Beruflich Dokumente
Kultur Dokumente
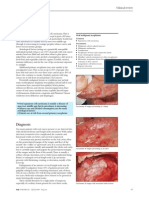
Parotid Mass
Hochgeladen von
Andre Eka Putra PrakosaCopyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Parotid Mass
Hochgeladen von
Andre Eka Putra PrakosaCopyright:
Verfügbare Formate
2006 WebMD, Inc. All rights reserved.
ACS Surgery: Principles and Practice
2 PAROTID MASS 1
2 HEAD AND NECK
PAROTID MASS
Ashok R. Shaha, M.D., F.A.C.S.
Approach to Evaluation of a Parotid Mass
There are three major salivary glands in the human bodythe
parotid gland, the submandibular gland, and the sublingual
glandof which the parotid gland is the largest. In addition, there
are approximately 600 to 800 minor salivary glands distributed
throughout the entire upper aerodigestive tract, starting from the
lip and extending to the lower end of the esophagus and up to the
pulmonary alveoli. Almost half of these minor salivary glands are
on the hard palate. Accordingly, any mass on the hard palate
should be considered a minor salivary gland tumor until proved
otherwise.1
The majority of salivary gland tumors originate in the parotid
gland. Approximately 75% to 80% of parotid tumors are
benign.2,3 In the evaluation and surgical treatment of parotid
tumors, it is essential to maintain awareness of the possibility of
temporary or permanent facial nerve injury [see Discussion, Principles of Facial Reanimation, below]. Because surgery necessarily
entails some risk of an injury to this structure or its branches, as
well as because most parotid masses do not give rise to significant
symptoms, many patients with parotid tumors may find the
prospect of surgical therapy difficult to accept.
Clinical Evaluation
HISTORY
Evaluation of a parotid mass begins
with a good clinical history. The most
important question is, how long has the
mass been present? If local pain and
swelling of recent onset (i.e., within the
past few days) are reported, infection or
obstruction is the likely cause. If the mass has been present for a
longer period (i.e., weeks to months), a neoplasm is more likely.
Unfortunately, the presentations of some nonneoplastic conditions resemble those of neoplasms, and distinguishing one type of
condition from the other can be challenging. Thus, the history
should continue with further questions focusing on local or systemic signs and symptoms, the presence of swelling or other masses in the salivary glands, and previous medical conditions (including skin cancer).
Classification
Major salivary gland masses can be classified as nonneoplastic,
lymphoepithelial, or neoplastic.4
Nonneoplastic The causes of nonneoplastic masses include
congenital, granulomatous, infectious or inflammatory, and noninflammatory conditions. Some congenital lesions (e.g., hemangioma or vascular malformation) present as a vague swelling in the
parotid region that has been present since childhood. One congenital lesion, a first branchial cleft cyst, presents as a mass inferior to the cartilaginous ear canal, with a cyst tract that can be either
medial or lateral to the facial nerve and may even divide the trunk
of the nerve. This cyst is most commonly noted in the second
through fourth decades in life.
Granulomatous diseases are frequently manifested by an
asymptomatic, gradual enlargement of a lymph node within the
gland; often, they cannot be readily distinguished from neoplasms.
In sarcoidosis, salivary gland involvement may cause duct obstruction, pain associated with the duct, xerostomia, or enlargement of
the gland. The diagnosis is supported by chest x-rays that show
bilateral hilar adenopathy and by elevated levels of angiotensinconverting enzyme (ACE).
As noted (see above), infectious or inflammatory diseases
involving the parotid, unlike neoplasms, tend to give rise to pain in
their early stages. Most such inflammatory conditions begin with
diffuse enlargement of one or more salivary glands. Although
parotitis is generally unilateral, it may be bilateral if a systemic
causative condition is involved, and other salivary glands may be
affected as well.The pain reported may be related to the presence
of a stone in the salivary duct or to diffuse obstructive parotitis.
Chronic parotitis may lead to recurrent infection and inflammation.When recurrent swelling of the salivary gland does occur, it is
directly related to eating and increased salivation.
Sialadenosis is a noninflammatory, nonneoplastic condition of
unknown origin that is characterized by diffuse enlargement of the
salivary gland, with no discrete mass or inflammation.
Lymphoepithelial Lymphoepithelial lesions may be divided
into lymphocytic infiltrative diseases and lymphomas. In many
cases, patients with lymphocytic infiltrative diseases (e.g., Mikulicz
disease, sicca complex, and Sjgren syndrome) have had their
conditions for long periods, and they may feel that they have
always been chubby, when in fact they have had chronic enlargement of both parotid glands. In patients with Sjgren syndrome,
malignant transformation to high-grade lymphoma is known to
occur. Lymphoepithelial cysts are benign cystic lesions that may
arise from lymph nodes or from lymphoid aggregates in the salivary gland. These lesions may be associated with HIV infection.
2006 WebMD, Inc. All rights reserved.
ACS Surgery: Principles and Practice
2 PAROTID MASS 2
2 HEAD AND NECK
Approach to Evaluation
of a Parotid Mass
Patient presents with parotid mass
Obtain clinical history.
