Beruflich Dokumente
Kultur Dokumente
Dengue
Hochgeladen von
Alvaro Andres Flores JimenezOriginaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Dengue
Hochgeladen von
Alvaro Andres Flores JimenezCopyright:
Verfügbare Formate
Diagnosis and Management of Dengue Fever in Children
Ashlesha Kaushik, Carol Pineda and Helen Kest
Pediatrics in Review 2010;31;e28
DOI: 10.1542/pir.31-4-e28
The online version of this article, along with updated information and services, is
located on the World Wide Web at:
http://pedsinreview.aappublications.org/content/31/4/e28
Pediatrics in Review is the official journal of the American Academy of Pediatrics. A monthly
publication, it has been published continuously since 1979. Pediatrics in Review is owned,
published, and trademarked by the American Academy of Pediatrics, 141 Northwest Point
Boulevard, Elk Grove Village, Illinois, 60007. Copyright 2010 by the American Academy of
Pediatrics. All rights reserved. Print ISSN: 0191-9601.
Downloaded from http://pedsinreview.aappublications.org/ at Eccles Health Sciences Lib on July 2, 2014
Article
infectious diseases
Diagnosis and Management of Dengue Fever
in Children
Ashlesha Kaushik, MD,*
Carol Pineda, MD,*
Helen Kest, MD, MPH,
CPH
Objectives
1.
2.
3.
4.
After completing this article, readers should be able to:
Describe the epidemiology and clinical spectrum of dengue viral infections.
Recognize when to consider dengue in the differential diagnosis of acute fever.
Discuss the diagnosis and management of this common tropical illness.
Identify other diseases that can mimic dengue viral infections.
Author Disclosure
Drs Kaushik, Pineda,
and Kest have
Case 1 Presentation
disclosed no financial
A 17-year-old Hispanic girl presents with a 5-day history of temperature of 39.4C to 40.5C
and a 4-day history of severe bifrontal and intermittent headaches. She also has a 3-day history
of malaise, generalized body aches, and mild epigastric pain. On the day of admission, she
develops a dark reddish-purple, nonpruritic, and nonblanching rash over her arms and thighs
and is brought to the emergency department. There is no cough, sore throat, vomiting, or
diarrhea. She denies illicit drug use, tick exposure, sexual activity, or allergies.
On physical examination, the girl appears alert, oriented, and in no acute distress. Her
temperature is 38.5C, heart rate is 119 beats/min, respiratory rate is 18 breaths/min, and
blood pressure is 130/68 mm Hg (90th to 95th percentile). Capillary refill time is less than
2 seconds. A petechial rash is present over her arms and anterior thighs. She has mild epigastric
tenderness, with no rebound tenderness, guarding, hepatosplenomegaly, or masses. Tourniquet
test is positive. Kernig and Brudzinski signs are negative. The remainder of the physical
examination findings are normal.
Her white blood cell count is 3.5103/mcL (3.510 9/L) with 59% neutrophils, 33%
lymphocytes, 6% monocytes, and 1% eosinophils; hemoglobin is 13.4 g/dL (134 g/L); hematocrit
is 39.6% (0.396); platelet count is 126103/mcL (12610 9/L); and erythrocyte sedimentation rate is 13 mm/hour. The electrolytes, urinalysis, and coagulation profile are normal.
The stool is negative for blood. Liver function test results include a protein concentration of
6.9 g/dL (69 g/L), albumin of 4 g/dL (40 g/L), aspartate aminotransferase of 39 U/L,
alanine aminotransferase of 18 U/L, alkaline phosphatase of 103 U/L, total bilirubin of
0.9 mg/dL (15.4 mcmol/L), and direct bilirubin of 0.3 mg/dL (5.1 mcmol/L). Thick and
thin smears are negative for malarial parasites.
The patient is admitted for monitoring, and intravenous hydration is started. On additional questioning, she states that she had returned from the Dominican Republic yesterday.
Additional serum testing reveals the diagnosis of classic dengue fever. Viral serology reveals
a dengue virus immunoglobin M (IgM) enzyme-linked immunosorbent assay (ELISA) titer
of 4.93 (negative, 1.11) and dengue virus IgG ELISA titer of 9.69 (negative, 1.11).
Platelet counts and hematocrit values are monitored every day. On the second hospital day, her
platelet count declines to 9610 3/mcL (9610 9/L) with no evidence of bleeding. The counts
increase to 10910 3/mcL (10910 9/L) on the third hospital day and 12110 3/mcL
(12110 9/L) on the following day. Her hematocrit value remains stable at 40% (0.40). Three
days after hospitalization, the fever resolves, the patient has stable vital signs, and she is
discharged.
relationships relevant
to this article. This
commentary does not
contain a discussion
of an unapproved/
investigative use of a
commercial product/
device.
Case 2 Presentation
A 15-year-old boy who has a 5-day history of a temperature of 39.0C to 39.5C with chills and
severe pain in both legs is admitted to the hospital for evaluation. Three days ago, he developed
*Department of Pediatrics, St Josephs Childrens Hospital, Patterson, NJ.
Division of Pediatric Infectious Disease, Department of Pediatrics, St. Josephs Childrens Hospital, Patterson, NJ.
e28 Pediatrics in Review Vol.31 No.4 April 2010
Downloaded from http://pedsinreview.aappublications.org/ at Eccles Health Sciences Lib on July 2, 2014
infectious diseases
nausea, headache, myalgias, and severe fatigue. He denies
any vomiting, somnolence, abdominal pain, or neck pain.
Findings on past and family histories are unremarkable.
Physical examination reveals a tired-looking adolescent
who has a temperature of 39.4C, heart rate of 95 beats/
min, respiratory rate of 16 breaths/min, and blood pressure
of 120/70 mm Hg (75th to 90th percentile). No neck
stiffness, rash, or lesions are apparent. After the blood
pressure cuff is removed, he develops petechiae all over his
antecubital fossa. He has shotty cervical lymphadenopathy.
All other physical findings are normal.
Initial laboratory results reveal a white blood cell count
of 3.6103/mcL (3.610 9/L) with 55% neutrophils, 29%
bands, 14% lymphocytes, and 10% monocytes; hemoglobin of
15.7 g/dL (157 g/L); hematocrit of 42.2% (0.422); and
platelet count of 87103/mcl (8710 9/L). Electrolytes
and fibrinogen values are normal. Prothrombin time is
15.2 seconds, international normalized ratio (INR) is 1.2,
and activated partial thromboplastin time is 41.2 seconds.
D-dimers are 3.3 mcg/mL (normal, 0.50 mcg/mL).
Thick and thin smears for malarial parasites are negative.
Liver function tests include a total protein of 6.2 g/dL
(62 g/L), albumin of 3.4 g/dL (34 g/L), aspartate aminotransferase of 587 U/L, alanine aminotransferase of
412 U/L, alkaline phosphatase of 157 U/L, total bilirubin
of 1 mg/dL (17.1 mcmol/L), and direct bilirubin of
0.2 mg/dL (3.4 mcmol/L).
Additional questioning reveals that the boy had been in
the Dominican Republic for 2 weeks and returned the day
his fever started. He is admitted with a diagnosis of suspected dengue hemorrhagic fever.
Viral studies sent on admission reveal an acute-phase
dengue virus IgM ELISA titer of 1.8 (negative, 1.11)
and a dengue virus IgG ELISA titer of 8.8 (negative,
1.11). On the second hospital day, he has one episode of
vomiting containing blood. Laboratory results reveal a
platelet count of 37103/mcL (3710 9/L), prothrombin
time of 16.2 seconds, INR of 1.2, and partial thromboplastin time of 42.9 seconds.
