Beruflich Dokumente
Kultur Dokumente
Suggested Soln To Foundation Organic
Hochgeladen von
黄维燕Originaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Suggested Soln To Foundation Organic
Hochgeladen von
黄维燕Copyright:
Verfügbare Formate
Practice Paper 7
Advo Education Group Pte Ltd
Reg. No. 200710217Z
Suggested Solution to Foundation Organic Chemistry
Alkanes
1.
(a)
A molecule can exhibit optical isomerism when it contains a chiral carbon atom which is
bonded to four different groups of atoms, giving rise to non-superimposable mirror
images. This pair of optical isomers/enantiomers rotate plane polarised light in opposite
directions.
(b)
2.
An asterisk is used to
identify a chiral carbon.
(a)
(i)
Note:
CH3 CH3
CH3C C [1]
CH3
(a)
(iii)
Equation for complete combustion of octane:
CH3 CH3
25
C8H18 +
O2 8CO2
2
+ 9H2O
[1]
Amount of octane burnt = 100/114.0
0.877 mol
Not= accepted
(ii)
CH3
Amount of oxygen reacted = 25/2 0.877 = 11.0 mol
[1]
(singly-branched and chiral)
H
Volume of oxygen reacted at r.t.p. = 11.0 24.0 = 263 dm3 [1]
* CH CH C CH CH CH CH
[1] CH CH
CH3CH2C CH2CH2CH2CH
OR
2
2
3
3
2
2
2
2
3
Since air contains 20% O2, 263 dm3 is only 20% ofCH
theCH
vol.CH
of air required for the
2
2
3
combustion.
Volume of air at r.t.p. = 100/20
(iv)
263 = 1.32 103 dm3 [1]
Any ONE of the following:
Carbon dioxide formed from combustion contributes to enhanced
greenhouse effect resulting in global warming.
Carbon monoxide formed from incomplete combustion of octane
combines with haemoglobin and prevents transportation of oxygen to all
parts of the body.
Unburnt hydrocarbons formed from incomplete combustion of octane
form photochemical smog /cause lung damage.
[1] for stating gas and source of gas
[1] for one environmental / health problem
(b)
(i)
A is Easy
with ADVO!
Alkanes do not cause ozone
depletion
unlike CFCs. [1]
Tel: (65) 6251 3359 / 8233 2753 www.advoedu.com 1 Goldhill Plaza #02-43 Singapore 308899
(ii)
Alkanes are highly flammable. [1]
Page39
2.
Advo Education Group Pte Ltd
Practice Paper 7
Reg. No. 200710217Z
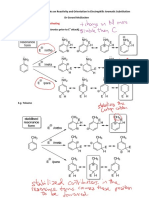
3.
(a)
(b)
Free radical substitution [1] ;
Excess butane or limited Br2, ultra-violet (UV) light
X:
[1]
Y:
Br
Br
H
HC C C C H
H C C C C * H
H
[1]
[1]
X exhibits optical isomerism as it has a
chiral carbon atom
Initiation step: (1)*
UV
Br
Br
2Br (2)
Propagation step: (1)*
(3)
Br + CH3CH2CH2CH3
CH3CHCH2CH3 + HBr (4)
CH3CHCH2CH3 + Br2
CH3CHCH2CH3
+ Br (5)
Br
Page39
(c)
Termination step: (1)*
A is Easy with ADVO!
Tel: (65) 6251 3359 / 8233 2753 www.advoedu.com 1 Goldhill Plaza #02-43 Singapore 308899
Advo Education Group Pte Ltd
Practice Paper 7
Reg. No. 200710217Z
+ Br
Br
Br2
CH3CHCH2CH3 + Br
(6) (7)
CH3CHCH2CH3
Br
CH3CHCH2CH3 + CH3CHCH2CH3
Give any 2 possible
equations
CH3CHCH2CH3
CH3CHCH2CH3
(1)*- labelling of all steps correctly
7 ()
5 6 ()
2 3 ()
1 ()
(2) - balanced eqn + half arrows + labelling of UV
(3) - correct radical formed with on correct C
(4) - correct balanced eqn
[3]
[2]
[1]
[0]
(5) - correct balanced eqn
(6)
(7)
3.
(d)
for each of any 2 possible
eqns, showing clearly the radicals involved.
Type of Stereoisomerism: Optical isomerism [1]
3 () [3]
Diagrams:
CH CH
2
() - correct structure
and bond linkages
CH CH
Br
CH
Br
() - correct mirror
image and bond
linkages
CH
() Non-superimposable mirror images
Position of bonds must be drawn accurately. The followings are NOT acceptable:
CH3
Page39
CH2CH3
A is Easy with ADVO!
Tel: (65) 6251 3359 / 8233 2753 www.advoedu.com 1 Goldhill Plaza #02-43 Singapore 308899
Advo Education Group Pte Ltd
Practice Paper 7
Reg. No. 200710217Z
(e)
X : Y = 2:3 [1]
Explanation:
Assuming that substitution is purely random, there are 4 possible H atoms (on 2 CH2)
to be substituted to form X while there are 6 possible H atoms (on 2 CH3 groups) to be
substituted to form Y.
Ratio of X : Y
=4:6
=2:3
Note:
Ha Hb
Substitute a Hb
atom by a Br atom
Hb
Ha
Hb
Ha
Hb
* C CH
H C CC
Ha C C C C Ha
Ha
Br
Br H
Substitute a Ha atom
by a Br atom
H C C C C H
Y
3.
(f)
Although molecule X has a chiral carbon atom, the reaction mixture contains an equal
amount / equimolar / 50:50 mixture of both optical isomers (enantiomers),
a racemic mixture is formed. The rotating power to rotate the plane-polarised light of
one isomer cancels that of the other. Thus, no optical activity is observed. [1]
Multiple-Choice Questions:
2.
(Answer: D)
A
Cl2 2Cl
CH3 + Cl
CH3 + HCl CH3Cl + H
CH3 + Cl2 CH3Cl + Cl (represents a propagation step between CH4 and Cl2)
(represents the initiation step)
CH3Cl
(represents a termination step)
(H radicals are not formed in the propagation step)
(Answer: B)
Oxides of nitrogen are formed via the reaction of nitrogen with oxygen at high temperatures of
the car engine. The high temperature is to provide the activation energy needed to break the
A is Easy with ADVO!
Tel: (65) 6251 3359 / 8233 2753 www.advoedu.com 1 Goldhill Plaza #02-43 Singapore 308899
Page39
1.
Advo Education Group Pte Ltd
Practice Paper 7
Reg. No. 200710217Z
strong NN in N2 and the strong O=O in O2.
3.
(Answer: D)
When methane reacts with chlorine in the presence of sunlight, free-radical substitution takes
place whereby radicals such as Cl and CH3 are produced as intermediates.
A
Radicals have high energy and are energetically unstable.
Radicals have no charge.
H radicals are not formed in the propagation step. Hence HCl cannot be produced in
this way. HCl is formed from the reaction of CH4 + Cl
CH3 + HCl and CH4 is the
reacting molecule (reactant) not the intermediate.
Radicals have unpaired electron they contain an odd number of electrons.