Perform physical examination of parotid region,
focusing on extent of parotid disease, localized
effects of lesion, and any motor or sensory deficits.
Diffuse enlargement is present
Diagnosis is obvious
Plan treatment (conservative
or surgical, as indicated).
Solitary mass is identified
Diagnosis is uncertain
Initiate further workup:
Imaging (CT, MRI, ultrasonography,
sialography, ?sialoendoscopy, ?PET)
FNA biopsy (routine use is controversial)
Lesion is benign
Lesion is malignant
Treat surgically with superficial
parotidectomy, preserving
facial nerve.
Treat surgically with superficial, total, or
radical parotidectomy, as necessary,
preserving facial nerve if possible.
If cervical lymphadenopathy is present,
consider elective neck dissection
(comprehensive or selective, as appropriate).
Provide postoperative radiotherapy for all
patients except those with T1 or T2 tumors
of low-grade histology and clear margins.
2006 WebMD, Inc. All rights reserved.
2 HEAD AND NECK
Primary lymphoma of the salivary gland is uncommon, occurring in fewer than 5% of patients with parotid masses.5 Suggestive
clinical features include (1) the development of a parotid mass in
a patient with a known history of malignant lymphoma, (2) the
occurrence of a parotid mass in a patient with an immune disorder (e.g., Sjgren syndrome, rheumatoid arthritis, or AIDS), (3)
the presence of a parotid mass in a patient with a previous diagnosis of benign lymphoepithelial lesion, (4) the finding of multiple
masses in one parotid gland or of masses in both parotid glands,
and (5) the association of a parotid mass with multiple enlarged
cervical lymph nodes unilaterally or bilaterally.6
Neoplastic Neoplastic masses may be present for years without causing any symptoms. Benign salivary tumors are more common in younger persons, whereas malignant parotid lesions are
more common in the fifth and sixth decades of life.7 The classic
presentation of a benign parotid tumor is that of an asymptomatic
parotid mass that has been present for months to years. Benign
neoplasms of the parotid include pleomorphic adenoma, basal cell
adenoma, myoepithelioma,Warthin tumor, oncocytoma, and cystadenoma. The observation of rapid growth in a long-standing pleomorphic adenoma is suggestive of malignant transformation. In a
2005 study of 94 patients with pleomorphic adenoma, malignant
transformation to carcinoma was documented in 8.5% of cases.8
Rapid tumor growth, metastasis to lymph nodes, deep fixation,
and facial nerve weakness are all strongly suggestive of malignant
disease and are indicators of a poor prognosis.9 Although pain is
more often experienced by patients with benign conditions, it is also
reported by some patients with infiltrative malignant tumors. In
the latter patients, pain is another indicator of a poor prognosis.10
The presence of facial nerve palsy should raise the index of suspicion for malignancy. Occasionally, patients present with classic
Bell palsy. This condition is usually of viral origin, and most patients recover over time. If Bell palsy persists, however, further investigation is required, including imaging studies to rule out any
obvious parotid lesion. Facial palsy occurring in association with
parotitis and anterior uveitis is known as Heerfordt syndrome and
is often seen in patients with sarcoidosis.
PHYSICAL EXAMINATION
The physical examination should focus on the extent of the disease in the parotid, the neck, and the parapharynx; the localized
effects of the tumor (including trismus); and the motor or sensory deficits resulting from neural invasion.
The mass is palpated to determine whether it is painless or
painful; whether it is soft, firm, hard, or cystic; and whether it is
mobile or fixed to deep tissue or skin.The skin of the scalp, the ear,
and the face is examined for lesions. The neck is palpated for
adenopathy. In the oral cavity, the ducts are examined for discharge or saliva after the glands are milked.The pharyngeal wall is
examined for deviation, and the jaw is examined for trismus.
The parotid occupies a large area, starting from the zygoma and
extending to the upper portion of the neck and behind the
mandible to what is commonly called the tail of the parotid.
Occasionally, the parotid tissue extends behind the earlobe, in
which case it may be misdiagnosed preoperatively as a nonsalivary
pathologic condition. Accessory parotid tissue is present along
Stensons duct in approximately 21% of persons.11 Patients sometimes present with a tumor (most commonly, a benign mixed
tumor) of this accessory tissue.12 Attempts to excise such masses
locally must be avoided, because of the high risk of injury to
branches of the facial nerve.
ACS Surgery: Principles and Practice
2 PAROTID MASS 3
The most common presentation of a parotid mass, whether
benign or malignant, is an asymptomatic swelling in the preauricular or retromandibular region. Occasionally, patients present with
metastases to the intraparotid or periparotid lymph nodes. There
are approximately 17 to 20 lymph nodes in the substance of the
parotid and along its tail, and there may be a smaller number of
lymph nodes within the deep lobe of the gland. These lymph
nodes may be directly affected by metastatic tumors originating
from the anterior scalp or the temporal, periocular, or malar
regions. Especially with elderly patients, it is extremely crucial to
obtain a detailed history of any previously excised skin lesions,
some of which may have been squamous cell carcinomas (SCCs)
that metastasized to the periparotid nodes. Generally, such metastasis involves multiple superficial lymph nodes and presents as diffuse enlargement of the parotid parenchyma. With the massive
involvement of the parotid gland, facial nerve palsy is not uncommon in this setting.The majority of metastases to the parotid gland
derive from cutaneous SCC or melanoma; however, metastatic
spread from the lung, the breast, and the kidney also is known to
occur.13
The location of the parotid mass is a very important diagnostic
factor. The classic presentation of a benign mixed tumor is as a
marblelike lesion in the parotid glanda firm, mobile mass that
commonly originates in the superficial portion of the gland and
generally is not fixed to the deeper structures or to the skin.
