Beruflich Dokumente
Kultur Dokumente
Carboxylic Acid
Hochgeladen von
Emman RevillaCopyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Carboxylic Acid
Hochgeladen von
Emman RevillaCopyright:
Verfügbare Formate
Carboxylic Acid
Prepared by: Emmanuel C. Revilla
REPORTER: Emmanuel C. Revilla
PHYSCI M15: BIOLOGICAL CHEMISTRY
Mrs. Clarissa F. Gregana
CARBOXYLIC ACID
INTRODUCTION
Carboxylic acids are a large group of chemical compounds that all have a certain
structure in common, composed of the three most important elements on earth carbon,
oxygen and hydrogen.
These various compounds make up the most abundant, naturally occurring organic
acids. The slightly stinging sourness of citrus fruits, such as lemons, comes from one example
called citric acid. Most of the compounds are relatively simple chemically, and as an acid, quite
weak. They readily dissolve into individual molecules in solvents, including water. Even the most
complex and stubborn of them are usually soluble in an alcohol solution.
Not only are they valuable in themselves, they also serve as starting materials for
preparing numerous acyl derivatives such as acid chlorides, esters, amides, and thioesters.
STRUCTURE AND PROPERTIES
Carboxylic acid are organic compounds containing a carboxyl group.
* A carboxyl
group is a carbonyl
group (C=O) with a
hydroxyl group (OH)
bonded to the carbonyl
carbon atom.
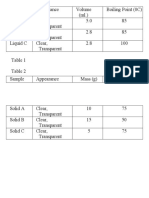
BOILING & MELTING POINT
1 | Page
FUNCTIONAL GROUPS:
Carboxylic Acid
Carboxylic acids are polar compounds because they possess a polar carbonyl group. (dipoledipole interactions)
Carboxylic acids also exhibit intermolecular hydrogen bonding since they possess a hydrogen
atom bonded to an electronegative oxygen atom.
They have the highest boiling and melting points among all known functional groups/organic
compounds.
SOLUBILITY IN WATER
Carboxylic acids form hydrogen bonds with
many water molecules. With 1-4 carbon atoms, they
are soluble in water.
ACIDITY
The most obvious property is implied by their name: carboxylic acids are acidic.
They are the most acidic class of compounds that contain only carbon, hydrogen, and oxygen.
Unsubstituted saturated monocarboxylic acids containing up to nine carbon atoms are liquids that
have strong, sharp odors. Acids with 10 or more carbon atoms in an unbranched chain are waxy solids
that are odorless (because of low volatility). Aromatic carboxylic acids, as well as dicarboxylic acids, are
also odorless solids.
PREPARATION OF CARBOXYLIC ACIDS
Many of the chemical reactions used for their preparation are oxidations.
2 | Page
FUNCTIONAL GROUPS:
Carboxylic Acid
MODERN APPLICATION/USE OF CARBOXYLIC ACID
Carboxylic acids are widely found in nature. You can find them as free acids like citric
acid, tannic acid and malic acid. Esters the products of acids and alcohols also contain
carboxylic acids. These include fats and oils, flavors of fruits and odors of flowers. Some
bacteria can also cause natural reactions in which these acids are formed. Some examples
include acetic acid from wine or cider, lactic acid found in sour milk and the butyric acid in rancid
butter.
Acetic Acid
This acid is found in vinegar and is responsible
for giving it the sour taste. Vinegar means sour
wine. It was discovered when bacteria reacted
with wine and turned it sour. It is one of the
simplest of carboxylic acids.
Tannic Acid
You may have heard of this acid, it has been
used for tanning. You will find it in the bark of a
number of trees. Tannic acid can be found as a
yellow or light brown powder, which is highly
soluble in water. It is used for the staining of
wood and is also used when dyeing cotton.
Salicylic Acid
This acid is derived from the bark of the willow
tree. Salicylic acid is used in acne creams to
help reduce acne. It derives its name from the
word Salix, which is Latin for Willow. Today this
acid is used as a food preservative.
Citric Acid
This acid is found in most citrus fruits. It gives
the tangy taste to lemons, limes, grape fruits
and oranges. Citric acid is a natural
preservative and used in both foods and soft
drinks. It is also used in some bathroom and
kitchen cleaning solutions.
Malic Acid
3 | Page
FUNCTIONAL GROUPS:
Carboxylic Acid
This acid is found in many unripe fruits like cue, to utilize the acids in, or chemically covert them
green apples. Malic acid includes green apples,into, a vast variety of applications. Perfumes,
plums, currants (seedless raisins) and a varietyindustrial bleaches, food preservatives and
pharmaceutical drugs are just a few more examples.
of other fruits.
Formic Acid
Formic acid is responsible for the sting of some
types of ants.