4.
(Answer: D)
Free radical substitution is not selective (or Substitution process is a random).
Both 2-chloropropane as well as 1-chloropropane would be formed from the substitution of
respective hydrogen atoms by the chlorine atom. In addition, there is the possibility of multisubstitution to form further chlorine-polysubstituted products.
Due to a mixture of products will be formed, the yield of 2-chloropropane is low.
5.
(Answer: C)
The more exothermic the Hr, the more stable the product and the more likely a reaction will
occur.
Using Hr = +BE(broken) BE(formed)
Option
Bonds formed
Bonds broken
Hr/ kJmol1
CH
CH
CC, HCl
CH, CCl
31
CC
CCl
10
CC
CH
+60
Thus, options A and D are eliminated. Single-step reaction (option C) is more favourable than
multiple-steps reaction (option B). Hence, option C.
(Answer: D)
Page39
6.
A is Easy with ADVO!
Tel: (65) 6251 3359 / 8233 2753 www.advoedu.com 1 Goldhill Plaza #02-43 Singapore 308899
Advo Education Group Pte Ltd
Practice Paper 7
Reg. No. 200710217Z
Both have same Mr since they have the same molecular formula.
7.
1.
Both have simple molecular structure. Smaller amount of energy is required to overcome
the weaker van der Waals forces between 2,2-dimethylpropane molecules than those
between pentane molecules due to branching which results in a smaller surface area
of contact between 2,2-dimethylpropane molecules.
Thus 2,2-dimethylpropane has a lower boiling point.
2.
Covalent bonds in simple molecules are not broken during boiling.
3.
Both are structural isomers (i.e same molecular formula but differ in the arrangement of
carbon chain). Thus, both contain the same number of electrons per molecule.
(Answer: A)
Let R1 = CH3CH2
and
R2 = (CH3)2CH
Possible alkane formed are R1 R1 ; R1 R2 ; R2 R2
1.
Equiv to R2 R2
2.
Equiv to R1 R2
3.
Equiv to R1 R1
Alkenes
(a) (i)
(ii)
Reduction
H2, Ni catalyst, 200oC
(iii)
(b) (i)
(ii)
Electrophilic addition
Cl2 in CCl4/inert organic solvent, absence of light
(iii)
(c) (i)
Page39
1.
Electrophilic addition
A is Easy with ADVO!
Tel: (65) 6251 3359 / 8233 2753 www.advoedu.com 1 Goldhill Plaza #02-43 Singapore 308899
Practice Paper 7
Advo Education Group Pte Ltd
Reg. No. 200710217Z
(ii)
HI(g), room temperature
(iii)
Apply Markovnikov's Rule for major product:
+
H of +H I (the atom bearing the partial positive charge) is added to the C of C=C with greater
no. of H atoms directly attached to it. That will result in formation of a more stable carbocation.
(d) (i)
(ii)
Electrophilic Addition
Steam/H2O(g), H3PO4 catalyst, 300oC, 65 atm
Apply
Markovnikov's
Rule for major
product.
(iii)
(e) (i)
(ii)
(Mild) Oxidation
COLD, alkaline/acidified KMnO4(aq)
(iii)
(i)
(ii)
(Strong) Oxidation
acidified KMnO4(aq), HEAT under reflux
(iii)
(f)
(i)
(ii)
Elimination
Ethanolic NaOH/KOH, heat under reflux
Apply Zaitsevs Rule for major product:
The major product is the most stable alkene, i.e. the alkene with the most number of substituents
(e.g. alkyl group) attached to the carbon-carbon double bond.
is Easy with
ADVO! > CH =CH
R2C=CR2 > R2C=CHR > R2C=CH2 > ARCH=CHR
> RCH=CH
2
2
2
Tel: (65) 6251 3359 / 8233 2753 www.advoedu.com 1 Goldhill Plaza #02-43 Singapore 308899
Page39
(iii)
Advo Education Group Pte Ltd
Practice Paper 7
Reg. No. 200710217Z
2.
(a)
Reddish brown Br2 decolourises.
(b)
2.
(c)
Electrophilic addition
(d)
(i)
Geometric isomerism
(d)
(ii)
Optical isomerism
Non-superimposable mirror images
(a)
1-methylcyclohexene is an alkene while 1-methylcyclohexane is an alkane.
(Preferred test simplest to carry out.)
Test: Add Br2(aq) to each compound separately (in the absence of light).
Obsn: For 1-methylcyclohexene, red-brown Br2(aq) decolourises
1-methylcyclohexane.
OR
but
not
A is Easy with ADVO!
Tel: (65) 6251 3359 / 8233 2753 www.advoedu.com 1 Goldhill Plaza #02-43 Singapore 308899
for
Page39
3.
Practice Paper 7
Advo Education Group Pte Ltd
Reg. No. 200710217Z
(b)
Test: Add acidified KMnO4(aq) to each compound separately and heat.
Obsn: Purple KMnO4 decolourises for 1-methylcyclohexene but not
1-methylcyclohexane.
Although propene and pent-2-ene are both alkenes, propene is a terminal alkene.
for
Test:
Add acidified KMnO4 to each compound separately and heat. Pass any gas evolved
through limewater.
Obsn: For both, purple KMnO4 decolourises.
For propene, CO2 gas evolved forms a white ppt with limewater but not for
pent-2-ene (no gas is evolved).
4.
Page39
Multiple-Choice Questions:
A is Easy with ADVO!
Tel: (65) 6251 3359 / 8233 2753 www.advoedu.com 1 Goldhill Plaza #02-43 Singapore 308899
Advo Education Group Pte Ltd
Practice Paper 7
Reg. No. 200710217Z
5.
+
Ethene undergoes electrophilic addition with HBr. The organic intermediate is the CH 3CH2+
carbocation, which is an electrophile.
Ans : C
Type of reaction: Elimination
Favorable as C = C
formed are alternate with
C C (hyperconjugation)
which helps to stabilise
the compound
Ans: D
Page39
6.
A is Easy with ADVO!
Tel: (65) 6251 3359 / 8233 2753 www.advoedu.com 1 Goldhill Plaza #02-43 Singapore 308899
Practice Paper 7
Advo Education Group Pte Ltd
Reg. No. 200710217Z
7.
The carbocation (electrophile) formed is unstable and will be attacked by ANY nucleophile present in
the mixture. In aqueous sodium nitrate, both H 2O (majority) and NO3 are nucleophiles, and the Br
formed from the slow step also acts as a nucleophile. Thus all three nucleophiles (H 2O, NO3 and Br )
attack the carbocation to form three different products.
Ans : D
Ans: C
A is Easy with ADVO!
Tel: (65) 6251 3359 / 8233 2753 www.advoedu.com 1 Goldhill Plaza #02-43 Singapore 308899
Page39
8.
Advo Education Group Pte Ltd
Practice Paper 7
Reg. No. 200710217Z
Arenes
1.