Parotid tumors that originate in the deep lobe may present as a
vague, diffuse swelling behind the angle of the mandible; more
often, however, they present as a swelling of the parapharyngeal
area accompanied by medial displacement of the tonsil or the soft
palate.
Although physical examination of a parotid mass is a simple
process in itself, it should be accompanied by a thorough evaluation of the head and neck that includes a detailed examination of
the oral cavity and the oropharyngeal, nasopharyngeal, hypopharyngeal, and laryngopharyngeal areas. Occasionally, a tumor of the
oropharynx presents as cervical lymphadenopathy or as metastatic disease in the tail of the parotid. In such cases, it may be difficult to determine whether the patient has a primary salivary gland
tumor or a metastatic lesion. The presence of any suspicious
pathologic condition in the oropharynx or the base of the tongue
is an indication for appropriate endoscopy and biopsy of the suspected primary site.
Physical findings suggestive of malignancy include a large and
fixed mass, facial nerve weakness, lymph node metastasis, and
skin involvement; patients with advanced parotid malignancies
may present with trismus. Whereas patients with benign parotid
tumors rarely exhibit facial nerve weakness, approximately 12%
to 15% of patients with parotid malignancies have facial nerve
dysfunction at presentation.14 The most common causes of facial
nerve weakness in this setting are adenoid cystic carcinoma,
poorly differentiated carcinoma, and SCC. Primary SCC of the
parotid is quite rare, and a diagnosis of SCC in the gland should
lead one to suspect that a tumor has metastasized to the parotid
lymph nodes. Before the diagnosis of primary SCC of the parotid is made, high-grade mucoepidermoid carcinoma and metastatic SCC must be excluded.
The presence of cervical lymph node metastasis may direct
ones attention to the parotid mass, though only about 20% of persons with parotid malignancies actually have clinically apparent
cervical lymph node metastases at the time of initial presentation.10 The parotid tumors most commonly associated with
metastatic disease to the lymph nodes at presentation are high-
2006 WebMD, Inc. All rights reserved.
ACS Surgery: Principles and Practice
2 PAROTID MASS 4
2 HEAD AND NECK
grade mucoepidermoid carcinoma, poorly differentiated carcinoma, and SCC. Lymph node metastases may also derive from highgrade adenocarcinomas or malignant mixed tumors.
Some patients are totally asymptomatic, with the only significant physical finding being a mass visible through the open mouth,
which is suggestive of either a deep-lobe parotid tumor or a parapharyngeal tumor.The majority of parotid tumors develop within
the superficial lobe of the parotidnot surprisingly, given that
between 80% and 90% of the parotid tissue is superficial to the
facial nerve. However, a significant minority of parotid masses
about 10%are found within the deep lobe. Most deep-lobe
parotid tumors are benign, in which case surgical treatment generally consists of a superficial parotidectomy with dissection and
preservation of the facial nerve, followed by removal of the tumor.
Occasionally, however, malignant deep-lobe parotid tumors do
occur. Such tumors frequently involve the facial nerve, and surgical treatment may require sacrifice of the facial nerve in select circumstances. Most patients who have undergone surgical treatment of a malignant deep-lobe tumor will require postoperative
radiation therapy.
Investigative Studies
The majority of parotid masses can
easily be evaluated with a careful history
and a thorough physical examination.
Nevertheless, it sometimes happens that
even after these measures have been carried out, there remains some clinical
uncertainty regarding whether the pathologic process is of parotid or of nonsalivary origin. A number of nonsalivary tumors (e.g., neurofibromas,
lipomas, lymphadenopathies, metastatic cutaneous lesions, and
lymphomas) are known to present in the parotid region on occasion. These tumors may be difficult to evaluate clinically, and further diagnostic studies may therefore be required.
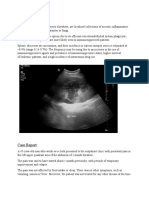
IMAGING
Generally, parotid masses are imaged with either computed
tomography or magnetic resonance imaging.15 CT is superior to
MRI for evaluation of the bony structures, whereas MRI may be
more helpful in distinguishing between inflammatory conditions
and salivary neoplasms. CT scanning is indicated in patients with
diffuse enlargement of the parotid gland, tumor extension beyond
the superficial lobe, facial nerve weakness, trismus, or deep-lobe
parotid tumors that are difficult to evaluate clinically. If the parotid
mass appears to be fixed to the deeper structures, it is appropriate
to proceed with CT to evaluate the extent and parapharyngeal
extension of the disease. MRI is indicated in patients with facial
nerve paralysis. Occasionally, it may be necessary to perform both
CT and MRI. Both imaging methods are helpful and accurate in
distinguishing deep-lobe parotid tumors from other parapharyngeal masses. They are also useful for evaluating suspicious lymph
nodes and the periphery of the mass (specifically with respect to
determining whether the lesion is encapsulated or has irregular
borders).