On the third hospital day, due to another episode of
hematemesis and a low platelet count of 21103/mcL
(2110 9/L), he receives a platelet transfusion. On the
same day, he develops diarrhea and mild lower abdominal
tenderness. He has a hemoglobin of 18.3 g/dL (183 g/L)
and hematocrit of 52.6% (0.53), which is a 20% increase
from the baseline. Liver function tests yield normal results
except for an albumin of 3.1 g/dL (31 g/L). Ultrasonography of the abdomen shows normal results, and stool examination for parasites and culture produces negative
results. Serum amylase and lipase values are normal. On
the fifth hospital day, the diarrhea subsides and the patient
dengue fever
is afebrile. By the sixth hospital day, the child is feeling better
and has stable vital signs. His hematocrit and platelet
counts have improved, and he is discharged the following
day.
Case 3 Presentation
An 11-year-old Hispanic boy develops a temperature of
39.1C with diarrhea. Two days later, the diarrhea subsides, but he develops severe abdominal pain and vomiting,
becomes increasingly lethargic, and is brought to the emergency department. His parents state that he has been complaining of severe back pain and headache. They deny any
medication use, previous hospitalization, or tick bites.
Physical examination reveals a sick-looking boy who is
somnolent but arousable. His temperature is 40.0C, heart
rate is 140 beats/min, respiratory rate is 26 breaths/min,
blood pressure is 80/50 mm Hg (5th percentile), and
Glasgow Coma Scale score is 12. He has a weak pulse, cold
extremities, and a capillary refill time of 6 to 8 seconds. His
abdomen is diffusely tender, with the liver enlarged 4 cm
below the right costal margin. A petechial rash is present
over his chest and trunk. Meningeal signs are absent. All
other physical findings are unremarkable.
Initial laboratory results reveal a white blood cell count
of 2.5103/mcL (2.510 9/L) with 58% neutrophils, 8%
bands, 10% lymphocytes, 18% atypical lymphocytes, and
4% monocytes; hemoglobin of 14 g/dL (140 g/L); hematocrit of 42% (0.42); and platelet count of 27103/mcL
(2710 9/L). Electrolyte values are normal, and urinalysis
shows trace blood. Coagulation profile reveals a prothrombin time of 17.8 seconds, activated partial thromboplastin
time of 44 seconds, D-dimers of 4.4 mcg/mL (normal,
0.50 mcg/mL), and fibrinogen value of 200 mg% (normal, 183 to 503 mg%). Liver function tests reveal a total
protein of 5.6 g/dL (56 g/L), albumin of 3 g/dL (30 g/L),
aspartate aminotransferase of 455 U/L, alanine aminotransferase of 324 U/L, alkaline phosphatase of 140 U/L,
total bilirubin of 1.2 mg/dL (20.5 mcmol/L), and direct
bilirubin of 0.1 mg/dL (1.7 mcmol/L). Smears for malarial parasites are negative.
The parents share their concern about multiple relatives
who had self-limiting fevers and body aches in the Caribbean, from where they had returned 4 days ago. The child
has been to the Caribbean twice within the past 2 years.
The clinical and laboratory picture suggest dengue shock
syndrome. The boy is admitted to the intensive care unit
and started on intravenous hydration. On the second hospital day, he has two episodes of vomiting containing blood
and develops frank hematuria. Laboratory results show a
platelet count of 14103/mcL (1410 9/L), prothrombin
time of 19.5 seconds, INR of 1.2, and partial thromboplasPediatrics in Review Vol.31 No.4 April 2010 e29
Downloaded from http://pedsinreview.aappublications.org/ at Eccles Health Sciences Lib on July 2, 2014
infectious diseases
dengue fever
tin time of 52 seconds. His hematocrit is 33% (0.33), a 22%
decrease from the initial value. He receives platelet and
fresh frozen plasma transfusions. There is no recurrence of
bleeding, and by the fourth hospital day, he is hemodynamically stable and has improvement in hematocrit and platelet counts (34% [0.34]and 90103/mcL [9010 9/L],
respectively). On day 5, he continues to be afebrile, is feeling
better, and is discharged. Viral studies sent on the third
hospital day are strongly positive for dengue virus infection, with a dengue virus IgM ELISA titer of 5.4 (negative, 1.11) and dengue virus IgG ELISA titer of 12
(negative, 1.11).
findings, and laboratory markers. There are four major
clinical syndromes: 1) undifferentiated fever, 2) dengue
fever, 3) dengue hemorrhagic fever (DHF), and 4) dengue shock syndrome (DSS). Most cases are mild. However, DHF case fatality rates can reach 20% if not treated
appropriately or in a timely manner. It is highly likely
that dengue cases are unreported in the United States
because physicians often do not include it in the differential diagnosis of travelers returning from endemic
areas. (6)(7)
Introduction
Dengue virus is an arbovirus of the flavivirus family that
has four different serotypes (DEN-1, -2, -3, and -4). Its
classification is based on biologic and immunologic characteristics. (8) Because there is no cross-protection between the different serotypes, lifetime immunity is obtained only after infection by each type. Therefore,
persons living in endemic areas may be infected more
than once with different serotypes. Genetic variation
within each serotype confers distinct virulence capacity
and epidemic potential that may result in epidemics by
the same serotype in different years and locations. (5)
After repeated infections, the chance of developing DHF
and DSS increases. (9)
According to the World Health Organization, about
50 million dengue infections and 25,000 deaths occur
worldwide annually, making dengue one of the most
important arthropod-borne viral diseases in humans. All
continents are endemic for dengue except Europe. An
estimated 2.5 billion of the worlds population live in
areas at risk for epidemic dengue transmission, and it
remains a leading cause of morbidity and mortality
among children in some Asian countries. Most of the
severe cases and deaths occur in children younger than
15 years of age. (1)(2)
The first reported epidemic occurred in the French
West Indies in the 17th century, (3) but it was the
Southeast Asia pandemic created by the ecological disruption that followed World War II that is credited for its
worldwide spread. Over the past several decades, the
gradually increasing incidence has been attributed to
multiple factors, including global demographic changes
with associated uncontrolled urbanization and population growth, overcrowding with inappropriate sanitation, infrastructural problems, lack of preventive programs for epidemic transmission, and poor mosquito
control efforts. (4)(5) Most cases in the United States are
imported from other countries. Currently, dengue fever
is the most common cause of fever in travelers returning
from certain high-risk areas that include the Caribbean,
Central America, and South Central Asia.
Areas bordering Mexico and southeastern states serve
as niches for imported and locally acquired cases of
dengue due to population migration. According to the
Centers for Disease Control and Prevention, Aedes aegypti and Aedes albopictus are the established vectors in
these areas and are a potential threat for dengue transmission throughout the United States.
Dengue fever is caused by the dengue virus and is
transmitted by the bite of an infective female Aedes
mosquito. The diagnosis is based on history, physical
Pathogenesis
Mosquito Cycle
A aegypti is the primary vector responsible for transmission; other vectors include A albopictus, A polynesiensis,
and A niveus. A aegypti is primarily a daytime feeder. It
breeds mainly in artificial water collections created by
poor sanitation or infrastructure such as jars, plates,
flowerpots, glass containers, drainpipes, and cupboards.