(a)
(b)
(c)
conc. HNO3, conc. H2SO4, 55 C
Electrophilic substitution
HNO3 + H2SO4
( 2)
1)
NO2+ + HSO(
+ H2O
4
( 4)
( 3)
( 1)
( 2)
( 3)
( 4)
( 5)
(a)
(i)
(ii)
I:
II:
I:
II:
limited Cl2, UV light
Fe/FeCl3/anhydrous AlCl3, heat
Free radical substitution
Electrophilic substitution
Page39
2.
( 5)
balanced equation for the generation of NO2 electrophile
full arrow from electron cloud of benzene ring to N of NO2+ electrophile
correct arenium ion with delocalisation of +ve charge over 5 sp2 C (not at the sp3 C)
full arrow from CH bond to the +ve charge of arenium ion
correct product formed with balanced eqn (+ HSO4 on arrow) and regeneration of
H2SO4 catalyst.
+
A is Easy with ADVO!
Tel: (65) 6251 3359 / 8233 2753 www.advoedu.com 1 Goldhill Plaza #02-43 Singapore 308899
Advo Education Group Pte Ltd
Practice Paper 7
Reg. No. 200710217Z
(b)
(c)
5
2
If Fe is used as catalyst,
Fe +
FeCl4 +
Cl +
If FeCl3 is used as catalyst,
If AlCl3 is used as catalyst,
FeCl3 + Cl2 FeCl4 +
AlCl3 + Cl2 AlCl4 +
Cl +
Cl +
Cl2
(d)
Check!
Draw displayed formula as requested by question: Show all bonds, trigonal
planar shape wrt carboxyl C and bent shape wrt to O of OH.
Page39
2.
A is Easy with ADVO!
Tel: (65) 6251 3359 / 8233 2753 www.advoedu.com 1 Goldhill Plaza #02-43 Singapore 308899
Practice Paper 7
Advo Education Group Pte Ltd
Reg. No. 200710217Z
3.
(a)
A
This is because electron-donating CH3 group is 2,4-directing (i.e. direct the NO2+ electrophile to the 2
or 4-position) and thus the 1,4-isomer will be one of the major products.
For Route B, electron-withdrawing CO2H group (unsaturated) is 3,5-directing (i.e. direct the NO 2+
electrophile to the 3-position) and thus the 1,4 isomer will be the minor product.
(b) (i)
(ii)
Halogen Derivatives
(a)
Reaction
I
II
III
(b)
Reaction
A
B
C
D
CH3CH2NH2
(c)
Reagents and Conditions
HBr(g)
limited Br2, UV light
PBr3, heat OR HBr, heat under reflux
Type of reaction
electrophilic addition
free radical substitution
substitution
Reagents and Conditions
ethanolic NaOH/KOH, heat under reflux
NaOH(aq)/KOH(aq), heat
NaCN/KCN, ethanol, heat under reflux
HCl(aq)/H2SO4(aq), heat under reflux
Type of reaction
elimination
nucleophilic substitution
nucleophilic substitution
acid hydrolysis
Page39
1.
A is Easy with ADVO!
Tel: (65) 6251 3359 / 8233 2753 www.advoedu.com 1 Goldhill Plaza #02-43 Singapore 308899
Advo Education Group Pte Ltd
Practice Paper 7
Reg. No. 200710217Z
1.
(d)
A primary alkyl halide usually undergoes SN2 mechanism unless otherwise stated (Both RX
and nucleophile are involved in the rate determining step so this is a SINGLE STEP
mechanism).
Reaction C
( 3)
( 1)
( 2)
( 4)
( 5)
Check!
( 1) on C and on Br of CBr bond
( 2) full arrow from lone pair on C atom of :CN to C (to show backside attack)
( 3) full arrow from CBr bond to Br
( 4) correct transition state showing the following:
dotted lines to show partial bond formation of CC bond and partial bond
breaking of CBr bond
square brackets with a net single NEGATIVE CHARGE
( 5) correct product (with inversion of stereochemistry) and balanced equation
Reaction E
( 3)
( 1)
( 5)
( 4)
Check!
( 1) on C and on Br of CBr bond
( 2) full arrow from lone pair on N atom of :NH3 to C (to show backside attack)
( 3) full arrow from CBr bond to Br
( 4) correct transition state showing the following:
dotted lines to show partial bond formation of CN bond and partial bond
breaking of CBr bond
square brackets with NO CHARGE
( 5) correct product (with inversion of stereochemistry) and balanced equation
Page39
( 2)
A is Easy with ADVO!
Tel: (65) 6251 3359 / 8233 2753 www.advoedu.com 1 Goldhill Plaza #02-43 Singapore 308899
Practice Paper 7
Advo Education Group Pte Ltd
Reg. No. 200710217Z
(a)
Since 3bromo3methylhexane is a tertiary RX, hence it follows the S N1 mechanism (Only
RX is involved in the rate determining step so the mechanism involves more than 1 step).
Reaction I
( 2)
( 3)
( 1)
( 5)
( 4)
( 5)
( 4)
Check!
( 1) on C and on Br of CBr bond
( 2) full arrow from CBr bond to Br
( 3) correct trigonal planar
of the carbocation + balanced equation
( 4) full arrow from lone pair on C atom of :OH to C+;
( 5) correct product (stereochemistry according to direction of OH attack on C+)
and balanced equation.
Reaction II
( 2)
( 3)
( 1)
( 5)
( 4)
( 5)
( 4)
A is Easy with ADVO!
Tel: (65) 6251 3359 / 8233 2753 www.advoedu.com 1 Goldhill Plaza #02-43 Singapore 308899
Page39
2.
Practice Paper 7
Advo Education Group Pte Ltd
Reg. No. 200710217Z
Check!
(1) on C and on Br of CBr bond
(2) full arrow from CBr bond to Br
( 3) correct trigonal planar
of the carbocation + balanced equation
( 4) full arrow from lone pair on N atom of :NH3 to C+;
( 5) correct product (stereochemistry according to direction of NH3 attack on C
and balanced equation (Note: Br on arrow and HBr as by-product).
2.
(b)
3bromo3methylhexane is a chiral alkyl halide. There is an equal probability for the
nucleophile :CN or :NH3 to attack EITHER side of the trigonal planar
of the
carbocation intermediate to form a racemic mixture (OR equal amounts of optical
isomers/enantiomers). The optical activity of the enantiomers cancel out one another and
thus, the resulting mixture exhibits no net optical activity.
3.
(a)
Chloroethane is more readily hydrolysed than chlorobenzene.
This is because p-p orbital overlap which results in the delocalisation of the lone pair of
electrons on Cl of chlorobenzene into the benzene ring, giving rise to partial double
bond character in C-Cl bond, thus strengthening the C-Cl bond (i.e. difficult to break).
Hence, chlorobenzene is resistant to nucleophilic attack.
(b)
Reactivity towards hydrolysis: chloroethane < bromoethane < iodoethane
From Data Booklet:
E(CCl) = +340 kJ mol1; E(CBr) = +280 kJ mol1; E(CI) = +240 kJ mol1
Since E(CCl) > E(CBr) > E(CI), the CX bond strength decreases from CCl bond to
CBr bond to CI bond. Hence the ease of breaking the CX bond increases from
chloroethane to bromoethane to iodoethane.