Ultrasonography can be useful for determining the location of
the lesion and for guiding fine-needle aspiration (FNA) biopsy. In
the past, technetium-99m scanning was commonly employed for
the diagnosis of oncocytoma and Warthin tumor.16
Various other diagnostic studies are available for evaluation of
parotid masses. At one time, sialography was commonly employed
to assess patients with suspected salivary stones, as well as to eval-
uate the ductal arrangements in patients with chronic sialoadenitis. Currently, however, sialography is rarely used, both because
there is a significant possibility of an infectious flare-up and
because the study actually yields only minimal information. In the
early 1980s, CT sialography became popular for a time; however,
it was quite a complicated procedure, and clinicians eventually
found that the information it yielded could easily be obtained from
spiral (helical) CT. Occasionally, surface-coil MRI may be helpful
for evaluating parotid masses deriving from subcutaneous processes. Sialoendoscopy has generated considerable interest, especially
in Europe and Germany17; however, this sophisticated technology
has not yet entered everyday practice. Although sialoendoscopy
may be of some value in the assessment of salivary stone disease
or chronic sialectasis, it is rarely performed in the United States at
present, and its eventual role in the evaluation of parotid masses
remains to be determined.
For a classic benign mixed tumor presenting in the form of a
small nodule in the superficial lobe of the parotid gland, imaging
is not required.
Currently, the role of positron emission tomography (PET) in
the initial evaluation of a parotid mass remains undefined. This
modality may, however, be of some value in the evaluation of suspected recurrent parotid cancers, lymph node metastases, or distant metastases.
FINE-NEEDLE ASPIRATION BIOPSY
Routine use of FNA biopsy in the evaluation of a parotid mass
[see Table 1] continues to be controversial. One reason for the
controversy is that cytologic evaluation is difficult. Another is
that the extent of surgical treatment can easily be defined by
means of the clinical assessment. The main argument against
routine use of FNA biopsy is based on the standard approach to
most parotid masses, which is a superficial parotidectomy that
includes identification and preservation of the facial nerve.
Nevertheless, FNA biopsy can be quite helpful, especially for the
purposes of preoperative consultation with patients regarding
the suspected pathologic process and the extent of surgical therapy. FNA biopsy has an overall accuracy that exceeds 90%,18,19
and it is considered a good investigational tool as long as it is
used in the appropriate clinical context.
In the case of a classic benign mixed tumor, which presents as
a mobile, confined, superficial nodule in the parotid gland, FNA
biopsy is not required for further treatment. However, in cases
where the clinical picture is not definitive and one cannot determine whether the pathologic process is of parotid origin or not,
FNA biopsy is extremely important. Besides distinguishing between benign and malignant conditions, FNA biopsy can also
distinguish between salivary and nonsalivary processes [see Table
Table 1
Uses of FNA Biopsy in Evaluation
of Parotid Mass
To identify suspected malignancy
To diagnose metastatic carcinoma
To identify suspected lymphoma
To distinguish between salivary and nonsalivary lesions
To facilitate conservative management of Warthin tumor or pleomorphic
adenoma in a poor-risk patient
To confirm preoperatively suspected malignancy in a patient with facial
palsy
To evaluate bilateral tumors
2006 WebMD, Inc. All rights reserved.
ACS Surgery: Principles and Practice
2 PAROTID MASS 5
2 HEAD AND NECK
Table 2 Salivary and Nonsalivary Pathologic
Processes Distinguished by FNA Biopsy
Salivary processes
Nonsalivary processes
Benign
Mixed tumor
Warthin tumor
Malignant
Primary salivary gland cancer
Metastatic disease in salivary gland
Cystic adenoma
SCC
Adenocarcinoma
Melanoma
Table 3 American Joint Committee on
Cancer TNM Clinical Classification of Major
Salivary Gland Tumors
TX: Primary tumor cannot be assessed
T0: No evidence of primary tumor
T1: Tumor 2 cm in greatest dimension without
extraparenchymal extension
T2: Tumor > 2 cm but 4 cm in greatest dimension without extraparenchymal extension
T3: Tumor having extraparenchymal extension
without seventh nerve involvement and/or >
4 cm but 6 cm in greatest dimension
T4: Tumor invades base of skull, seventh nerve,
and/or > 6 cm in greatest dimension
Primary tumor (T)
Lipoma
Sebaceous cyst
Lymph node pathology
Benign melanoma
Metastatic cancer
Lymphoma
2].20 A variety of different lymph node pathologies, benign
parotid tumors, and even suspected malignant parotid tumors
can be differentiated by means of FNA biopsy. Furthermore,
lymphoepithelial lesions of the parotid, benign mixed tumors,
and Warthin tumors are easily diagnosed with this procedure.
