoch keine Bewertungen
- GCFGCGCFGFDGDokument15 SeitenGCFGCGCFGFDGZabrinaRuizNoch keine Bewertungen
- Cold ElectricityDokument16 SeitenCold Electricityi2lovejesusNoch keine Bewertungen
- %uhdngrzqri DVHRXV, Qvxodwlrq: 1.1 Ionisation of GasesDokument21 Seiten%uhdngrzqri DVHRXV, Qvxodwlrq: 1.1 Ionisation of GasesBalakrushna SahuNoch keine Bewertungen
- Photoelectric EffectDokument19 SeitenPhotoelectric EffectSonu SinghalNoch keine Bewertungen
- 101 - Electron TheoryDokument2 Seiten101 - Electron TheorySeagullian DaveNoch keine Bewertungen
- A-Level Chemistry NotesDokument10 SeitenA-Level Chemistry NotesHannah Bryson-JonesNoch keine Bewertungen
- Teacher's Guide Cosmic Rays and Cloud Chambers: Background InformationDokument14 SeitenTeacher's Guide Cosmic Rays and Cloud Chambers: Background InformationlocutorNoch keine Bewertungen
- Periodic Properties of The ElementsDokument57 SeitenPeriodic Properties of The ElementstalktotiffanychengNoch keine Bewertungen
- Unit 2Dokument49 SeitenUnit 2mexebi1971Noch keine Bewertungen
- Electric Glow DischargeDokument12 SeitenElectric Glow DischargebismuthsunilNoch keine Bewertungen
- Corona DischargeDokument1 SeiteCorona DischargemayureshNoch keine Bewertungen
- The Photoelectric Effect (Notes)Dokument10 SeitenThe Photoelectric Effect (Notes)Chathumi LelwalaNoch keine Bewertungen
- Periodic Table IIDokument2 SeitenPeriodic Table IIPrinceblesed EdemNoch keine Bewertungen
- Radiation & Radioactivity and Half LifeDokument27 SeitenRadiation & Radioactivity and Half LifedwyphyNoch keine Bewertungen
- Photoelectric Effect EssayDokument4 SeitenPhotoelectric Effect EssayCong NguyenNoch keine Bewertungen
- Current SolutionDokument12 SeitenCurrent SolutionSwarnavo PramanikNoch keine Bewertungen
- Ionic BondsDokument1 SeiteIonic BondsDivya ShahNoch keine Bewertungen
- Unit-3 Solar Energy: What Is It and How Does It Work?Dokument13 SeitenUnit-3 Solar Energy: What Is It and How Does It Work?djkasjdhfNoch keine Bewertungen
- Dual Nature of Matter and Radiation NotesDokument23 SeitenDual Nature of Matter and Radiation Notesanilpatel39Noch keine Bewertungen
- Inspeções Por Corona - UVDokument8 SeitenInspeções Por Corona - UVJaques ValleNoch keine Bewertungen
- Lecture 01 2Dokument17 SeitenLecture 01 2সায়েম মুহাম্মাদNoch keine Bewertungen
- Periodic TrendsDokument4 SeitenPeriodic TrendsBalcy GayodanNoch keine Bewertungen
- EG1108 - DCmotors JHBJHBJHB JHBJHBJHBJDokument19 SeitenEG1108 - DCmotors JHBJHBJHB JHBJHBJHBJSulaiman JafferyNoch keine Bewertungen
- English Literature PIDokument1 SeiteEnglish Literature PIZia AteeqNoch keine Bewertungen
- English Literature PIIDokument1 SeiteEnglish Literature PIIZia AteeqNoch keine Bewertungen
- PQR Pres.Dokument7 SeitenPQR Pres.Zia AteeqNoch keine Bewertungen
- Solar Tracker Using ATMEGA8 Micro Controller.Dokument18 SeitenSolar Tracker Using ATMEGA8 Micro Controller.Zia AteeqNoch keine Bewertungen
- 8Dokument7 Seiten8Zia AteeqNoch keine Bewertungen
- Computer ScienceDokument2 SeitenComputer ScienceZia AteeqNoch keine Bewertungen
- NAB Past-Paper Solved 2015Dokument9 SeitenNAB Past-Paper Solved 2015Shafeeque MemonNoch keine Bewertungen
- 08 B Lecture Transmission Lines - ProblemsDokument7 Seiten08 B Lecture Transmission Lines - ProblemsZia AteeqNoch keine Bewertungen
- English EssayDokument1 SeiteEnglish EssayAtif KhanNoch keine Bewertungen