OR
As atomic radius of X increases from C l to Br to I atom, CX bond length increases
and thus the CX bond strength decreases from CCl bond to CBr bond to CI bond.
Hence the ease of breaking the CX bond increases from chloroethane to
bromoethane to iodoethane.
1. Add NaOH(aq) to the compounds separately and heat.
2. Add excess HNO3(aq) to remove excess NaOH.
3. Add AgNO3(aq) to resulting mixture
For chlorobenzene, no ppt is formed.
For chloroethane, white ppt of AgCl is formed.
For iodoethane, yellow ppt of AgI is formed.
Page39
4.
A is Easy with ADVO!
Tel: (65) 6251 3359 / 8233 2753 www.advoedu.com 1 Goldhill Plaza #02-43 Singapore 308899
Practice Paper 7
Advo Education Group Pte Ltd
Reg. No. 200710217Z
5.
From molecular formula of M (C3H6O), M is CH3CH2CHO, an aldehyde
hence L is CH3CH2CH2OH, a primary alcohol ().
Therefore, K is CH3CH2CH2Cl.
() and
Explain your reasoning: Check you have all the ()s!
State clearly the type of reaction a compound has undergone and what functional group or
characteristic structure you can deduce a particular compound possess or do not possess (if no
reaction).
Hydroxy Compounds
substitution
(a)
(b)
substitution
(c)
(d)
redox reaction
(e)
redox reaction
(f)
(g)
Page39
1.
A is Easy with ADVO!
Tel: (65) 6251 3359 / 8233 2753 www.advoedu.com 1 Goldhill Plaza #02-43 Singapore 308899
Practice Paper 7
Advo Education Group Pte Ltd
Reg. No. 200710217Z
(h)
acid-base reaction
oxidation
(i)
(j)
oxidation
(k)
oxidation
(l)
(m)
electrophilic substitution
(n)
electrophilic substitution
Why phenol undergoes electrophilic substitution under milder conditions as compared to benzene?
(o)
electrophilic substitution
(p)
condensation
(q)
elimination
A is Easy with ADVO!
Tel: (65) 6251 3359 / 8233 2753 www.advoedu.com 1 Goldhill Plaza #02-43 Singapore 308899
Page39
1.
Practice Paper 7
Advo Education Group Pte Ltd
Reg. No. 200710217Z
(a)
(i)
redox reaction
(ii)
acid-base reaction
(iii
)
substitution
(iv
)
condensation
(v)
condensation
(vi
)
(a)
oxidation
(vii)
electrophilic substitution of phenol
and electrophilic addition of alkene
(b)
Rxn with reagent (iii): white fumes of HCl evolved.
Rxn with reagent (vi): orange K2Cr2O7 turns green.
Rxn with reagent (vii): red-brown Br2(aq) decolourises and white ppt. is formed.
A is Easy with ADVO!
Tel: (65) 6251 3359 / 8233 2753 www.advoedu.com 1 Goldhill Plaza #02-43 Singapore 308899
Page39
2.
Practice Paper 7
Advo Education Group Pte Ltd
Reg. No. 200710217Z
3.
(a)
(b)
conc. H2SO4, heat under reflux
(c)
(a)
Test: Add neutral FeCl3(aq) to each compound separately.
Obsn: For phenol, violet colouration is observed but not for phenylmethanol.
OR
Test: Add aqueous Br2 to each compound separately.
Obsn: For phenol, red-brown Br2(aq) decolourises but not for phenylmethanol.
OR
Test: Add PCl5 to each compound separately.
Obsn: For phenylmethanol, white fumes of HCl is formed but not for phenol.
OR
Test: Add acidified K2Cr2O7(aq) to each compound separately and heat.
Obsn: For phenylmethanol, orange K2Cr2O7 turns green but not for phenol.
The test of acidified KMnO4 with heating is not suitable as phenol may be oxidised by KMnO 4
(oxidation of phenol is not in syllabus).
(b)
Test:
Add acidified KMnO4(aq) (OR acidified K2Cr2O7(aq)) to each compound separately
and heat.
n
Obs : For butan-1-ol, purple KMnO4 decolourises (or orange K2Cr2O7 turns green) but
not for 2-methylpropan-2-ol.
(c)
Butan-2-ol has the
characteristics structure of
CH(OH)CH3 which can
be identified using
iodoform test.
Test:
Add alkaline aqueous I2 (or aqueous I2 and aqueous NaOH) to each compound
separately, and heat.
Obsn: For butan-2-ol, a pale yellow ppt. of CHI3 is formed but not for butan-1-ol.
A is Easy with ADVO!
Tel: (65) 6251 3359 / 8233 2753 www.advoedu.com 1 Goldhill Plaza #02-43 Singapore 308899
Page39
4.
Advo Education Group Pte Ltd
Practice Paper 7
Reg. No. 200710217Z
5.
Check!
Explain your
reasoning. Make
sure you have all
the ().
( 1)
6. The p-p orbital overlap results in the delocalisation of the lone pair of electrons on O atom into
( 3)
( 2)
the benzene ring, which disperses the negative charge and stabilises
Hence, phenol is a stronger acid than ethanol.
( 1)
( 2)
Electronegative Cl group is electron-withdrawing and thus disperses the negative charge and
( 3)
stabilises
Check! When explaining relative acidity,
( 1) state the reason clearly (e.g. electron-donating group/ electron-withdrawing group/ p-p orbital
overlap)
( 2) disperses or intensifies negative charge
( 3) stabilises or destabilises <structure of anion>
A is Easy with ADVO!
Tel: (65) 6251 3359 / 8233 2753 www.advoedu.com 1 Goldhill Plaza #02-43 Singapore 308899
Page39
Hence, 4-chlorophenol is a stronger acid than phenol.
Practice Paper 7
Advo Education Group Pte Ltd
Reg. No. 200710217Z
Carbonyl Compounds
(a)
(b)
oxidation
(c)
(d)
(e)
reduction
(f)
(e)
nucleophilic
addition
(f)
Page39
1.
A is Easy with ADVO!
Tel: (65) 6251 3359 / 8233 2753 www.advoedu.com 1 Goldhill Plaza #02-43 Singapore 308899
Practice Paper 7
Advo Education Group Pte Ltd
Reg. No. 200710217Z
2.
(a)
Page39
3.
A is Easy with ADVO!
Tel: (65) 6251 3359 / 8233 2753 www.advoedu.com 1 Goldhill Plaza #02-43 Singapore 308899
Advo Education Group Pte Ltd
Practice Paper 7
Reg. No. 200710217Z
3.
(b)
(c)
Generation of nucleophile: HCN H+ + CN ( 1)
4.
( 2) both partial charges
( 4)
( 3)
( 5)
Check!
( 6)
( 1) generation of nucleophile
( 2) for both + on C and on O
( 3) full arrow from lone pair on C of CN to C+ of C=O (check correct nucleophile)
( 4) full arrow from C=O to O
( 5) correct intermediate with negative charge on O
( 6) balanced equation with HCN for fast step and CN generated.
Page39
There is equal probability for the :CN nucleophile to attack EITHER side (top or bottom) of the
trigonal planar >C= of butanone, producing a racemic mixture/equal amount of both optical
isomers.