Although transmission is year round, the rainy season
creates ideal larval habitats and ecologically suitable
niches for mosquito breeding and subsequent endemicity. (10)
The life cycle begins when an uninfected female mosquito takes blood from an infected person during the
viremic phase of illness. Within the mosquitos digestive
system, the virus replicates for 8 to 12 days (extrinsic
incubation period). When this infective mosquito bites
again, it transmits the virus to another person by injecting its salivary fluid. Once the virus is in the body, it
replicates in target organs and is released into the blood
(intrinsic incubation period). Symptoms appear 3 to
14 days after inoculation and may last up to 7 days or
more. Dengue should not be considered in the differential diagnosis of a patient who develops fever more than
2 weeks after leaving a dengue endemic area. (2)
e30 Pediatrics in Review Vol.31 No.4 April 2010
Downloaded from http://pedsinreview.aappublications.org/ at Eccles Health Sciences Lib on July 2, 2014
infectious diseases
Clinical Presentation of Dengue Infection
Infection with dengue viruses in children can have varied
presentations, ranging from asymptomatic to severe
shock and death. Table 1 lists definitions of probable and
confirmed dengue syndromes. (2)(11)
Undifferentiated Fever
Patients are mildly symptomatic, with nonspecific flulike
symptoms. This pattern usually occurs during a primary
infection with dengue viruses and may be the most
common manifestation.
Dengue Fever
Classic dengue fever is characterized by abrupt onset of
high-grade fever (temperature of 38.9C to 40.6C)
associated with headache (especially retroorbital pain
that worsens with eye movement), severe myalgia, arthralgia, nausea/vomiting, altered taste sensation (often
described as metallic), and sometimes a rash. (2)(12)(13)
The constellation of symptoms of severe and incapacitating body ache, back pain, and arthralgia often is called
break bone fever. Fever may last from 2 days to 1 week
Table 1.
dengue fever
and occasionally is described as having two peaks or
being saddle-backed, that is, the initial 2 to 5 days of
fever are followed by 1 to 2 days of defervescence, after
which the temperature may rise again. (8)(14)(15)
Dengue fever rash may be erythematous, macular, or
maculopapular, and lymphadenopathy may be present.
Infants and young children usually present with nonspecific symptoms such as fever, runny nose, rash, and
diarrhea; older children and adults have the classic break
bone fever, as described previously. Dengue fever can
have hemorrhagic manifestations without including the
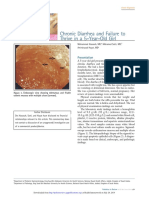
entire constellation of DHF. The hemorrhagic manifestations associated with dengue fever include a positive
tourniquet test (Figure), petechiae/purpura, mucosal
bleeding, and gastrointestinal bleeding. (8)(14) The
child in the first case had petechiae and a positive tourniquet test.
Most patients who have dengue fever recover uneventfully. Rarely, patients may present with uncommon
manifestations such as seizures, paresis, meningitis, and
mental status changes that can include lethargy, somnolence, and coma.
WHO and CDC Definitions of Dengue Clinical Syndromes
(2)(11)
Type
Clinical Case Definition
Dengue Fever
Probable dengue fever: Fever of 2 to 7 days duration, with two or more of the
following:
Headache, retroorbital pain, myalgia, arthralgia, rash, hemorrhagic manifestations,
leukopenia, and supportive serology or occurrence at the same location and time as
other confirmed cases of dengue.
Confirmed dengue fever: Confirmed by laboratory criteria (isolation of the dengue
virus, demonstration of the dengue virus antigen, serology, or genomic sequence).
All of the following criteria must be fulfilled:
1. Fever or history of acute fever, lasting 2 to 7 days, occasionally biphasic
2. Hemorrhagic manifestations in the form of at least one of the following:
- A positive tourniquet test
- Petechiae, ecchymosis, or purpura
- Bleeding from the mucosa or injection sites
- Hematemesis, melena, hematochezia, hematuria, increased menstrual flow
3. Thrombocytopenia (<100103/mcL [100109/L])
4. Objective evidence of plasma leakage caused by increased vascular permeability, as
evidenced by one or more of the following:
- A rise in the hematocrit (defined as >20% over baseline)
- A drop in hematocrit following volume replacement treatment <20% of baseline
- Low albumin or
- Pleural effusion, ascites, or other effusions
DHF plus evidence of circulatory failure manifested by shock or all of the following:
- Rapid and weak pulse
- Narrow pulse pressure (<20 mm Hg) or hypotension for age (systolic pressure
<80 mm Hg for children younger than 5 years of age or <90 mm Hg for
children 5 years of age and older)
- Cold, clammy skin and altered mental status
Dengue Hemorrhagic Fever
(DHF)
Dengue Shock Syndrome
(DSS)
CDCCenters for Disease Control and Prevention, WHOWorld Health Organization
Pediatrics in Review Vol.31 No.4 April 2010 e31
Downloaded from http://pedsinreview.aappublications.org/ at Eccles Health Sciences Lib on July 2, 2014
infectious diseases
dengue fever
Grades of Dengue
Hemorrhagic Fever (11)
Table 2.
Grades
Definitions
Grade I
Fever and nonspecific constitutional
symptoms, with a positive
tourniquet test being the only
hemorrhagic manifestation
Grade I manifestations plus
spontaneous bleeding
Circulatory failure manifested as
rapid /weak pulse, with cold skin,
restlessness, and narrow pulse
pressure or hypotension
Profound shock with nondetectable
pulse or blood pressure
Grade II
Grade III*
Grade IV*
Figure. Positive tourniquet test.
* Grades III and IV constitute dengue shock syndrome.
Dengue Hemorrhagic Fever
DHF is a potentially fatal illness marked by high fever,
hemorrhagic manifestations, and evidence of plasma leakage (Table 1). DHF begins with the sudden onset of a high
temperature that lasts 2 to 7 days, with accompanying chills,
flulike constitutional symptoms, and a flushed face. As the
fever subsides, patients may recover or progress to a state of
plasma leakage. Features of plasma leakage include ascites,
pleural effusion (right-sided in most cases), and rarely,
pericardial effusion associated with a high mortality. (8)(16)
If untreated, the condition may deteriorate rapidly to profound shock and death within hours. (12) The hemorrhagic
manifestations of DHF include skin hemorrhages such as
petechiae, purpura, and ecchymoses; bleeding from mucous
membranes (epistaxis, gingival bleeding); and bleeding from
the gastrointestinal, vaginal, and urinary tracts. These manifestations usually occur after the fever subsides, with the gastrointestinal tract being the most common site of bleeding.
DHF is classified into four grades according to severity
(Table 2). Laboratory abnormalities associated with DHF
include thrombocytopenia (100103/mcL [100109/
L]), leukopenia, prolonged prothrombin time and activated partial thromboplastin time, elevated fibrin degradation products, low serum albumin, and elevated liver
enzymes, as in the patient in case 2. Atypical lymphocytosis
(15%) and electrolyte abnormalities also may be seen.
Recovery from DHF usually is uneventful, marked by a
return of appetite and often a recovery rash (erythematous
petechial rash with islands of clearing). (2)(8) In a few
patients, symptoms such as weakness and malaise may persist for several weeks after the acute illness has subsided.
Dengue Shock Syndrome
Most patients who have DHF do not develop DSS. DSS
occurs during defervescence 3 to 6 days after the onset of
symptoms and has a high mortality rate of 10% to 47%.