Oxalic Acid
This acid was originally derived from the wood Hexanoic Acid
sorrel plant Oxalis. It is now made artificiallyIt has the foul odor associated with dirty socks and
and is used in bleaching and cleansinglocker rooms. It also contributes to the unpleasant
odor of ginkgo seeds.
solutions.
Acrylic Acid
Acrylic acid and its esters readily combine with
Amino acids
This acids are also among natures most important themselves by reacting at their double bond, forming
carboxylic acids. They are nicknamed the building homopolymers or copolymers which are used in the
blocks of proteins. Proteins, in turn, create the
manufacture of various plastics, coatings, adhesives,
nearly infinite diversity of organic tissues, from hair, elastomers, as well as floor polishes, and paints.
skin, heart to tree bark. Scientists have taken this
TESTS FOR CARBOXYLIC ACIDS
1. Litmus Test:
Carboxylic acids turn blue litmus red.
2. Sodium Bicarbonate Test:
To a small portion of the organic compound taken in a test tube, a pinch of solid sodium bicarbonate is
added. Evolution of carbon dioxide with brisk effervescence shows the presence of carboxylic acid.
Alcohols do not give this test.
3. Ester Test:
In this test, the compound to be tested is warmed with small quantity of ethyl alcohol and 2-3 drops of
concentrated sulphuric acid. The formation of sweet smelling vapors indicates the presences of some
carboxylic acid. The sweet smelling vapors are due to the formation of some ester by reaction between
the acid and ethyl alcohol.
4.Fluorescein Reaction:
A little of the substance is heated with Conc.H2SO4 & Resorcinol in a dry test tube. It is cooled & then
poured into a beaker containing excess of NaOH. A red solution with intense green fluorescence.
Presence of dicarboxylic acid.
5. Anhydride formation:
A little of the acid is heated in a dry china dish covered with an inverted funnel whose stem is closed. It
is then cooled. White shiny needles are deposited on the sides of the funnel. Presence of dicarboxylic
acid
4 | Page
FUNCTIONAL GROUPS:
Carboxylic Acid
REFERENCES
http://humantouchofchemistry.com/fruity-facts-about-carboxylic-acid.htm
http://www.ck12.org/book/From-Vitamins-to-Baked-Goods%3A-Real-Applications-of-OrganicChemistry/section/6.3/
http://www.preservearticles.com/201101022309/uses-of-carboxylic-acids.html
http://academics.wellesley.edu/Chemistry/chem211lab/Orgo_Lab_Manual/Appendix/ClassificationT
ests/carboxylic.html
http://www.preservearticles.com/201101022310/tests-for-carboxylic-acids.html
http://www.docbrown.info/page13/ChemicalTests/ChemicalTestsf.htm#KEYWORDS
http://vlab.amrita.edu/?sub=2&brch=191&sim=345&cnt=1
http://mchem.weebly.com/uploads/9/2/0/5/9205056/functional_group_tests.pdf
http://www.britannica.com/science/carboxylic-acid/Reactions
5 | Page
Das könnte Ihnen auch gefallen
- Activity 5 Breathe In, Breathe OutDokument3 SeitenActivity 5 Breathe In, Breathe OutEmman Revilla100% (1)
- Directions: Label The Parts and Answer The Questions BelowDokument1 SeiteDirections: Label The Parts and Answer The Questions BelowEmman Revilla100% (1)
- Respiratory System ReviewDokument3 SeitenRespiratory System ReviewEmman Revilla0% (1)
- Circulatory and Respiratory SystemsDokument15 SeitenCirculatory and Respiratory SystemsEmman RevillaNoch keine Bewertungen
- Activity 1 4pics-1 WordDokument1 SeiteActivity 1 4pics-1 WordEmman Revilla100% (4)
- Solutions: Complete The Crossword Puzzle BelowDokument1 SeiteSolutions: Complete The Crossword Puzzle BelowEmman RevillaNoch keine Bewertungen
- Learning Activity Sheet: Pre-Assessment: I. True or FalseDokument2 SeitenLearning Activity Sheet: Pre-Assessment: I. True or FalseEmman RevillaNoch keine Bewertungen
- ACTIVITY 3 What A Bunch of GrapesDokument1 SeiteACTIVITY 3 What A Bunch of GrapesEmman RevillaNoch keine Bewertungen
- ACTIVITY 2 Define MeDokument1 SeiteACTIVITY 2 Define MeEmman RevillaNoch keine Bewertungen
- Learning Activity Sheet: Pre-Assessment: I. True or FalseDokument2 SeitenLearning Activity Sheet: Pre-Assessment: I. True or FalseEmman RevillaNoch keine Bewertungen