Note: Reject trigonal planar butanone. Only the shape wrt carbonyl C is trigonol planar but the
shape wrt the each of the other carbon atoms is tetrahedral so the whole molecule is NOT planar.
A is Easy with ADVO!
Tel: (65) 6251 3359 / 8233 2753 www.advoedu.com 1 Goldhill Plaza #02-43 Singapore 308899
Practice Paper 7
Advo Education Group Pte Ltd
Reg. No. 200710217Z
5. (a) Pentan3one is a ketone and pentane is an alkane.
Test:
Add 2,4-DNPH to each compound separately.
n
Obs : For pentan3one, an orange ppt is formed but not for pentane.
(b) Pentan2one is a ketone with COCH3 structural unit and pentan3one does not have the
structural unit.
Test:
Add alkaline I2(aq) (or aqueous I2, aqueous NaOH) to each compound separately
and heat.
n
Obs : For pentan2one, a pale yellow ppt of CHI3 is formed upon cooling but not for
pentan3one.
(c) Pentan-3-one is a ketone and pentanal is an aliphatic aldehyde.
Test:
Add Tollens reagent to each compound separately and heat.
Obsn: For pentanal, a silver mirror is formed but not for pentan3one.
OR
Test:
Add Fehlings solution to each compound separately and heat.
n
Obs : For pentanal, a red-brown ppt is formed but not for pentan3one.
OR
Test:
Add acidified KMnO4(aq) (OR acidified K2Cr2O7(aq)) to each compound separately
and heat.
n
Obs : For pentanal, purple KMnO4 decolourises (or orange K2Cr2O7 turns green) but not
for pentan-3-one.
(d) Pentan-3-one is a ketone and pentan-3-ol is a secondary alcohol.
Test:
Add 2,4-DNPH to each compound separately.
n
Obs : For pentan3one, an orange ppt is formed but not for pentan-3-ol.
OR
Test:
Add PCl5 to each compound separately.
n
Obs : For pentan3ol, white fumes of HCl is formed but not for pentan-3-one.
OR
Test:
Page39
Obsn:
Add acidified KMnO4(aq) (OR acidified K2Cr2O7(aq)) to each compound separately
and heat.
For pentan-3-ol, purple KMnO4 decolourises (or orange K2Cr2O7 turns green) but
not for pentan-3-one.
A is Easy with ADVO!
Tel: (65) 6251 3359 / 8233 2753 www.advoedu.com 1 Goldhill Plaza #02-43 Singapore 308899
Practice Paper 7
Advo Education Group Pte Ltd
Reg. No. 200710217Z
Page39
6.
Carboxylic Acid & Derivatives
A is Easy with ADVO!
Tel: (65) 6251 3359 / 8233 2753 www.advoedu.com 1 Goldhill Plaza #02-43 Singapore 308899
Advo Education Group Pte Ltd
Practice Paper 7
Reg. No. 200710217Z
1.
(a)
(b)
2.
(a)
(i)
(ii)
(i)
(ii)
(iii
)
I: acidified KMnO4(aq)/K2Cr2O7(aq), heat under reflux
II: acidified KMnO4(aq)/K2Cr2O7(aq), heat under reflux
III: HCl(aq)/H2SO4(aq), heat under reflux
I: oxidation
II: oxidation
LiAlH4 in dry ether
A: CH3COCl
B: CH3COOCH2CH3
IV: reduction
V: substitution
VI: condensation
VII: acid-carbonate reaction
CH3COCl + H2O
CH3COOH + HCl
R
CH3COCl + NH3
CH3CONH2 + HCl
S
III: acid hydrolysis
C: CH3COO-Na+
CH3COCl + CH3OH CH3COOCH3 + HCl
T
(b)
Check!
Question asks for
displayed formula.
(c)
(d)
PCl5, room temp. OR PCl3, heat OR SOCl2, heat
Ease of hydrolysis in decreasing order (easiest hardest): CH3COCl, CH3CH2Cl, C6H5Cl
C6H5Cl does not undergo hydrolysis because p-p orbital overlap results in delocalisation of
lone pair of electrons on Cl atom into the benzene ring, causes partial double bond
character in C-Cl bond and thus strengthening the C-Cl bond, making it difficult to break.
CH3COCl is more readily hydrolysed than CH3CH2Cl because the carbon in COCl group is
highly electron deficient as it is bonded to 2 electronegative atoms, O and C l as
compared to CH3CH2Cl which has only one Cl bonded to its reactive carbon. Hence, the
carbon in COCl group is more susceptible to nucleophilic attack.
(a)
(b)
secondary alcohol, carboxylic acid
(i)
substitution
(ii)
substitution
Page39
3.
A is Easy with ADVO!
Tel: (65) 6251 3359 / 8233 2753 www.advoedu.com 1 Goldhill Plaza #02-43 Singapore 308899
Practice Paper 7
Advo Education Group Pte Ltd
Reg. No. 200710217Z
3.
(b)
(iii
)
(iv
)
redox reaction
acid-base reaction
(v)
oxidation
(vi
)
condensation
(vii)
condensation
(a)
Page39
4.
A is Easy with ADVO!
Tel: (65) 6251 3359 / 8233 2753 www.advoedu.com 1 Goldhill Plaza #02-43 Singapore 308899
Practice Paper 7
Advo Education Group Pte Ltd
Reg. No. 200710217Z
4.
(b)
(c)
(a)
(i)
(ii)
CH3CH2CO2H
CH3CH2CH2OH
CH3CH2CO2 + H+
CH3CH2CH2O + H+
The pp orbital overlap results in the delocalisation of lone pair of electrons on O
over the two O atoms in CH 3CH2CO2. This disperses the negative charge and
stabilises CH3CH2CO2.
Whereas, the electron-donating CH2CH2CH3 group in propanol intensifies the negative
charge and destabilises CH3CH2CH2O.
CH3CH2CO2H
CH3CH2CO2 + H+
CH3CH(CH3)CO2H
CH3CH(CH3)CO2 + H+
Electron-donating CH3/alkyl group intensifies the negative charge and destabilises
CH3CH(CH3)COO.
Hence CH3CH(CH3)COOH is a weaker acid than CH3CH2COOH.
Page39
5.
A is Easy with ADVO!
Tel: (65) 6251 3359 / 8233 2753 www.advoedu.com 1 Goldhill Plaza #02-43 Singapore 308899
Practice Paper 7
Advo Education Group Pte Ltd
Reg. No. 200710217Z
5.
(a)
(iii) CH3CH2CO2H
CH3CH2CO2 + H+
CH3CH(OH)CO2H
CH3CH(OH)CO2 + H+
Electronegative O of OH group is electron-withdrawing and thus disperses the
negative charge and stabilises CH3CH(OH)CO2.
Hence CH3CH(OH)COOH is a stronger acid than CH3CH2COOH.