- Real Experience Using Power Quality Data To Improve Power Distribution ReliabilityDokument11 SeitenReal Experience Using Power Quality Data To Improve Power Distribution ReliabilityKARAM ZAKARIANoch keine Bewertungen
- Philosophy P IDokument1 SeitePhilosophy P INawal Asif KhattakNoch keine Bewertungen
- Chapter 2Dokument41 SeitenChapter 2Prem KumarNoch keine Bewertungen
- Chapter 1Dokument26 SeitenChapter 1Harshavardhan GuptaNoch keine Bewertungen
- Sect6 3 4 332Dokument8 SeitenSect6 3 4 332Zia AteeqNoch keine Bewertungen
- POWER QUALITY EVENTS AND MITIGATIONDokument54 SeitenPOWER QUALITY EVENTS AND MITIGATIONSundhar SivaNoch keine Bewertungen
- 6.3.2. Forward ConverterDokument12 Seiten6.3.2. Forward ConverterZia AteeqNoch keine Bewertungen
- Lecture For Reading 2Dokument40 SeitenLecture For Reading 2Zia AteeqNoch keine Bewertungen
- 6.2. A Short List of Converters: Fundamentals of Power Electronics Chapter 6: Converter CircuitsDokument8 Seiten6.2. A Short List of Converters: Fundamentals of Power Electronics Chapter 6: Converter CircuitsZia AteeqNoch keine Bewertungen
- 6.3.2. Forward ConverterDokument12 Seiten6.3.2. Forward ConverterZia AteeqNoch keine Bewertungen
- Lecture For Reading 2Dokument40 SeitenLecture For Reading 2Zia AteeqNoch keine Bewertungen
- 6.2. A Short List of Converters: Fundamentals of Power Electronics Chapter 6: Converter CircuitsDokument8 Seiten6.2. A Short List of Converters: Fundamentals of Power Electronics Chapter 6: Converter CircuitsZia AteeqNoch keine Bewertungen
- Biodata FormatDokument1 SeiteBiodata FormatSristi PaulNoch keine Bewertungen
- Sect6 1 4Dokument9 SeitenSect6 1 4Zia AteeqNoch keine Bewertungen
- 3p ResumDokument2 Seiten3p ResumLSara Martín GNoch keine Bewertungen
- Electric Field (E) Due To Point ChargesDokument7 SeitenElectric Field (E) Due To Point ChargesZia AteeqNoch keine Bewertungen
- 6.1. Circuit ManipulationsDokument11 Seiten6.1. Circuit ManipulationsZia AteeqNoch keine Bewertungen
- Solar Tracker Using ATMEGA8 Micro Controller.Dokument18 SeitenSolar Tracker Using ATMEGA8 Micro Controller.Zia AteeqNoch keine Bewertungen
- Energy Processes: Understanding Plasma, Insulators and BreakdownDokument28 SeitenEnergy Processes: Understanding Plasma, Insulators and BreakdownZia AteeqNoch keine Bewertungen
- Practical-Characteristics of Waste WaterDokument19 SeitenPractical-Characteristics of Waste WatersarfaNoch keine Bewertungen
- Chapter 1 - Introduction: Sr. No. QuestionsDokument15 SeitenChapter 1 - Introduction: Sr. No. QuestionsAkashNoch keine Bewertungen
- MCWD Water Safety Forum 2018Dokument31 SeitenMCWD Water Safety Forum 2018Shirlee Alicor L MacheteNoch keine Bewertungen
- Pressure Drop Calculation for Item No. 2432 at Varying Clog ConditionsDokument1 SeitePressure Drop Calculation for Item No. 2432 at Varying Clog ConditionsKailas NimbalkarNoch keine Bewertungen
- Cap 9-Reservoir and Stream Flow RoutingDokument25 SeitenCap 9-Reservoir and Stream Flow RoutingLívia M. Pinheiro RosalemNoch keine Bewertungen
- MIT2 25F13 EquationSheetDokument2 SeitenMIT2 25F13 EquationSheetMauricio Andrés Gutiérrez BravoNoch keine Bewertungen
- Seminar On Methods of IrrigationDokument25 SeitenSeminar On Methods of IrrigationRavi MalikNoch keine Bewertungen
- Vectra GL BrochureDokument4 SeitenVectra GL BrochureYohan GalarragaNoch keine Bewertungen
- Guidelines For The Avoidance of Vibration Induced Fatigue Failure in Process PDokument5 SeitenGuidelines For The Avoidance of Vibration Induced Fatigue Failure in Process PFSAAVEDRAFNoch keine Bewertungen