(2)(17) Warning signs of DSS include severe abdominal
pain; persistent vomiting (with or without blood);
abrupt change of temperature from fever to hypothermia; and altered mental status, including irritability,
somnolence, or obtundation. In DSS, capillary leakage
and loss of intravascular volume result in shock rather
than in hemorrhage (Table 1). (11)
Complications of Dengue Infections
Severe dengue complications include liver dysfunction, encephalitis, cardiomyopathy (usually reversible), pancreatitis,
acalculous cholecystitis, peripheral neuropathy, and acute
renal failure. (8)(13)(18) Liver involvement is one of the
most important gastrointestinal manifestations, especially
with infection by DEN-3 and DEN-4 serotypes, (19) and it
can present as acute hepatitis with elevated liver enzyme
values (aspartate aminotransferase being more significantly
elevated than alanine aminotransferase), jaundice, altered
mental status, seizures, and severe hypoglycemia. Transaminase values are highest on day 9 of illness and normalize
within 3 weeks. (18) Central nervous system (CNS) disease
is attributed to various factors, including direct viral invasion of the CNS, liver failure, electrolyte disturbances, and
cerebral edema. Some case series have reported a high
mortality rate (up to 20%) in children who develop encephalopathy. (20) (21)
Diagnosis
Dengue infection can be diagnosed via serologic methods, virus isolation, or molecular methods (Table 3).
Table 4 shows the characteristics of similar infections that
should be in the differential diagnosis of dengue fever.
e32 Pediatrics in Review Vol.31 No.4 April 2010
Downloaded from http://pedsinreview.aappublications.org/ at Eccles Health Sciences Lib on July 2, 2014
infectious diseases
Table 3.
dengue fever
Methods of Laboratory Diagnosis of Dengue Infection
Diagnostic Method
Serology:
ELISA
Hemagglutination inhibition test
Complement fixation tests
Antigen capture enzyme
immunosorbent assay
Virus Isolation:
Mosquito cell cultures
Mosquito inoculation:
Toxorhynchites amboinensis
or Aedes albopictus are
used commonly for inoculation
Molecular Methods:
RT-PCR for viral RNA
Comments
The IgM ELISA is the most common test for serologic diagnosis. Sensitivity is
83.9% to 98.4% and specificity is 100%. IgM antibodies remain detectable
from day 5 to 4 to 5 weeks of illness. (2)(22)
Virus isolation methods are employed to determine the serotype of the
infecting virus. Because this procedure takes 2 weeks and is costly, it is
used primarily for research purposes. Mosquito inoculation technique is
more sensitive than cell cultures and is the preferred method of virus
isolation. (23)
RT-PCR is a rapid method of diagnosis (allowing detection within 24 hours).
It is more sensitive than virus isolation and useful in the early phase of
illness when antibodies are not circulating. However, this method is costly
and needs expertise. (2)(24)
ELISAenzyme-linked immunosorbent assay, IgMimmunoglobulin M, RT-PCRreverse transcription polymerase chain reaction
Table 4.
Differential Diagnosis of Dengue Fever
Disease
Classic Signs and Symptoms
Differentiating Features of Dengue
Influenza
Fever, headache, myalgias, malaise, respiratory tract
symptoms. (25)
Malaria
High fever, chills, rigor, sweats that may be
paroxysmal. Vomiting, diarrhea, cough, arthralgias,
abdominal and back pain, hepatosplenomegaly,
anemia, and thrombocytopenia are common. (26)
Fever, headache, malaise, anorexia, abdominal pain,
hepatosplenomegaly, rose spots, altered mental
status. (27)
Initial phase: Fever, chills, headache, vomiting, transient
rash, myalgias of calf and lumbar regions, and
conjunctival discharge (nonpurulent). Second phase:
meningitis, liver disease, and renal failure. (28)
Typhoid fever
Leptospirosis
Meningococcemia Fever, chills, malaise, prostration, rash (macular,
maculopapular, petechial). Can progress to fulminant
with disseminated intravascular coagulation, purpura,
shock, and death. (29)
Chikungunya
Rubella
Fever, rash (petechial or maculopapular) of trunk or
limbs, arthralgias, arthritis. Other: Headache,
conjunctivitis, and photophobia.
Travel history is usually positive for Africa or Asia. (30)
Rash, posterior auricular or suboccipital
lymphadenopathy, headache, conjunctivitis,
polyarthritis. (31)
Similar to undifferentiated fever form
Dengue should be included in differential
diagnosis when there is a history of travel
to endemic area
Fever can have a biphasic pattern but is
not cyclic
Thrombocytopenia may be present in
both; diagnostic studies are needed
Typical break bone features are absent
Severe disease may be difficult to
differentiate from DSS, and diagnostic
studies may be needed
Biphasic presentation in leptospirosis and
eye findings
Usually exposure to animals/farms
Travel history to high-risk areas may be
absent in leptospirosis
DHF or shock due to dengue may be
undistinguishable from meningococcemia;
it is reasonable to manage as
meningococcemia, and dengue fever
should be considered as a possibility in
returned travelers
Febrile illness in dengue does not last as
long and is not followed by arthralgic
disease, which is prolonged in
chikungunya
Rash does not have the cephalocaudal
distribution of rubella; similar to
undifferentiated fever form
DHFdengue hemorrhagic fever, DSSdengue shock syndrome
Pediatrics in Review Vol.31 No.4 April 2010 e33
Downloaded from http://pedsinreview.aappublications.org/ at Eccles Health Sciences Lib on July 2, 2014
infectious diseases
dengue fever
Treatment
Treatment is supportive. Fever is controlled with acetaminophen. Nonsteroidal anti-inflammatory agents should be
avoided due to their anticoagulant properties and risk of
Reye syndrome in children. Most cases of dengue fever
are mild and occur as undifferentiated fever or classic
dengue fever.
Early recognition and treatment decreases morbidity
and mortality. Home therapy with adequate fluid intake
and bed rest should be reinforced. Patients do not have
to be admitted to the hospital or receive intravenous
fluids unless they present with severe vomiting, dehydration, bleeding, altered mental status, clinical deterioration, or evidence of DHF or DSS. Patients who have
DHF and can be managed as outpatients include those
who have platelet counts of at least 50103/mcL
(50109/L), no active bleeding (besides the petechiae),
and a hematocrit that is not elevated. Patients who have
DHF and those who are in shock should be treated in
an intensive care setting. Hematologic, cardiovascular,
and fluid and electrolyte status should be observed and
supported. The platelet transfusion threshold in DHF
is controversial, and transfusion is required only in patients who have severe thrombocytopenia or hemorrhagic manifestations. (8) When dengue is considered in
a differential diagnosis, acute-phase (0 to 5 days) and
convalescent-phase samples (14 to 21 days) should be
collected and sent for viral isolation and serology.
travel, global migration, and climate change continue to
affect this global reemergence. (32) Dengue never
should be overlooked in countries whose prevalence is
low, such as the United States. Dengue fever continues
to appear, and the possibility of epidemics exists even
after several years of sporadic outbreak.
Summary
Today, dengue is considered among the most
important arthropod-borne viral diseases in humans.