CH3CHClCO2H
CH3CHClCO2 + H+
CH2ClCH2CO2H
CH2ClCH2CO2 + H+
Electronegative Cl atom is further away from COO in CH2ClCH2CO2 than in
CH3CHClCO2 and thus electron-withdrawing effect of CH2CH2Cl is weaker than
CHClCH3. The negative charge on CH2ClCH2CO2 is less dispersed and hence
CH2ClCH2CO2 is less stable than the CH3CHClCO2 anion.
Hence, 3-chloropropanoic acid is a weaker acid than 2-chloropropanoic acid.
(a)
Test:
Add Na2CO3(aq) or NaHCO3(aq) to each compound separately and pass any gas
produced through limewater.
Obsn: For ethanoic acid, CO2 gas evolved form white ppt with limewater but not for
phenol.
OR
Test: Add PCl5 to each compound separately.
Obsn: For ethanoic acid, white fumes of HCl are given off but not for phenol.
OR
Test: Add neutral FeCl3(aq) to each compound separately.
Obsn: For phenol, a violet colouration is observed but not for ethanoic acid.
OR
Test: Add Br2(aq) to each compound separately.
Obsn: For phenol, red-brown Br2(aq) decolourises but not for ethanoic acid.
(b)
Test:
1. Add NaOH(aq) to each compound separately and heat.
2. To the resulting mixture, add alkaline I2 (aq) (OR I2(aq), NaOH(aq) ) and heat.
Obs: For CH3COOCH(CH3)CH2CH3, pale yellow ppt of CHI3 is formed upon cooling but not
for CH3COOC(CH3)3.
Page39
6.
(b)
A is Easy with ADVO!
Tel: (65) 6251 3359 / 8233 2753 www.advoedu.com 1 Goldhill Plaza #02-43 Singapore 308899
Advo Education Group Pte Ltd
Practice Paper 7
Reg. No. 200710217Z
6.
(c)
Test:
Add aqueous AgNO3 to each compound separately.
Obsn: For
OR
Test:
, white ppt. of AgCl is formed, but not for
Add 2,4-DNPH to each compound separately.
Obsn: For
OR
Test:
, orange ppt is formed, but not for
(a)
, a silver mirror is formed, but not for
C
H
35.8
4.5
35.812.0 = 2.98
4.51.0 = 4.5
1
1.5
4
6
Empirical formula of R (diacid) is C4H6O5.
ethanol, conc.H2SO4,
heat under reflux
(condensation)
()
(b) R: diacid
C4H6O5
2,4-DNPH
(condensation) ()
V is an aldehyde or ketone ()
H
C C
HO
V:
O
C
H H
O
C
CH3CH2O
OH
OH
T: OH group ()
Fehlings or
Tollens reagent
No oxidation
V: not an aldehyde ()
Is a ketone ()
Since V is a ketone,
T is a secondary alcohol ()
T is a primary or
secondary alcohol
T:
H OH
O
C
CH3CH2O
C C
O
OCH2CH3
Page39
H2(g)
acidified K2Cr2O7
(oxidation) ()
T is a diester or
contains 2 ester groups
()
Orange ppt.
59.7
59.716.0 = 3.73
1.25
5
Na(s)
(redox reaction) ()
T
C8H14O5
% mass
Amount/mol
Mole ratio
R:
Add Tollens reagent, to each compound separately and heat.
Obsn: For
7.
C
OCH2CH3
A is Easy with ADVO!
Tel: (65) 6251 3359 / 8233 2753 www.advoedu.com 1 Goldhill Plaza #02-43 Singapore 308899
Advo Education Group Pte Ltd
Practice Paper 7
Reg. No. 200710217Z
Organic Nitrogen Compounds
(a)
(b)
2.
(i)
(ii)
(i)
(ii)
(iii
)
I: excess NH3, ethanol, heat in sealed tube
II: LiAlH4 in dry ether OR H2, Ni, heat
I: Nucleophilic substitution
II: Reduction
+
CH3CH2NH3 Cl
CH3COCl
III: Acid-base reaction
IV: Condensation
(a)
(b)
I: conc HNO3, conc H2SO4, 55 C
II: 1. Sn, conc HCl, heat
2. NaOH(aq)
(i)
O
R
Note: The following equation is not accepted as it only shows that phenylamine is a weak
base. It does not show that phenylamine is acting as a base (i.e. react with an acid).
(ii)
Relative basic strength in increasing order (weakest strongest): C , A , B
Compare B (i.e. aliphatic amine) versus A & C (i.e. aromatic amines):
The pp orbital overlap results in the delocalisation of the lone pair of electrons on
N atom into the benzene ring, making the lone pair less available for protonation.
Hence, A and C are weaker bases than B.
Compare A versus C:
Electrondonating CH3/alkyl group in A makes the lone pair of electrons on N atom
more available for protonation.
Hence, A is a stronger base than C.
(c)
Page39
1.
A is Easy with ADVO!
Tel: (65) 6251 3359 / 8233 2753 www.advoedu.com 1 Goldhill Plaza #02-43 Singapore 308899
Practice Paper 7
Advo Education Group Pte Ltd
Reg. No. 200710217Z
3.
(a)
(b)
(c)
Proteins
(a)
Thought Process involved :
1) Assign pKa value to the respective NH2/ COOH group in glutamic acid.
-NH2 is a basic group, hence the pKa of its conjugate acid, -NH3+ is 9.5.
COOH is stronger acid than side chain COOH because of the electron
withdrawing N atom of NH2 group is closer to the-COO and hence, dispersing
the negative charge of-COO more, making -COO more stable than side
chain -COO.
2)To determine the major species present at the respective pH values, check:
pH > pKa predominantly exists as its conjugate base form;
pH < pKa predominantly exists as its acidic form.
pH 1
pH < pKa of all 3 groups
predominantly acidic form
pH 3
pH > pKa of COOH
predominantly conjugate base form
pH 7
pH 11
pH > pKa of COOH AND side chain pH > pKa of all 3 groups
predominantly conjugate base form
COOH
predominantly conjugate base form
A is Easy with ADVO!
Tel: (65) 6251 3359 / 8233 2753 www.advoedu.com 1 Goldhill Plaza #02-43 Singapore 308899
Page39
4.
Practice Paper 7
Advo Education Group Pte Ltd
Reg. No. 200710217Z
(b)
Thought Process involved :
Isoelectric point is the pH at which the amino acid exists only as zwitterion and thus
will not migrate under the influence of an electric field.
At pH > 2.1, the zwitterion is the major species present.
At pH > 4.1, the net singly negative charged species is the major species present.
Hence, the zwitterion is solely present in solution when pH of solution is between 2.1
and 4.1.
pI of glutamic acid is any value between 2.1 and 4.1 (e.g. 3.1).
5.
Working:
argvaltyr
aspargval
valtyrile
ilehispro
prophe
aspargvaltyrilehisprophe
6.
(a)
(b)
If alkaline hydrolysis is employed, the three amino acids will exist as the following form in
alkaline medium:
Page39
(c)
peptide bond/linkage
HCl(aq)/H2SO4(aq) of moderate concentration, prolonged heating under reflux
OR
NaOH(aq)/KOH(aq) of moderate concentration, prolonged heating under reflux
Note: Moderate concentration and prolonged heating are needed to break the numerous
covalent bonds.