- LE TemplateDokument1 SeiteLE TemplateEmman RevillaNoch keine Bewertungen
- Activity 1 - How Long Can You Hold Your BreathDokument2 SeitenActivity 1 - How Long Can You Hold Your BreathEmman RevillaNoch keine Bewertungen
- Tarpapel Element and CompoundDokument3 SeitenTarpapel Element and CompoundEmman RevillaNoch keine Bewertungen
- Las 1Dokument3 SeitenLas 1Emman RevillaNoch keine Bewertungen
- Sample PropertiesDokument2 SeitenSample PropertiesEmman RevillaNoch keine Bewertungen
- Properties of Pure Substances and MixturesDokument2 SeitenProperties of Pure Substances and MixturesEmman RevillaNoch keine Bewertungen
- Form Carbohydrate That Is Usable by The BodyDokument5 SeitenForm Carbohydrate That Is Usable by The BodyEmman RevillaNoch keine Bewertungen
- Scientific Method Big ExampleDokument2 SeitenScientific Method Big ExampleEmman RevillaNoch keine Bewertungen
- Parts of The Heart LectureDokument2 SeitenParts of The Heart LectureEmman RevillaNoch keine Bewertungen
- Effects of Cigarette SmokingDokument16 SeitenEffects of Cigarette SmokingEmman RevillaNoch keine Bewertungen
- Activity 2 - Volcano Model MakingDokument1 SeiteActivity 2 - Volcano Model MakingEmman RevillaNoch keine Bewertungen
- Pics DNADokument4 SeitenPics DNAEmman RevillaNoch keine Bewertungen
- Financial statements guide business profit analysisDokument18 SeitenFinancial statements guide business profit analysisEmman RevillaNoch keine Bewertungen
- Handout DnaDokument3 SeitenHandout DnaEmman RevillaNoch keine Bewertungen
- ENERGYDokument63 SeitenENERGYEmman RevillaNoch keine Bewertungen
- Lesson Plan MathDokument9 SeitenLesson Plan MathEmman Revilla87% (23)
- Trachea Nasal Cavity Bronchioles Pharynx Oral Cavity Diaphragm Alveoli Bronchi Lungs LarynxDokument3 SeitenTrachea Nasal Cavity Bronchioles Pharynx Oral Cavity Diaphragm Alveoli Bronchi Lungs LarynxEmman RevillaNoch keine Bewertungen
- Preparing Questions For A Qualitative Research InterviewDokument9 SeitenPreparing Questions For A Qualitative Research InterviewEmman RevillaNoch keine Bewertungen
- Financial statement overview for business analysisDokument12 SeitenFinancial statement overview for business analysisEmman RevillaNoch keine Bewertungen
- Origin and Structure of EarthDokument9 SeitenOrigin and Structure of EarthEmman RevillaNoch keine Bewertungen
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeVon EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeBewertung: 4 von 5 Sternen4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingVon EverandThe Little Book of Hygge: Danish Secrets to Happy LivingBewertung: 3.5 von 5 Sternen3.5/5 (399)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryVon EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryBewertung: 3.5 von 5 Sternen3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceVon EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceBewertung: 4 von 5 Sternen4/5 (894)
- The Yellow House: A Memoir (2019 National Book Award Winner)Von EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Bewertung: 4 von 5 Sternen4/5 (98)
- Shoe Dog: A Memoir by the Creator of NikeVon EverandShoe Dog: A Memoir by the Creator of NikeBewertung: 4.5 von 5 Sternen4.5/5 (537)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureVon EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureBewertung: 4.5 von 5 Sternen4.5/5 (474)
- Never Split the Difference: Negotiating As If Your Life Depended On ItVon EverandNever Split the Difference: Negotiating As If Your Life Depended On ItBewertung: 4.5 von 5 Sternen4.5/5 (838)
- Grit: The Power of Passion and PerseveranceVon EverandGrit: The Power of Passion and PerseveranceBewertung: 4 von 5 Sternen4/5 (587)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaVon EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaBewertung: 4.5 von 5 Sternen4.5/5 (265)
- The Emperor of All Maladies: A Biography of CancerVon EverandThe Emperor of All Maladies: A Biography of CancerBewertung: 4.5 von 5 Sternen4.5/5 (271)