It is transmitted by the bite of an infective female
Aedes mosquito. (2)(6)
According to the World Health Organization and
Centers for Disease Control and Prevention, the
incidence of dengue in the United States is
increasing and may be underreported due to
inadequate disease recognition and low index of
suspicion. (1)(7)
Children younger than 15 years of age are at highest
risk for severe disease and death. (2)(25)
The spectrum of dengue viral infections includes
four categories: undifferentiated fever, dengue fever,
DHF, and DSS. (1)(11)
DHF and shock forms should be managed
aggressively in an intensive care setting to prevent
morbidity and mortality. (2)(12)
Dengue should be considered in the differential
diagnosis of any child who develops fever within
2 weeks of travel to endemic areas. (2)
Prevention/Infection Control
References
Pretravel counseling for all patients visiting endemic
regions should emphasize measures to prevent humanvector contact. Using repellants containing N,Ndiethyl-3-methylbenzamide (DEET), wearing protective clothing (long-sleeved shirts and pants) during the
mosquito-biting period (morning and afternoon), and
using bed nets can minimize mosquito bites. Insect
repellants can be used safely in children older than 2
months of age. Travelers also can reduce their risk by
staying in screened or air-conditioned areas when possible and avoiding potential mosquito breeding sites.
Eliminating mosquito breeding by covering water containers and eliminating standing water can prevent the
transmission of dengue virus.
Worldwide epidemic control involves aggressive initiatives targeting prevention of dengue transmission and
includes educating the medical community and improving public health infrastructure that provides integrated
A aegypti control and an active, laboratory-based surveillance system that has a rapid response contingency plan
for epidemic prevention. However, factors such as air
1. World Health Organization. Dengue and Dengue Hemorrhagic
Fever. Publication No. 117. Geneva, Switzerland: World Health
Organization; 2008
2. Division of Vector Borne Infectious Disease. Dengue Fever.
Atlanta, Ga: Centers for Disease Control and Prevention; 2008
3. Howe GM. World Geography of Human Diseases. New York,
NY: Academic Press; 1977
4. Guzman MG, Kouri G. Dengue: an update. Lancet Infect Dis.
2002;2:33 42
5. World Health Organization. Initiative of vaccine research:
vector-borne viral infections. The World Health Report. 2003
6. Centers for Disease Control and Prevention. Travel-associated
dengueUnited States, 2005. MMWR Morb Mort Wkly Rep. 2006;
55:700 702
7. Centers for Disease Control and Prevention. Travel-associated
dengue infections, 20012004. MMWR Morb Mort Wkly Rep.
2005;54:556 558
8. Malavige GN, Fernando S, Fernando DJ, Seneviratne SL. Dengue viral infections. Postgrad Med J. 2004;80:588 601
9. Halstead SB. Dengue haemorrhagic fevera public health
problem and a field for research. Bull WHO. 1980;58:121
10. Promprou S, Jaroensutasinee M, Jaroensutasinee K. Climatic
factors affecting dengue hemorrhagic fever: incidence in southern
Thailand. Dengue Bulletin. 2005;29
11. World Health Organization. Dengue in the Context of Inte-
e34 Pediatrics in Review Vol.31 No.4 April 2010
Downloaded from http://pedsinreview.aappublications.org/ at Eccles Health Sciences Lib on July 2, 2014
infectious diseases
grated Management of Childhood Illness. Geneva, Switzerland:
World Health Organization; 2005
12. World Health Organization. Dengue Haemorrhagic Fever:
Diagnosis, Treatment, Prevention and Control. 2nd ed. Geneva,
Switzerland: World Health Organization; 1997
13. World Health Organization. Regional Guidelines of Dengue:
DHF Prevention and Control. Geneva, Switzerland: World Health
Organization; 1999
14. Ahmed FU, Mahmood CB, Sharma JD, et al. Dengue fever
and dengue haemorrhagic fever in children: the 2000 outbreak in
Chittagong, Bangladesh. Dengue Bulletin. 2001;25:3339
15. Narayanan M, Aravind MA, Thilothammal N, et al. Dengue
fever epidemic in Chennaia study of clinical profile and outcome.
Indian Pediatr. 2002;39:10271033
16. Kalayanarooj S, Chansiriwongs V, Nimmannitya S. Dengue
patients at the Childrens Hospital, Bangkok: 19951999. Dengue
Bulletin. 2002;26:33 43
17. Kabra SK, Jain Y, Pandey RM, et al. Dengue haemorrhagic
fever in children in the 1996 Delhi epidemic. Trans R Soc Trop Med
Hyg. 1999;93:294 298
18. Gulati S, Maheshwari A. Atypical manifestations of dengue.
Trop Med Int Health. 2007;12:10871095
19. Kalayanarooj S, Nimmannitya S. Clinical and laboratory presentations of dengue patients with different serotypes. Dengue
Bulletin. 2000;24:5359
20. Cam BV, Fonsmark L, Hue NB, et al. Prospective case-control
study of encephalopathy in children with dengue hemorrhagic
fever. Am J Trop Med Hyg. 2001;65:848 851
21. Lum LC, Lam SK, Choy YS, et al. Dengue encephalitis: a true
entity? Am J Trop Med Hyg. 1996;54:256 259
22. Guzman MG, Kouri G, Soler M. Dengue 2 virus enhancement
in asthmatic and non-asthmatic individuals. Mem Inst Oswaldo
Cruz. 1992;87:559 564
23. Guzman MG, Kouri G. Advances in dengue diagnosis. Clin
Diagn Lab Immunol. 1996;3:621 627
24. De Paula SO, Pires Neto RJ, Correa JA, et al. The use of reverse
dengue fever
transcription polymerase chain reaction (RT-PCR) for the rapid
detection and identification of dengue virus in an endemic region: a
validation study. Trans R Soc Trop Med Hyg. 2002;96:266 299
25. American Academy of Pediatrics. Influenza. In: Pickering LK,
Baker CJ, Kimberlin DW, Long SS, eds. Red Book: 2009 Report of
the Committee on Infectious Diseases. 28th ed. Elk Grove Village, Ill:
American Academy of Pediatrics; 2009:400 412
26. American Academy of Pediatrics. Malaria. In: Pickering LK,
Baker CJ, Kimberlin DW, Long SS, eds. Red Book: 2009 Report of
the Committee on Infectious Diseases. 28th ed. Elk Grove Village, Ill:
American Academy of Pediatrics; 2009:438 444
27. American Academy of Pediatrics. Epidemic typhus. In: Pickering LK, Baker CJ, Kimberlin DW, Long SS, eds. Red Book: 2009
Report of the Committee on Infectious Diseases. 28th ed. Elk Grove
Village, Ill: American Academy of Pediatrics; 2009:711712
28. American Academy of Pediatrics. Leptospirosis. In: Pickering
LK, Baker CJ, Kimberlin DW, Long SS, eds. Red Book: 2009 Report
of the Committee on Infectious Diseases. 28th ed. Elk Grove Village,
Ill: American Academy of Pediatrics; 2009:427 428
29. American Academy of Pediatrics. Meningococcal infections.
In: Pickering LK, Baker CJ, Kimberlin DW, Long SS, eds. Red
Book: 2009 Report of the Committee on Infectious Diseases. 28th ed.