FNA biopsy is particularly useful when a parotid mass is likely
to be the result of metastasis.
Occasionally, in an elderly person whose overall physical condition is poor, a conservative approach can be taken when a
Warthin tumor is diagnosed by means of FNA biopsy. Similarly,
in a person with a long-standing benign mixed tumor, observation may be appropriate if the patient is not a candidate for surgical intervention and if FNA biopsy confirms that the lesion is
benign.
FNA biopsy findings that suggest lymphoma may help one
avoid unnecessary parotidectomy and risk to the facial nerve.
Often, it is hard to achieve a definitive diagnosis of lymphoma by
means of FNA biopsy alone. In such cases, core biopsy or open
biopsy can be performed to establish the diagnosis. Core biopsy of
a parotid mass is a difficult procedure (unless the mass is very large
and very superficial), and it is generally contraindicated on the
grounds that it may cause bleeding or facial nerve injury. Nevertheless, if core biopsy can be performed safely, it can be a good
choice in cases where lymphoma is suspected on the basis of FNA
biopsy.21 Open incisional biopsy can also be performed safely
when done in conjunction with continuous monitoring of the
facial nerve.22
Benign lymphoepithelial lesions related to HIV disease are
readily diagnosed by means of FNA biopsy, especially if they are
multiply recurrent cystic lesions in the tail of the parotid or if they
occur bilaterally. In general, HIV-related pathology is quite easy to
diagnose with FNA; HIV-infected patients with benign lymphoepithelial lesions may be treated by providing appropriate
management of the underlying illness.
The crucial points for clinicians with respect to FNA biopsy in
the setting of a parotid mass are (1) that this investigative study will
not make a definitive diagnosis of parotid malignancy and (2) that
one therefore should not make decisions about how to manage the
facial nerve solely on the basis of an FNA-suggested malignant
diagnosis. Any decisions regarding the approach to the facial nerve
should also be based on preoperative assessment of facial nerve
function and intraoperative evaluation of the nerve in relation to
the tumor.
NX: Regional lymph nodes cannot be assessed
N0: No regional lymph node metastasis
N1: Metastasis in a single ipsilateral lymph node,
3 cm in greatest dimension
N2: Metastasis in a single ipsilateral lymph node,
> 3 cm but 6 cm in greatest dimension; or
in multiple ipsilateral lymph nodes, none > 6
cm in greatest dimension; or in bilateral or
contralateral lymph nodes, none > 6 cm in
greatest dimension
N2a: Metastasis in a single ipsilateral lymph
node > 3 cm but 6 cm in greatest
dimension
N2b: Metastasis in multiple ipsilateral lymph
nodes, none > 6 cm in greatest
dimension
N2c: Metastasis in bilateral or contralateral
lymph nodes, none > 6 cm in greatest
dimension
N3: Metastasis in a lymph node > 6 cm in greatest dimension
Regional lymph nodes (N)
MX: Distant metastasis cannot be assessed
M0: No distant metastasis
M1: Distant metastasis
Distant metastasis (M)
Staging and Prognosis
For cancers of the parotid gland (as well as those of other major
salivary glands), staging is accomplished by means of the familiar
tumor-node-metastasis (TNM) system developed by the American Joint Committee on Cancer (AJCC) and the International
Union Against Cancer (UICC) [see Tables 3 and 4].23
The prognostic factors in the management of parotid gland
tumors must be understood in relation to the stage the disease has
reached, the need for postoperative radiation therapy, and the
overall long-term outcome [see Table 5]. Such factors include age
Table 4 American Joint Committee on Cancer
Staging System for Major Salivary Gland Tumors
T
Stage
Stage I
T1, T2
N0
M0
Stage II
T3
N0
M0
Stage III
T1, T2
N1
M0
Stage IV
T4
T3, T4
Any T
Any T
N0
N1
N2, N3
Any N
M0
M0
M0
M1
2006 WebMD, Inc. All rights reserved.
ACS Surgery: Principles and Practice
2 PAROTID MASS 6
2 HEAD AND NECK
Table 5
Age at diagnosis
Pain at presentation
T stage
N stage
Skin invasion
Prognostic Factors for Salivary
Gland Tumors
Facial nerve dysfunction
Perineural growth
Positive surgical margins
Soft tissue invasion
Treatment type
at diagnosis, pain at presentation, TNM staging, skin invasion,
facial nerve dysfunction, perineural growth of the primary tumor,
positive surgical margins in the final pathology report, soft tissue
invasion by the primary tumor, treatment type, and extranodal
spread of the metastatic disease in the neck. To make definitive
decisions regarding treatment, it is necessary to analyze these
prognostic factors critically in individual patients. An example of
such critical analysis is the prognostic index devised by the Dutch
Head and Neck Cooperative Group for patients with parotid cancer.24,25 In this index, a weighted combination of the parameters of
age, pain, tumor size, nodal stage, skin invasion, facial nerve dysfunction, perineural growth, and positive surgical margins is
employed to compute a prognostic score. The score is then used
to assign patients to one of four groups, each of which is associated with an expected recurrence-free percentage.