During the reaction (i.e. hydrolysis), the amide bond is cleaved to form acidic COOH and
basic NH2.
If acid hydrolysis is employed, the three amino acids will exist as the following form in acidic
medium:
A is Easy with ADVO!
Tel: (65) 6251 3359 / 8233 2753 www.advoedu.com 1 Goldhill Plaza #02-43 Singapore 308899
Practice Paper 7
Advo Education Group Pte Ltd
Reg. No. 200710217Z
6.
(d)
When a small amount of acid is added, H+ is removed as follows:
When a small amount of base is added, OH is removed as follows:
(a)
(b)
(c)
Primary structure of a protein refers to the sequence of the amino acid residues in a
polypeptide chain.
Check!
No H attached to N
Check! Draw a section of a protein NOT tripeptide, as requested in question.
helix is a regular spiral configuration of the polypeptide chain, which is stabilised by
hydrogen bonds between the BACKBONE C=O group of one amino acid residue and
the BACKBONE NH group of the 4th amino acid residue further down the chain.
Page39
7.
A is Easy with ADVO!
Tel: (65) 6251 3359 / 8233 2753 www.advoedu.com 1 Goldhill Plaza #02-43 Singapore 308899
Practice Paper 7
Advo Education Group Pte Ltd
Reg. No. 200710217Z
(c)
(d)
Check!
correct structure of helix, showing:
C=O and NH group of one peptide bond
involved in the formation of hydrogen
bonds
N and C must be on the polypeptide
backbone
polypeptide chain must be labeled.
at least 1 hydrogen bond correctly
drawn:
on N and on H of NH bond
on O and on C of C=O
dotted line between lone pair on O of C=O
and H of NH bond
clear labeling of hydrogen bonds
(reject Hbond)
pleated sheet consists of adjacent polypeptide strands stabilised by hydrogen bonds
between the BACKBONE C=O group of one strand and the BACKBONE NH group of
the adjacent strand.
Check!
correct structure of pleated sheet,
showing:
C=O and NH group of one peptide bond
involved in the formation of hydrogen
bonds
N and C must be on the polypeptide
backbone
Strands of polypeptide chain must be
labeled.
at least 1 hydrogen bond correctly
drawn:
on N and on H of NH bond
on O and on C of C=O
dotted line between lone pair on O of C=O
and H of NH bond
clear labeling of hydrogen bonds
(reject Hbond)
Page39
7.
A is Easy with ADVO!
Tel: (65) 6251 3359 / 8233 2753 www.advoedu.com 1 Goldhill Plaza #02-43 Singapore 308899
Practice Paper 7
Advo Education Group Pte Ltd
Reg. No. 200710217Z
(a)
(i)
(ii)
Formed by oxidation of the SH groups in the cysteine residues:
Check! Disulfide bridge is a covalent bond (solid line).
Type of R
group
Diagram illustrating R group interaction
interaction
ionic
interaction
Note: When acidic COOH comes close to basic NH2, acid-base
reaction will take place. COOH will become COO and NH2 will
become NH3+. These oppositely charged groups will form ionic
interaction with one another (NOT hydrogen bond!).
Any one of the following:
hydrogen bond
(between polar
R groups except
between NH2
and COOH)
van der Waals
forces
(between nonpolar alkyl R
groups)
(iii) Lysine, serine, aspartic acid and asparagine. [1m]
Their side chains are hydrophilic and thus able to form hydrogen bond or ion
dipole interaction with water molecules surrounding the watersoluble globular
protein.
A is Easy with ADVO!
Tel: (65) 6251 3359 / 8233 2753 www.advoedu.com 1 Goldhill Plaza #02-43 Singapore 308899
Page39
8.
Practice Paper 7
Advo Education Group Pte Ltd
Reg. No. 200710217Z
8.
(a)
(b)
(iv) They can be found in the interior of the watersoluble globular protein.
van der Waals forces occur between the residues with hydrophobic/nonpolar R
groups in the polypeptide chain.
(i) Denaturation results from the disruption of the interactions that maintain the shape
of a protein, leading to possible destruction of the secondary, tertiary and
quaternary structures. This disruption alters the conformation/shape of the protein
and causes the protein to lose its ability to perform its specific function.
(ii) Heavy metal ions, such as Ag+, form ionic interaction with COO which brings about
the formation of insoluble protein salt (i.e. precipitation of protein). This disrupts the
ionic interaction in the tertiary and quaternary structures, resulting in the
denaturation of the protein.
Heavy metal ions have a high affinity for sulfur and will bind tightly to SH group of
cysteine residue. This disrupts the disulfide bridges in tertiary and quaternary
structures, resulting in the denaturation of the protein.
Discuss the
disruption of
each R group
interaction
separately.
At high pH, COOH becomes COO while at low pH, NH2 becomes NH3+. This
disrupts the hydrogen bonds in the tertiary and quaternary structures, resulting in
the denaturation of the protein.
Note: pH changes WILL NOT disrupt hydrogen bonds in secondary structures as these
hydrogen bonds are formed between C=O and N-H group of NEUTRAL amide linkage/
peptide bond.
Extreme heat will disrupt the weak interactions such as the van der Waals forces in the
tertiary and quaternary structures and the hydrogen bonds in the secondary, tertiary
and quaternary structures of the protein.
This alters the conformation/shape of the active site of the enzyme. As a result,
substrate molecule cannot bind to the active site and hence the enzyme loses its catalytic
activity, i.e. the enzyme is denatured.
Page39
(c)
At low pH, COO becomes COOH while at high pH, NH3+ becomes NH2. This
disrupts the ionic interactions in the tertiary and quaternary structures, resulting in
the denaturation of the protein.
A is Easy with ADVO!