Elk Grove Village, Ill: American Academy of Pediatrics; 2009:
455 463
30. American Academy of Pediatrics. Arboviruses. In: Pickering
LK, Baker CJ, Kimberlin DW, Long SS, eds. Red Book: 2009 Report
of the Committee on Infectious Diseases. 28th ed. Elk Grove Village,
Ill: American Academy of Pediatrics; 2009:214 220
31. American Academy of Pediatrics. Rubella. In: Pickering LK,
Baker CJ, Kimberlin DW, Long SS, eds. Red Book: 2009 Report of
the Committee on Infectious Diseases. 28th ed. Elk Grove Village, Ill:
American Academy of Pediatrics; 2009:579 584
32. Patz JA, Martens WJM, Focks DA, Jetten TH. Dengue
fever epidemic potential as projected by general circulation
models of global climate change. Environ Health Perspect. 1998;
106:147153
Pediatrics in Review Vol.31 No.4 April 2010 e35
Downloaded from http://pedsinreview.aappublications.org/ at Eccles Health Sciences Lib on July 2, 2014
Diagnosis and Management of Dengue Fever in Children
Ashlesha Kaushik, Carol Pineda and Helen Kest
Pediatrics in Review 2010;31;e28
DOI: 10.1542/pir.31-4-e28
Updated Information &
Services
including high resolution figures, can be found at:
http://pedsinreview.aappublications.org/content/31/4/e28
References
This article cites 17 articles, 5 of which you can access for free at:
http://pedsinreview.aappublications.org/content/31/4/e28#BIBL
Subspecialty Collections
This article, along with others on similar topics, appears in the
following collection(s):
Infectious Diseases
http://pedsinreview.aappublications.org/cgi/collection/infectious_dise
ases_sub
International Child Health
http://pedsinreview.aappublications.org/cgi/collection/international_c
hild_health_sub
Permissions & Licensing
Information about reproducing this article in parts (figures, tables) or
in its entirety can be found online at:
http://pedsinreview.aappublications.org/site/misc/Permissions.xhtml
Reprints
Information about ordering reprints can be found online:
http://pedsinreview.aappublications.org/site/misc/reprints.xhtml
Downloaded from http://pedsinreview.aappublications.org/ at Eccles Health Sciences Lib on July 2, 2014
Das könnte Ihnen auch gefallen
- NBME 2 Block 1-4 EditedDokument88 SeitenNBME 2 Block 1-4 EditedMayank Gogna100% (2)
- NBME 2 BlocksDokument112 SeitenNBME 2 Blocks3592648Noch keine Bewertungen
- NBME Step 2 Form 3Dokument45 SeitenNBME Step 2 Form 3bostickdrew16100% (1)
- Part 1 Sample Questions MRCPDokument33 SeitenPart 1 Sample Questions MRCPCharan Pal Singh100% (1)
- What Is Infusion Pump?Dokument4 SeitenWhat Is Infusion Pump?BMTNoch keine Bewertungen
- Nbme 2 Block 1-4Dokument112 SeitenNbme 2 Block 1-4lk0704Noch keine Bewertungen
- MRCP Part 2 Sample Questions PDFDokument83 SeitenMRCP Part 2 Sample Questions PDFamesbNoch keine Bewertungen
- NBME 2 Block 1-4 All IncludedDokument112 SeitenNBME 2 Block 1-4 All Included3592648Noch keine Bewertungen
- Nbme 2Dokument98 SeitenNbme 2oskie7Noch keine Bewertungen
- Complementary and Alternative Medical Lab Testing Part 6: Liver and GallbladderVon EverandComplementary and Alternative Medical Lab Testing Part 6: Liver and GallbladderNoch keine Bewertungen
- Substance Abuse in Pregnancy Effects and ManagementDokument14 SeitenSubstance Abuse in Pregnancy Effects and ManagementKhoirunnisa NovitasariNoch keine Bewertungen
- Fischer's BrainX Internal Medicine Board Practice Exams (Q&A Separate)Dokument482 SeitenFischer's BrainX Internal Medicine Board Practice Exams (Q&A Separate)Pratap TetaliNoch keine Bewertungen
- Alert Medical Series: Internal Medicine Alert I, II, IIIVon EverandAlert Medical Series: Internal Medicine Alert I, II, IIINoch keine Bewertungen
- 100 BCQ MRCP QuestionsDokument31 Seiten100 BCQ MRCP QuestionsMatin Ahmad Khan100% (1)
- 68th AACC Annual Scientific Meeting Abstract eBookVon Everand68th AACC Annual Scientific Meeting Abstract eBookNoch keine Bewertungen
- PracticeExam 2 QsDokument24 SeitenPracticeExam 2 QsBehrouz YariNoch keine Bewertungen
- 86 MRCP Part 2 Sample QuestionsDokument86 Seiten86 MRCP Part 2 Sample QuestionsJa Gh50% (2)
- PracticeExam 1 QsDokument16 SeitenPracticeExam 1 QsBehrouz YariNoch keine Bewertungen
- Kanker PayudaraDokument60 SeitenKanker PayudaranoviNoch keine Bewertungen
- September 2018: Frcpath Questions Hemato-OncologyDokument24 SeitenSeptember 2018: Frcpath Questions Hemato-OncologySyed Danish Ali100% (1)
- Dengue Fever in ChildrenDokument10 SeitenDengue Fever in ChildrenMuhammad NajihNoch keine Bewertungen
- Dengue Pedia CaseDokument10 SeitenDengue Pedia CaseSam Raven AndresNoch keine Bewertungen
- Crim Em2013-948071Dokument2 SeitenCrim Em2013-948071m.fahimsharifiNoch keine Bewertungen
- Nephrology Best RDokument6 SeitenNephrology Best Rfrabzi100% (1)
- Edisi 2 Artcl 6Dokument4 SeitenEdisi 2 Artcl 6jackbayNoch keine Bewertungen
- DIC - Case Study (Blood 2)Dokument2 SeitenDIC - Case Study (Blood 2)Aen BridgetteNoch keine Bewertungen
- NBME 2 Block 1-4 EditedDokument83 SeitenNBME 2 Block 1-4 EditedنبيلالمسلاتيNoch keine Bewertungen
- MRCP 1 On Examination ITU: Haemoglobin 130 White Cell Count 3.2 ×10 Platelets MCVDokument17 SeitenMRCP 1 On Examination ITU: Haemoglobin 130 White Cell Count 3.2 ×10 Platelets MCVCherryNoch keine Bewertungen
- AML Case Study AnalysisDokument14 SeitenAML Case Study AnalysisIssaiah Nicolle CeciliaNoch keine Bewertungen
- NBME 2 Block 1-4Dokument112 SeitenNBME 2 Block 1-4Daniel EspinosaNoch keine Bewertungen
- Penyakit Rongga MulutDokument9 SeitenPenyakit Rongga MulutMuhammad HernandyNoch keine Bewertungen
- Oxford University PressDokument9 SeitenOxford University PresssarafraunaqNoch keine Bewertungen
- Microbiology Infectious Disease Case StudiesDokument18 SeitenMicrobiology Infectious Disease Case StudiesKayeNoch keine Bewertungen
- Adrenal Carcinoma - A Case StudyDokument3 SeitenAdrenal Carcinoma - A Case StudyНиколина ГроздановскаNoch keine Bewertungen
- Nursing Care of a Client with Gastrointestinal BleedingDokument3 SeitenNursing Care of a Client with Gastrointestinal BleedingnicoleNoch keine Bewertungen
- Exam 19 BloodDokument8 SeitenExam 19 BloodPurwa RaneNoch keine Bewertungen