Management
Treatment of a parotid mass depends
on the nature and extent of the lesion [see
Table 6]. Malignant parotid masses are
treated surgically according to established
oncologic principles [see Surgical Therapy,
below], with postoperative radiation therapy added as necessary [see Radiation
Therapy, below]. Generally, benign parotid masses are managed surgically as well, with exploration of the
parotid gland, evaluation of the neoplasm, and appropriate
parotidectomy with identification and preservation of the facial
nerve and its branches. In the case of a suspected benign mixed
tumor, enucleation is contraindicated because of the possibility of
tumor spillage, incomplete removal of the tumor, injury to the
capsule and resultant seeding of the tumor into the parotid tissue,
or inadvertent injury to the branches of the facial nerve. Enucleation of such a tumor is very likely to result in local recurrence, which
invariably is much more difficult to manage than the original
lesion was and poses a high risk of injury to the facial nerve.
SURGICAL THERAPY
Parotidectomy with or without Facial Nerve Reconstruction
The minimum surgical procedure for a parotid mass is superficial parotidectomy with identification and preservation of the
facial nerve [see 2:6 Parotidectomy]. Subtotal parotidectomy may be
required for larger tumors that involve the deep lobe of the parotid
gland; however, true total parotidectomy with preservation of the
facial nerve is almost impossible, because of the parotid gland tissues surrounding the nerve. If the tumor involves the facial nerve
and is directly infiltrating into it, a diagnosis of malignancy should
be explored, and only if the diagnosis is confirmed should the
facial nerve be sacrificed. Preoperative facial nerve weakness generally indicates that the tumor is involving the nerve, in which case
due consideration should be given to the sacrifice and subsequent
reconstruction of this structure.
Reconstruction of the facial nerve can be performed with a
nerve graft from the greater auricular nerve, from the sural
nerve in the leg, or from the ansa hypoglossi. In view of the
technical complexity of nerve grafting, preoperative consultation with a plastic surgeon may be necessary. The functional
results of nerve grafting vary considerably, depending on the
age of the patient, the extent of the disease, and the identification of and appropriate anastomosis to the peripheral branches
of the facial nerve. If postoperative radiation therapy is envisioned, its potential deleterious effects on nerve regeneration
should be kept in mind.
If the tumor involves only an isolated branch of the facial nerve,
one may opt for selective sacrifice of that branch, along with nerve
grafting. If the tumor extends beyond the parotid gland and
involves the infratemporal fossa, the ascending ramus of the
mandible, or the mastoid process, a much more extensive surgical
procedure (e.g., composite resection, lateral temporal bone resection, or radical parotidectomy with sacrifice of the entire facial
nerve) becomes necessary. Such patients invariably require postoperative radiation therapy [see Radiation Therapy, below]. However, in the majority of patients who present with an isolated parotid mass and a functioning facial nerve, every attempt should be
made to preserve the nerve.26 It is rarely necessary to sacrifice a
functioning facial nerve: the only indication for doing so is a situation in which the entire tumor cannot be resected without sacrifice of the main trunk or the branches of the facial nerve and there
is concern about leaving any gross tumor behind.
Intraoperative Frozen-Section Examination
The role of intraoperative frozen-section examination in the
evaluation of a parotid mass, like that of FNA biopsy, is the subject of considerable debate. Nevertheless, frozen-section examination has been found to be useful for distinguishing salivary processes from nonsalivary processes and benign disease from malignant disease. In 80% to 90% of cases, the findings from intraoperative frozen-section examination correlate with the final pathologic diagnosis.27 This study is also helpful in making a definitive
diagnosis of Warthin tumor. If frozen-section examination shows a
benign mixed tumor and this finding agrees with ones clinical
judgment, lateral superficial parotidectomy should be sufficient. If
frozen-section examination shows high-grade mucoepidermoid
carcinoma, selective neck dissection may be considered (mainly
for staging purposes, to determine whether any of the deep jugular nodes are positive). If frozen-section examination provides a
definitive diagnosis of malignancy, further decisions about the
extent of parotidectomy and possible selective neck dissection can
be made accordingly; if not, the procedure originally planned can
be performed, with any further interventions (if required) dictated
by the final pathologic diagnosis. Obviously, the decision whether
to sacrifice the facial nerve must not be based solely on whether
the frozen section shows a benign or a malignant process.
Table 6
Principles of Treatment of Parotid Tumors
Adequate local excision of tumor, based on extent of primary lesion
Preservation of facial nerve if possible
Elective neck dissection reserved for selected patients
Postoperative radiotherapy when indicated (in appropriate fields)