- U-2, Pp-I, Carewell PharmaDokument14 SeitenU-2, Pp-I, Carewell PharmaSai sowjanya RaaviNoch keine Bewertungen
- Sistem Pengendalian Banjir Dan DrainaseDokument43 SeitenSistem Pengendalian Banjir Dan DrainaseSiswo YuwonoNoch keine Bewertungen
- Sediment Tank and Rapid Sand Filter Design DetailsDokument6 SeitenSediment Tank and Rapid Sand Filter Design DetailsEzdee100% (2)
- Spillway DesignDokument4 SeitenSpillway DesignBondoc, Miles Jerome Z.Noch keine Bewertungen
- Boiling Liquid Expanding Vapor ExpDokument3 SeitenBoiling Liquid Expanding Vapor ExppetalbdNoch keine Bewertungen
- GLO-T800-PRO-EVA-TEC-000-00001-00 H01 (GLO Fuel Gas System Study Report For Gendalo FPU)Dokument100 SeitenGLO-T800-PRO-EVA-TEC-000-00001-00 H01 (GLO Fuel Gas System Study Report For Gendalo FPU)Tifano Khristiyanto100% (3)
- VIESSMANN - Technical Guide Steam BoilersDokument343 SeitenVIESSMANN - Technical Guide Steam BoilersTanes Yimsuan100% (1)
- Manual Abanico Refrescante de Aire HoneywellDokument68 SeitenManual Abanico Refrescante de Aire HoneywellleochardoNoch keine Bewertungen
- Project Report Suface Facility (Group 17)Dokument18 SeitenProject Report Suface Facility (Group 17)FINAL YEAR PETROLEUM 2020 BATCHNoch keine Bewertungen
- Hospital Plant and Building ServicesDokument5 SeitenHospital Plant and Building ServicesLevin WasikeNoch keine Bewertungen
- Dew Point: Constant PressureDokument5 SeitenDew Point: Constant PressureDavid IonescuNoch keine Bewertungen
- Environmental Science Project Report Solutions for Local Water IssuesDokument11 SeitenEnvironmental Science Project Report Solutions for Local Water IssuesshiveshNoch keine Bewertungen
- Water Filtration: MaterialsDokument3 SeitenWater Filtration: MaterialsGwyneth Pearl Javier100% (1)
- Streeter PhelpsDokument7 SeitenStreeter Phelpsjantskie100% (1)
- Conventional SeparatorsDokument27 SeitenConventional SeparatorsMax Singh100% (1)
- Pnaaw239 PDFDokument165 SeitenPnaaw239 PDFSamErnesto007Noch keine Bewertungen
- Chapter 9 - Hydroelectric Power PlantDokument122 SeitenChapter 9 - Hydroelectric Power PlantWawNoch keine Bewertungen
- Construction of A Deep Tube Well - A Case StudyDokument33 SeitenConstruction of A Deep Tube Well - A Case Studyanirbanpwd76Noch keine Bewertungen
- Solucionario Química Capitulo 12 - Whitten - 10 EdiciónDokument21 SeitenSolucionario Química Capitulo 12 - Whitten - 10 EdiciónDanielNoch keine Bewertungen
- Gas Permeability MeasurementDokument18 SeitenGas Permeability MeasurementAnusha Anu100% (1)
- Flow MeasurementsDokument1 SeiteFlow MeasurementsSUPAPORN KLABKLAYDEENoch keine Bewertungen
- Dark Matter and the Dinosaurs: The Astounding Interconnectedness of the UniverseVon EverandDark Matter and the Dinosaurs: The Astounding Interconnectedness of the UniverseBewertung: 3.5 von 5 Sternen3.5/5 (69)
- Quantum Physics: What Everyone Needs to KnowVon EverandQuantum Physics: What Everyone Needs to KnowBewertung: 4.5 von 5 Sternen4.5/5 (48)
- Summary and Interpretation of Reality TransurfingVon EverandSummary and Interpretation of Reality TransurfingBewertung: 5 von 5 Sternen5/5 (5)
- Quantum Spirituality: Science, Gnostic Mysticism, and Connecting with Source ConsciousnessVon EverandQuantum Spirituality: Science, Gnostic Mysticism, and Connecting with Source ConsciousnessBewertung: 4 von 5 Sternen4/5 (6)