Tel: (65) 6251 3359 / 8233 2753 www.advoedu.com 1 Goldhill Plaza #02-43 Singapore 308899
Das könnte Ihnen auch gefallen
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryVon EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryBewertung: 3.5 von 5 Sternen3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Von EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Bewertung: 4.5 von 5 Sternen4.5/5 (119)
- Never Split the Difference: Negotiating As If Your Life Depended On ItVon EverandNever Split the Difference: Negotiating As If Your Life Depended On ItBewertung: 4.5 von 5 Sternen4.5/5 (838)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaVon EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaBewertung: 4.5 von 5 Sternen4.5/5 (265)
- The Little Book of Hygge: Danish Secrets to Happy LivingVon EverandThe Little Book of Hygge: Danish Secrets to Happy LivingBewertung: 3.5 von 5 Sternen3.5/5 (399)
- Grit: The Power of Passion and PerseveranceVon EverandGrit: The Power of Passion and PerseveranceBewertung: 4 von 5 Sternen4/5 (587)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyVon EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyBewertung: 3.5 von 5 Sternen3.5/5 (2219)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeVon EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeBewertung: 4 von 5 Sternen4/5 (5794)
- Team of Rivals: The Political Genius of Abraham LincolnVon EverandTeam of Rivals: The Political Genius of Abraham LincolnBewertung: 4.5 von 5 Sternen4.5/5 (234)
- Shoe Dog: A Memoir by the Creator of NikeVon EverandShoe Dog: A Memoir by the Creator of NikeBewertung: 4.5 von 5 Sternen4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerVon EverandThe Emperor of All Maladies: A Biography of CancerBewertung: 4.5 von 5 Sternen4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreVon EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreBewertung: 4 von 5 Sternen4/5 (1090)
- Her Body and Other Parties: StoriesVon EverandHer Body and Other Parties: StoriesBewertung: 4 von 5 Sternen4/5 (821)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersVon EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersBewertung: 4.5 von 5 Sternen4.5/5 (344)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceVon EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceBewertung: 4 von 5 Sternen4/5 (890)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureVon EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureBewertung: 4.5 von 5 Sternen4.5/5 (474)
- The Unwinding: An Inner History of the New AmericaVon EverandThe Unwinding: An Inner History of the New AmericaBewertung: 4 von 5 Sternen4/5 (45)
- The Yellow House: A Memoir (2019 National Book Award Winner)Von EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Bewertung: 4 von 5 Sternen4/5 (98)
- Chapter 14 - Acids and Bases: Name: Clas S: Dat eDokument38 SeitenChapter 14 - Acids and Bases: Name: Clas S: Dat e鄭子玄Noch keine Bewertungen
- On Fire: The (Burning) Case for a Green New DealVon EverandOn Fire: The (Burning) Case for a Green New DealBewertung: 4 von 5 Sternen4/5 (73)
- Hydrocarbons Chapter 13 ReviewDokument70 SeitenHydrocarbons Chapter 13 ReviewGururaj Vasisth100% (3)
- Nandesari DataDokument47 SeitenNandesari DataVaibhavNoch keine Bewertungen
- VanadiumDokument12 SeitenVanadiumEkha Kirei100% (1)
- Prelab 6 Cyclohexyl ChlorideDokument5 SeitenPrelab 6 Cyclohexyl ChlorideAndrea RonquilloNoch keine Bewertungen
- Common Foundation Organic Q in A LevelDokument21 SeitenCommon Foundation Organic Q in A Level黄维燕Noch keine Bewertungen
- Hydrometallurgy Del Cu PDFDokument528 SeitenHydrometallurgy Del Cu PDFAnonymous FfIxH2o9100% (1)
- Practice Paper 10 (Paper 3) - AnsDokument11 SeitenPractice Paper 10 (Paper 3) - Ans黄维燕Noch keine Bewertungen
- Common Foundation Inorganic Q in A LevelDokument17 SeitenCommon Foundation Inorganic Q in A Level黄维燕Noch keine Bewertungen
- Practice Paper 9 (Paper 3) - AnsDokument6 SeitenPractice Paper 9 (Paper 3) - Ans黄维燕Noch keine Bewertungen
- Suggested Soln To Foundation InorganicDokument21 SeitenSuggested Soln To Foundation Inorganic黄维燕Noch keine Bewertungen
- Suggested Soln To Foundation Physical ChemistryDokument29 SeitenSuggested Soln To Foundation Physical Chemistry黄维燕Noch keine Bewertungen
- Common Foundation Physical Q in A Level (Repaired)Dokument39 SeitenCommon Foundation Physical Q in A Level (Repaired)黄维燕Noch keine Bewertungen
- 1 Metallurgy SB 2023Dokument39 Seiten1 Metallurgy SB 2023Bella CakieNoch keine Bewertungen
- Week 1 4 Chem134 Lec ModuleDokument56 SeitenWeek 1 4 Chem134 Lec ModuleMay Ann RiveraNoch keine Bewertungen
- Complet Analyz of Ghori CementDokument3 SeitenComplet Analyz of Ghori CementSarbaz BanozaiNoch keine Bewertungen
- MohsinDokument4 SeitenMohsinWaqas AzizNoch keine Bewertungen
- Amine DPP 02Dokument4 SeitenAmine DPP 02Dharmvir TantyNoch keine Bewertungen
- The Chemistry of Chromyl CompoundsDokument61 SeitenThe Chemistry of Chromyl Compoundsbkoska2005Noch keine Bewertungen
- Is 4737Dokument18 SeitenIs 4737Milagros WieczorekNoch keine Bewertungen
- Chemical Formulae of Ionic CompoundsDokument2 SeitenChemical Formulae of Ionic CompoundsYoung XYeeNoch keine Bewertungen
- The Effect of Substituents on Reactivity and Orientation in EASDokument5 SeitenThe Effect of Substituents on Reactivity and Orientation in EASMatthew Ng100% (1)
- Light Mineral Oil - USP-NFDokument2 SeitenLight Mineral Oil - USP-NFelenitabastos100% (1)
- Chemistry Investigatory Project: Amount of Acetic Acid Present in VinegarDokument14 SeitenChemistry Investigatory Project: Amount of Acetic Acid Present in VinegarM AkshithaNoch keine Bewertungen
- Polystyrene-coated paper feasibility for sustainable packagingDokument3 SeitenPolystyrene-coated paper feasibility for sustainable packagingJobeth Presto AlonzoNoch keine Bewertungen
- LCU Biochemistry Lecture on Water, Acids, and BuffersDokument3 SeitenLCU Biochemistry Lecture on Water, Acids, and BuffersAnn Ross FernandezNoch keine Bewertungen
- Experiments With Ammonium AmalgamDokument162 SeitenExperiments With Ammonium AmalgamangelofgloryNoch keine Bewertungen
- Experiment - Salt Analysis Ammonium AcetateDokument1 SeiteExperiment - Salt Analysis Ammonium AcetateprafullNoch keine Bewertungen
- Aromatic CompoundsDokument30 SeitenAromatic CompoundsMA Masum HossainNoch keine Bewertungen
- KimiaDokument28 SeitenKimiazannubaqotrunnadhaNoch keine Bewertungen
- Textile Wet Processing UNIT-3Dokument7 SeitenTextile Wet Processing UNIT-3Chaarvi SaranyaNoch keine Bewertungen
- SCH3UO Review IdeasDokument4 SeitenSCH3UO Review IdeasAmanjotBrarNoch keine Bewertungen
- Distribution of Acetic Acid Between Two Immiscible Solution by Simple Simple Methods PapooDokument11 SeitenDistribution of Acetic Acid Between Two Immiscible Solution by Simple Simple Methods PapooHasnain SaifiNoch keine Bewertungen
- Super Coolant: Top 1 Oil Products Company Product Data SheetDokument3 SeitenSuper Coolant: Top 1 Oil Products Company Product Data SheetMukhlis Agung PrasetyoNoch keine Bewertungen
- Disintegrants Concentration in Granules (%W/W) Special CommentsDokument3 SeitenDisintegrants Concentration in Granules (%W/W) Special CommentskiranstNoch keine Bewertungen
- Lesson2 Solutions and Their Properties UpdatedDokument52 SeitenLesson2 Solutions and Their Properties Updated68r8x492qgNoch keine Bewertungen
- Lab Report Experiment 2 Determination of Ka Value of A Weak AcidDokument17 SeitenLab Report Experiment 2 Determination of Ka Value of A Weak AcidarisyahariffNoch keine Bewertungen