Prognosis determined primarily by stage and grade of tumor
2006 WebMD, Inc. All rights reserved.
ACS Surgery: Principles and Practice
2 PAROTID MASS 7
2 HEAD AND NECK
100
Indications for Postoperative Radiation
Therapy for Parotid Cancer
Aggressive, highly malignant tumor
Invasion of adjacent tissues outside parotid capsule
Regional lymph node metastases
Deep-lobe cancer
Gross residual tumor after resection
Recurrent tumor after resection
Invasion of facial nerve by tumor
75
Percent
Table 7
50
25
Neck Dissection
Overall, about 20% of patients with malignant parotid tumors
present with clinically detectable cervical lymphadenopathy
(cN+).10 There is wide agreement that these patients require either
a comprehensive or a modified neck dissection [see 2:7 Neck
Dissection], depending on the extent of the disease, but there continues to be controversy regarding the management of patients
with salivary malignancies who have no clinically detectable cervical lymphadenopathy (cN0). In one study, approximately 14% of
the patients were in cN+ status; however, approximately 12% were
in cN0 status but presented with pathologically positive nodes
(pN+).28 In view of the low frequency of occult metastasis in the
group as a whole, the investigators generally did not recommend
routine elective treatment of the neck. They did find that certain
histologic grades were associated with a higher incidence of
metastatic disease to the neck nodes: the incidence of occult
metastasis was about 49% in patients with high-grade lesions,
compared with 7% in those with intermediate-grade or low-grade
lesions. In addition, the incidence of having occult metastatic disease was more than 20% in patients with tumors larger than 4 cm,
compared with 4% in those with smaller tumors.
The incidence of lymph node metastasis is affected not only by
the size and histologic grade of the tumor but also by the histologic
type, the primary tumor stage, the presence of facial nerve palsy,
the patients age, the extraparotid extension of the disease, and the
degree of perilymphatic invasion.29 In a multivariate analysis, the
variables that showed the highest correlation with the incidence of
lymph node metastasis were the histologic type (i.e., adenocarcinoma, undifferentiated carcinoma, high-grade mucoepidermoid
carcinoma, SCC, or salivary duct carcinoma) and the T stage.30
Thus, elective neck dissection may be considered in patients
with advanced-stage primary tumors, those whose tumors are of
high histologic grades, and those whose tumors are of certain specific histologic types. A selective neck dissection may be performed
to remove the lymph nodes of the submandibular triangle, level II,
level III, and the upper part of level V for the purposes of staging.29
However, a comprehensive neck dissection that encompasses levels I through V may be necessary in patients who present with clinically obvious cervical metastases.
10
Time (Years)
Stage I, II
Stage I, II with RT
Stage III, IV
Stage III, IV with RT
Figure 1 Depicted is the impact of postoperative radiation therapy
(RT) on overall outcome for patients with stage I or II tumors and
patients with stage III or IV tumors.
RADIATION THERAPY
Patients who present with advanced-stage (stage III or IV) disease, a large primary tumor, close margins, perineural spread, soft
tissue extension, facial nerve dysfunction, or cervical lymph node
metastasis invariably require postoperative radiation therapy [see
Table 7].31,32 In general, this means that postoperative radiation
therapy is indicated for all patients except those with T1 or T2
malignant tumors of low-grade histology and clear margins.Thus,
the decision whether to employ such therapy depends largely on
the intraoperative findings and the final pathology report. A 1990
analysis showed that postoperative radiation therapy had a major
impact on overall outcome in patients with stage III or IV tumors
but conferred no statistically significant benefit on patients with
stage I or II tumors [see Figure 1].33
Currently, there is substantial interest in the potential role of
neutron therapy as a primary treatment modality for parotid
malignancies, especially advanced inoperable parotid cancers and
adenoid cystic carcinomas. A group from the University of
Washington demonstrated that neutron therapy exerted a beneficial effect when used as the sole treatment of advanced parotid
cancers.34 Although these results are extremely promising, the
patients treated with this modality clearly experienced an
increased incidence of complications. Nevertheless, for patients
with inoperable parotid cancer, neutron therapy may be the best
option currently available.
Discussion
Implications of Histopathologic Classification of Parotid
Gland Cancer
Alhough considerable controversy and debate continue to surround the histopathologic classification of malignant parotid
tumors, most clinicians currently prefer either the classification
system of the Armed Forces Institute of Pathology35 or that of the
World Health Organization.36 The most common types of parotid
tumor are mucoepidermoid carcinoma, adenoid cystic carcinoma,
adenocarcinoma, malignant mixed tumor, acinic cell carcinoma,
and primary SCC [see Figure 2].
As noted (see above), primary SCC of the parotid gland is quite
rare, and most diagnoses of parotid SCC represent skin cancer
2006 WebMD, Inc. All rights reserved.
ACS Surgery: Principles and Practice
2 PAROTID MASS 8
2 HEAD AND NECK
cystic carcinoma is low; however, the incidence of distant metastasis (especially pulmonary metastasis) appears to be high.41 It is
noteworthy that even when adenoid cystic carcinoma patients have
pulmonary metastases, they tend to do remarkably well. Quite
often, the metastatic disease in the lungs remains dormant for
months or even years. At present, the role of chemotherapy in the
management of adenoid cystic carcinoma and parotid tumors is
supported only by anecdotal evidence and remains investigational.