- The Tao of Physics: An Exploration of the Parallels between Modern Physics and Eastern MysticismVon EverandThe Tao of Physics: An Exploration of the Parallels between Modern Physics and Eastern MysticismBewertung: 4 von 5 Sternen4/5 (500)
- The Physics of God: How the Deepest Theories of Science Explain Religion and How the Deepest Truths of Religion Explain ScienceVon EverandThe Physics of God: How the Deepest Theories of Science Explain Religion and How the Deepest Truths of Religion Explain ScienceBewertung: 4.5 von 5 Sternen4.5/5 (23)
- Packing for Mars: The Curious Science of Life in the VoidVon EverandPacking for Mars: The Curious Science of Life in the VoidBewertung: 4 von 5 Sternen4/5 (1395)
- Lost in Math: How Beauty Leads Physics AstrayVon EverandLost in Math: How Beauty Leads Physics AstrayBewertung: 4.5 von 5 Sternen4.5/5 (125)
- A Brief History of Time: From the Big Bang to Black HolesVon EverandA Brief History of Time: From the Big Bang to Black HolesBewertung: 4 von 5 Sternen4/5 (2193)
- Quantum Physics for Beginners Who Flunked Math And Science: Quantum Mechanics And Physics Made Easy Guide In Plain Simple EnglishVon EverandQuantum Physics for Beginners Who Flunked Math And Science: Quantum Mechanics And Physics Made Easy Guide In Plain Simple EnglishBewertung: 4.5 von 5 Sternen4.5/5 (18)
- Strange Angel: The Otherworldly Life of Rocket Scientist John Whiteside ParsonsVon EverandStrange Angel: The Otherworldly Life of Rocket Scientist John Whiteside ParsonsBewertung: 4 von 5 Sternen4/5 (94)
- Midnight in Chernobyl: The Story of the World's Greatest Nuclear DisasterVon EverandMidnight in Chernobyl: The Story of the World's Greatest Nuclear DisasterBewertung: 4.5 von 5 Sternen4.5/5 (409)
- The Magick of Physics: Uncovering the Fantastical Phenomena in Everyday LifeVon EverandThe Magick of Physics: Uncovering the Fantastical Phenomena in Everyday LifeNoch keine Bewertungen
- The End of Everything: (Astrophysically Speaking)Von EverandThe End of Everything: (Astrophysically Speaking)Bewertung: 4.5 von 5 Sternen4.5/5 (155)
- In Search of Schrödinger’s Cat: Quantum Physics and RealityVon EverandIn Search of Schrödinger’s Cat: Quantum Physics and RealityBewertung: 4 von 5 Sternen4/5 (380)
- Too Big for a Single Mind: How the Greatest Generation of Physicists Uncovered the Quantum WorldVon EverandToo Big for a Single Mind: How the Greatest Generation of Physicists Uncovered the Quantum WorldBewertung: 4.5 von 5 Sternen4.5/5 (8)
- Paradox: The Nine Greatest Enigmas in PhysicsVon EverandParadox: The Nine Greatest Enigmas in PhysicsBewertung: 4 von 5 Sternen4/5 (57)
- Bedeviled: A Shadow History of Demons in ScienceVon EverandBedeviled: A Shadow History of Demons in ScienceBewertung: 5 von 5 Sternen5/5 (5)
- The Holographic Universe: The Revolutionary Theory of RealityVon EverandThe Holographic Universe: The Revolutionary Theory of RealityBewertung: 4.5 von 5 Sternen4.5/5 (76)
- Starry Messenger: Cosmic Perspectives on CivilizationVon EverandStarry Messenger: Cosmic Perspectives on CivilizationBewertung: 4.5 von 5 Sternen4.5/5 (158)
- Black Holes: The Key to Understanding the UniverseVon EverandBlack Holes: The Key to Understanding the UniverseBewertung: 4.5 von 5 Sternen4.5/5 (13)
- The Sounds of Life: How Digital Technology Is Bringing Us Closer to the Worlds of Animals and PlantsVon EverandThe Sounds of Life: How Digital Technology Is Bringing Us Closer to the Worlds of Animals and PlantsBewertung: 5 von 5 Sternen5/5 (5)