- Dengue Perimyocarditis: A Case Report: PL GohDokument3 SeitenDengue Perimyocarditis: A Case Report: PL GohDenny IntanNoch keine Bewertungen
- Continuous Assessment: Antimicrobial Therapy Questions and Case StudiesDokument6 SeitenContinuous Assessment: Antimicrobial Therapy Questions and Case StudiesfekaduNoch keine Bewertungen
- Journal Presentation: The New England Journal of Medicine Case Records of The Massachusetts General HospitalDokument49 SeitenJournal Presentation: The New England Journal of Medicine Case Records of The Massachusetts General HospitalSomnath SenguptaNoch keine Bewertungen
- A Case of Emphysematous Pyelonephritis: Grand RoundDokument3 SeitenA Case of Emphysematous Pyelonephritis: Grand RoundEriet HidayatNoch keine Bewertungen
- Methimazole Drug Induced Agranulocytosis DefinitionDokument21 SeitenMethimazole Drug Induced Agranulocytosis DefinitionReyza ReyzaNoch keine Bewertungen
- Iloilo Doctors' College Nursing Students Care for Client with Multiple Organ Dysfunction Syndrome (MODSDokument22 SeitenIloilo Doctors' College Nursing Students Care for Client with Multiple Organ Dysfunction Syndrome (MODSMissy U. TorrechillaNoch keine Bewertungen
- Case Study of Pediatric CareDokument4 SeitenCase Study of Pediatric CareazafatimatuzzahraNoch keine Bewertungen
- Evaluation of Stress Hormones in Traumatic Brain Injury Patients With Gastrointestinal BleedingDokument7 SeitenEvaluation of Stress Hormones in Traumatic Brain Injury Patients With Gastrointestinal BleedingIvonne SoelionoNoch keine Bewertungen
- Clinical and Laboratory Manifestations of Typhoid Fever at Persahabatan Hospital, JakartaDokument6 SeitenClinical and Laboratory Manifestations of Typhoid Fever at Persahabatan Hospital, JakartaFebyan AbotNoch keine Bewertungen
- Topic 9 Ankit Int MedDokument4 SeitenTopic 9 Ankit Int MedAnkit Kumar PatelNoch keine Bewertungen
- Fatal Toxic Shock Syndrome From An Intrauterine DeviceDokument3 SeitenFatal Toxic Shock Syndrome From An Intrauterine DeviceSiti Ro'AinunNoch keine Bewertungen
- Acute Fatty Liver of Pregnancy: A Case ReportDokument0 SeitenAcute Fatty Liver of Pregnancy: A Case ReportnajmulNoch keine Bewertungen
- Clin Cases RF GR 67-15506Dokument4 SeitenClin Cases RF GR 67-15506simina intunericNoch keine Bewertungen
- Exam Tests Part 1Dokument57 SeitenExam Tests Part 1Temitope AdeosunNoch keine Bewertungen
- Anexa 5 - Cerere de Acordare A Vizei AnualeDokument11 SeitenAnexa 5 - Cerere de Acordare A Vizei AnualecosminNoch keine Bewertungen
- Epidemic Poststreptococcal Glomerulonephritis: Principal Discussant: BERNARDO RODRIGUEZ-ITURBEDokument8 SeitenEpidemic Poststreptococcal Glomerulonephritis: Principal Discussant: BERNARDO RODRIGUEZ-ITURBEdrian pamungkasNoch keine Bewertungen
- A Prothrombotic Thrombocytopenic Disorder Resembling Heparin-Induced Thrombocytopenia Following Coronavirus-19 VaccinationDokument8 SeitenA Prothrombotic Thrombocytopenic Disorder Resembling Heparin-Induced Thrombocytopenia Following Coronavirus-19 VaccinationJoseph Adinolfi Jr.100% (1)
- Cardiac Leptospirosis: Iralphuaborque Md14thbatch Hds DocharlabardaDokument15 SeitenCardiac Leptospirosis: Iralphuaborque Md14thbatch Hds DocharlabardaJr. CesingNoch keine Bewertungen
- (KASUS-ENDOKRIN) (2023-10-24) A HYPEROSMOLAR HYPERGLYCEMIC STATE (HHS) PATIENT WITH NEUROLOGICAL MANIFESTATION INVOLUNTARY MOVEMENT (Biyan Maulana)Dokument11 Seiten(KASUS-ENDOKRIN) (2023-10-24) A HYPEROSMOLAR HYPERGLYCEMIC STATE (HHS) PATIENT WITH NEUROLOGICAL MANIFESTATION INVOLUNTARY MOVEMENT (Biyan Maulana)ANoch keine Bewertungen
- MalariaDokument9 SeitenMalariaDANIELLE GRACE SABERONNoch keine Bewertungen
- Rotavirus Fatal CasesDokument7 SeitenRotavirus Fatal CasesylliafwnNoch keine Bewertungen
- 1213 0pdffileDokument5 Seiten1213 0pdffilecomedyvideo2220Noch keine Bewertungen
- The Cleveland Clinic Manual of Dynamic Endocrine TestingVon EverandThe Cleveland Clinic Manual of Dynamic Endocrine TestingNoch keine Bewertungen
- Fast Facts: Blastic Plasmacytoid Dendritic Cell Neoplasm: Shedding light on a rare diseaseVon EverandFast Facts: Blastic Plasmacytoid Dendritic Cell Neoplasm: Shedding light on a rare diseaseNoch keine Bewertungen
- Pituitary Tumors: A Clinical CasebookVon EverandPituitary Tumors: A Clinical CasebookLisa B. NachtigallNoch keine Bewertungen
- 10 1378@chest 09-2690Dokument9 Seiten10 1378@chest 09-2690Alvaro Andres Flores JimenezNoch keine Bewertungen
- Right Ventricular Failure in Septic Shock: Characterization, Incidence and Impact On Fluid ResponsivenessDokument8 SeitenRight Ventricular Failure in Septic Shock: Characterization, Incidence and Impact On Fluid ResponsivenessAlvaro Andres Flores JimenezNoch keine Bewertungen
- Choosing A Mass Immunization Program Against Meningococcal BDokument4 SeitenChoosing A Mass Immunization Program Against Meningococcal BAlvaro Andres Flores JimenezNoch keine Bewertungen
- 10 1056@NEJMra1805377Dokument11 Seiten10 1056@NEJMra1805377mariano villavicencioNoch keine Bewertungen
- Bishnoi2020 Article EvaluationOfFactorsDeterminingDokument6 SeitenBishnoi2020 Article EvaluationOfFactorsDeterminingAlvaro Andres Flores JimenezNoch keine Bewertungen
- 10 1056@NEJMc1905875Dokument2 Seiten10 1056@NEJMc1905875Majo CoronadoNoch keine Bewertungen
- Ventilación Protectiva y Diafragmática 2020Dokument13 SeitenVentilación Protectiva y Diafragmática 2020Brian Antonio Veramatos LopezNoch keine Bewertungen
- Lactate-Guided Resuscitation Saves Lives: We Are Not Sure: EditorialDokument3 SeitenLactate-Guided Resuscitation Saves Lives: We Are Not Sure: EditorialAlvaro Andres Flores JimenezNoch keine Bewertungen
- Update in Clinical Psychopharmacology: Peter A. Demaria, JR., M.D., FasamDokument52 SeitenUpdate in Clinical Psychopharmacology: Peter A. Demaria, JR., M.D., FasamZubair Mahmood KamalNoch keine Bewertungen
- Good News and Bad News - 4cmenb Vaccine For Group B: Neisseria MeningitidisDokument3 SeitenGood News and Bad News - 4cmenb Vaccine For Group B: Neisseria MeningitidisAlvaro Andres Flores JimenezNoch keine Bewertungen
- 10 1056@NEJMicm1906186Dokument1 Seite10 1056@NEJMicm1906186Alya RosadynaNoch keine Bewertungen