- On Fire: The (Burning) Case for a Green New DealVon EverandOn Fire: The (Burning) Case for a Green New DealBewertung: 4 von 5 Sternen4/5 (73)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersVon EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersBewertung: 4.5 von 5 Sternen4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnVon EverandTeam of Rivals: The Political Genius of Abraham LincolnBewertung: 4.5 von 5 Sternen4.5/5 (234)
- The Unwinding: An Inner History of the New AmericaVon EverandThe Unwinding: An Inner History of the New AmericaBewertung: 4 von 5 Sternen4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyVon EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyBewertung: 3.5 von 5 Sternen3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreVon EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreBewertung: 4 von 5 Sternen4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Von EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Bewertung: 4.5 von 5 Sternen4.5/5 (119)
- Her Body and Other Parties: StoriesVon EverandHer Body and Other Parties: StoriesBewertung: 4 von 5 Sternen4/5 (821)
- T3-Ias 33 - Case 1 - KeyDokument2 SeitenT3-Ias 33 - Case 1 - KeyTrúc ĐoànNoch keine Bewertungen
- BloombergDokument2 SeitenBloombergJosé IgnacioNoch keine Bewertungen
- MSCCH06Dokument404 SeitenMSCCH06Swapnil KumarNoch keine Bewertungen
- Bond Price Sensitivity AnalysisDokument5 SeitenBond Price Sensitivity AnalysisZhenyi ZhuNoch keine Bewertungen
- Latest A4VDokument10 SeitenLatest A4VStephen L. SmithNoch keine Bewertungen
- IR SpectrosDokument97 SeitenIR SpectrosManu Jose100% (3)
- 1 Solution Assignment 1Dokument4 Seiten1 Solution Assignment 1No NoNoch keine Bewertungen
- AFME Guide To Infrastructure FinancingDokument99 SeitenAFME Guide To Infrastructure Financingfunction_analysisNoch keine Bewertungen
- Chapter 17 SolutionsDokument40 SeitenChapter 17 Solutionseongl39Noch keine Bewertungen
- IONS AND IONIC BONDING – EXAM QUESTIONSDokument2 SeitenIONS AND IONIC BONDING – EXAM QUESTIONSAhmadElgindy50% (8)
- Equity Index-Linked Notes ExplainedDokument2 SeitenEquity Index-Linked Notes ExplainedOwenNoch keine Bewertungen
- ARE - CDS Exam GuideDokument22 SeitenARE - CDS Exam GuideiamarrNoch keine Bewertungen
- Chemistry Form 6 Sem 2 04 Notes STPM 2014/2013Dokument27 SeitenChemistry Form 6 Sem 2 04 Notes STPM 2014/2013Raj Nittiya SugumaranNoch keine Bewertungen
- 09-13-12 Hillsborough Solid WasteDokument157 Seiten09-13-12 Hillsborough Solid WasteSeanWMNFNoch keine Bewertungen
- Unit 1 Multiple ChoiceDokument13 SeitenUnit 1 Multiple ChoiceJinJinKiraieNoch keine Bewertungen
- Types of CapitalDokument24 SeitenTypes of CapitalAnkita N VyasNoch keine Bewertungen
- Chem IA NUMERO 5Dokument4 SeitenChem IA NUMERO 5BrittanyNoch keine Bewertungen
- AUF SOM DEPARTMENT OF BIOCHEMISTRY UNIT EXAM IN NUCLEIC ACIDSDokument3 SeitenAUF SOM DEPARTMENT OF BIOCHEMISTRY UNIT EXAM IN NUCLEIC ACIDSAlbert TayagNoch keine Bewertungen
- ExamView Pro - DEBT FINANCING - TST PDFDokument15 SeitenExamView Pro - DEBT FINANCING - TST PDFShannon ElizaldeNoch keine Bewertungen
- Practice Exam I - Organic ChemsitryDokument14 SeitenPractice Exam I - Organic ChemsitryDave KowalczykNoch keine Bewertungen
- Chapter 14Dokument56 SeitenChapter 14Ahmed HalawaNoch keine Bewertungen
- MPW Standard Spec 2010Dokument528 SeitenMPW Standard Spec 2010Tim Ranf63% (8)
- 01 StereochemistryDokument6 Seiten01 StereochemistryGundum Bodyz100% (1)
- Studies On Bound Water in PvaDokument4 SeitenStudies On Bound Water in PvasggdgdNoch keine Bewertungen
- CH 11Dokument12 SeitenCH 11Cary Lee33% (3)
- Student Guide Lesson TwelveDokument7 SeitenStudent Guide Lesson Twelveapi-344266741Noch keine Bewertungen
- Hill Country SnackDokument8 SeitenHill Country Snackkiller dramaNoch keine Bewertungen
- Revision QuestionDokument4 SeitenRevision QuestionAishah SisNoch keine Bewertungen
- Transition Metal ChemistryDokument6 SeitenTransition Metal ChemistryOli RaynerNoch keine Bewertungen
- Synthesis and Characterization of Alkane, Alkene and AlkyneDokument9 SeitenSynthesis and Characterization of Alkane, Alkene and Alkynesapphirerk100% (3)