- Case 3-2020: A 44-Year-Old Man With Weight Loss, Diarrhea, and Abdominal PainDokument10 SeitenCase 3-2020: A 44-Year-Old Man With Weight Loss, Diarrhea, and Abdominal PainAlvaro Andres Flores JimenezNoch keine Bewertungen
- Perfusion Abstractbook Supplement EuroELSO 2021 Optimal..Dokument89 SeitenPerfusion Abstractbook Supplement EuroELSO 2021 Optimal..Alvaro Andres Flores JimenezNoch keine Bewertungen
- 10 1056@NEJMicm1909329Dokument1 Seite10 1056@NEJMicm1909329Alvaro Andres Flores JimenezNoch keine Bewertungen
- Bupropion Overdose PediatricsDokument22 SeitenBupropion Overdose PediatricsAlvaro Andres Flores JimenezNoch keine Bewertungen
- Tribute ofDokument10 SeitenTribute ofAlvaro Andres Flores JimenezNoch keine Bewertungen
- Bupropion 2011 Efectos CardiovascularesDokument10 SeitenBupropion 2011 Efectos CardiovascularesAlvaro Andres Flores JimenezNoch keine Bewertungen
- Tiempo de Velocidad Integral y Minuto Distancia para El Calculo Del Volumen Latido y Gasto Cardiaco.Dokument9 SeitenTiempo de Velocidad Integral y Minuto Distancia para El Calculo Del Volumen Latido y Gasto Cardiaco.Alvaro Andres Flores JimenezNoch keine Bewertungen
- Curso Básico de EKGDokument84 SeitenCurso Básico de EKGmanuel panchoNoch keine Bewertungen
- Babinsky Sign PoetryDokument1 SeiteBabinsky Sign PoetryAlvaro Andres Flores JimenezNoch keine Bewertungen
- DR Jekyll and Mr. Hyde Chapter 8Dokument9 SeitenDR Jekyll and Mr. Hyde Chapter 8Alvaro Andres Flores JimenezNoch keine Bewertungen
- Prone Position in ARDS Patients: Why, When, How and For WhomDokument12 SeitenProne Position in ARDS Patients: Why, When, How and For WhomCornelia PredoiNoch keine Bewertungen
- Caso 6 Errores Congenitos Del MetabolismoDokument11 SeitenCaso 6 Errores Congenitos Del MetabolismoAlvaro Andres Flores JimenezNoch keine Bewertungen
- Pharmacokinetics of Bupropion and Its Metabolites in Haemodialysis Patients Who SmokeDokument8 SeitenPharmacokinetics of Bupropion and Its Metabolites in Haemodialysis Patients Who SmokeAlvaro Andres Flores JimenezNoch keine Bewertungen
- Shigella PediatricsDokument52 SeitenShigella PediatricsAlvaro Andres Flores JimenezNoch keine Bewertungen
- Clinical Manifestations and Diagnosis of Adult Still's Disease - UpToDateDokument19 SeitenClinical Manifestations and Diagnosis of Adult Still's Disease - UpToDateAlvaro Andres Flores JimenezNoch keine Bewertungen
- Pancreatitis Aguda Severa 2016Dokument12 SeitenPancreatitis Aguda Severa 2016Cyliane AnscechiNoch keine Bewertungen
- Cardiopatias en RN ,,,la InfanciaDokument11 SeitenCardiopatias en RN ,,,la InfanciaAlvaro Andres Flores JimenezNoch keine Bewertungen
- Treatment EpilepsiDokument11 SeitenTreatment EpilepsiRajabSaputraNoch keine Bewertungen
- CCIM Telemedicine Guidelines for ASU PractitionersDokument33 SeitenCCIM Telemedicine Guidelines for ASU PractitionersNishantNoch keine Bewertungen
- Hahn El 2016Dokument9 SeitenHahn El 2016ka waiiNoch keine Bewertungen
- BPH - PlanDokument5 SeitenBPH - PlanSomesh GuptaNoch keine Bewertungen
- GMDSS Exam Schedule For Year 2022Dokument7 SeitenGMDSS Exam Schedule For Year 2022Mani ThapaNoch keine Bewertungen
- CDC 5435DS1 PDFDokument211 SeitenCDC 5435DS1 PDFbodeadumitru9261Noch keine Bewertungen
- Cintya - Jurnal SDHDokument6 SeitenCintya - Jurnal SDHCintya Dyah ayu SNoch keine Bewertungen
- Case Write Up on Moderate Acne VulgarisDokument9 SeitenCase Write Up on Moderate Acne VulgarisAmbhi GanaNoch keine Bewertungen
- AnnobibliDokument7 SeitenAnnobibliapi-317692053Noch keine Bewertungen
- University of Dentistry Medicine .: Department of Dermatology and D VenerologyDokument4 SeitenUniversity of Dentistry Medicine .: Department of Dermatology and D VenerologyEriola FrrokuNoch keine Bewertungen
- Aerobic Dancing BenefitsDokument4 SeitenAerobic Dancing BenefitsDal.giNoch keine Bewertungen
- Revised Hypergly NCPDokument15 SeitenRevised Hypergly NCPDacillo GailleNoch keine Bewertungen
- Kleinth Quinto - Week6 EAPP - Performance ActivityDokument9 SeitenKleinth Quinto - Week6 EAPP - Performance ActivityAehjhae DancelNoch keine Bewertungen
- First Floor Plan: Covered Area 1028.00 SQ MDokument1 SeiteFirst Floor Plan: Covered Area 1028.00 SQ MNit56122Noch keine Bewertungen
- Peptic Ulcer Disease: Learning ObjectivesDokument7 SeitenPeptic Ulcer Disease: Learning ObjectivesMahendraNoch keine Bewertungen
- Newborn Care: A Newborn Baby or Animal Is One That Has Just Been BornDokument26 SeitenNewborn Care: A Newborn Baby or Animal Is One That Has Just Been BornJenny-Vi Tegelan LandayanNoch keine Bewertungen
- Family Nursing and Home NursingDokument35 SeitenFamily Nursing and Home NursingSamjhana Neupane100% (1)
- Medical Emergencies in The Dental Practice Poster: Revised and UpdatedDokument8 SeitenMedical Emergencies in The Dental Practice Poster: Revised and UpdatedMelissa Gabriela Sánchez SaucedoNoch keine Bewertungen
- Psychiatric TriageDokument30 SeitenPsychiatric TriageastroirmaNoch keine Bewertungen
- Mens Health Philippines 2013Dokument2 SeitenMens Health Philippines 2013Kallyx Kiel ManjiroNoch keine Bewertungen
- Hexyon LeafletDokument49 SeitenHexyon LeafletEllaNoch keine Bewertungen
- Gene Therapy: Science, Technology & SocietyDokument25 SeitenGene Therapy: Science, Technology & SocietyNicki Lyn Dela CruzNoch keine Bewertungen
- Bum Run GradDokument10 SeitenBum Run GradsabyasachiNoch keine Bewertungen
- AIDS and Oral Health - Jaypee Brothers 1st Edition (2006) PDFDokument151 SeitenAIDS and Oral Health - Jaypee Brothers 1st Edition (2006) PDFErisa BllakajNoch keine Bewertungen
- Safety and Health at Work: Kyung-Taek RimDokument8 SeitenSafety and Health at Work: Kyung-Taek RimAkbarNoch keine Bewertungen
- 04 Clinical Assessment and Differential Diagnosis of A ChildDokument31 Seiten04 Clinical Assessment and Differential Diagnosis of A ChildAmit KavimandanNoch keine Bewertungen
- 2019 Externship Survey 07242019 FINALDokument494 Seiten2019 Externship Survey 07242019 FINALddNoch keine Bewertungen
- CHN2 Module 1Dokument3 SeitenCHN2 Module 1Alexa Ann I. GUBATANGANoch keine Bewertungen