Other
Adenocarcinoma
Mucoepidermoid
Adenoid
Cystic
Acinic
Cell
Malignant
Mixed
Squamous
Figure 2 Shown are the approximate relative frequencies of the
most common types of parotid gland tumors.
that has metastasized to the periparotid lymph nodes. Primary
SCC has a high malignant potential, and radical surgical extirpation (with preservation of the facial nerve when possible), followed
by planned postoperative radiotherapy, is the treatment of
choice.37
Mucoepidermoid carcinomas are best divided into low-grade,
intermediate-grade, and high-grade tumors.38 For high-grade
tumors, selective node dissection may be appropriate, with due
consideration given to postoperative radiation therapy. For the
majority of low-grade tumorsprovided that they are properly
excised with appropriate superficial parotidectomypostoperative
radiation therapy is unnecessary, because the incidence of local
recurrence is lower than 5%.
Adenoid cystic carcinoma is a unique salivary gland tumor with
a classic Swiss-cheese appearance under the microscope.There is a
very high incidence of perineural spread with skip metastasis along
the facial nerve and its branches, and the incidence rises with higher T stages.39 The incidence of local recurrence is also very high,
and thus, postoperative radiation therapy should be provided to
patients who are at high risk for relapse (i.e., those with close or
positive margins and those with perineural invasion). Such therapy
appears to reduce the incidence of local recurrence.40 The incidence of cervical lymph node metastasis in patients with adenoid
Principles of Facial Reanimation
Every surgeon who performs parotid surgery, especially surgery
for parotid cancer, should be familiar with the principles of facial
reanimation. If the facial nerve dysfunction is recognized before
the operation, appropriate arrangements may be made for plastic
surgical consultation and facial nerve grafting. If the proximal
stump of the facial nerve can be identified and the peripheral
branches detected at the time of the operation, a nerve graft repair
can be performed. The main donor nerves used in facial nerve
grafting are the greater auricular nerve, the ansa hypoglossi, and
the sural nerve. Of these, the greater auricular nerve is the preferred source for a graft: harvesting is relatively easy, the diameter
matches that of the facial nerve, and the arborization of the distal
branches allows for a greater number of facial nerve grafts.42 The
use of loupes or a microscope helps in the placement of the 9-0
nylon stitches employed in facial nerve grafting.
Regeneration of the facial nerve may take 3 to 6 months.There
is some controversy regarding whether postoperative radiation
therapy has a beneficial effect on functional outcome after nerve
grafting: some studies cite detrimental effects,43 whereas others
report adequate functional outcomes.42,44 In any case, if it is feasible to perform nerve grafting, every attempt should be made to do
so. Currently, hypoglossal nerve transfer is rarely performed,45
because various effective alternatives (e.g., fascia lata slings and
Gore-Tex slings) are available. A consultation with a facial plastic
surgeon may be quite helpful in the management of patients with
total facial nerve palsy.
One of the most important considerations in addressing facial
nerve paralysis is how to prevent exposure keratopathy and other
ocular complications. Placement of a gold weight or a palpebral
spring on the upper eyelid may be considered as a primary rehabilitative measure aimed at preventing corneal ulceration and
opacification.46 In the past, lateral tarsorrhaphy was commonly
employed for this purpose, but currently, as a consequence of the
superior results obtained with the gold weight and the palpebral
spring, it is rarely performed. Other, simpler measures for preventing or minimizing ocular complications include the use of artificial tears and an eye patch during the day and the use of ointment with proper taping to keep the eyelid closed at night.
References
5. Batsakis JG: Primary lymphomas of the major salivary glands. Ann Otol Rhinol Laryngol 95:107,
1986
9. Wong DS: Signs and symptoms of malignant parotid tumours: an objective assessment. J R Coll Surg
Edinb 46:91, 2001
6. Barnes L, Myers EN, Prokopakis EP: Primary malignant lymphoma of the parotid gland. Arch Otolaryngol Head Neck Surg 124:573, 1988
10. Spiro RH, Huvos AG, Strong EWL: Cancer of the
parotid gland: a clinicopathologic study of 288 primary cases. Am J Surg 130:452, 1975
3. Spiro RH: Salivary neoplasms: overview of a 35year experience with 2,807 patients. Head Neck
Surg 8:177, 1986
7. Kane WJ, McCaffrey TV, Olsen KD, et al: Primary
parotid malignancies. A clinical and pathologic review. Arch Otolaryngol Head Neck Surg 117:307,
1991
11. Frommer J:The human accessory parotid gland: its
incidence, nature, and significance. Oral Surg Oral
Med Oral Pathol 43:671, 1977
4. Fu YS, Wenig BM, Abemayor E, et al: Head and
Neck Pathology With Clinical Correlations.
Churchill Livingstone, New York, 2001, p 234
8. Friedrich RE, Li L, Knop J, et al: Pleomorphic adenoma of the salivary glands: analysis of 94 patients.
Anticancer Res 25:1703, 2005
12. Rodino W, Shaha AR: Surgical management of
accessory parotid tumors. J Surg Oncol 54:153,
1993
1. Beckhardt RN,Weber RS, Zane R, et al: Minor salivary gland tumors of the palate: clinical and pathologic correlates of outcome. Laryngoscope 105:1155,
1995
2. Eneroth CM: Salivary gland tumors in the parotid
gland, submandibular gland and the palate region.
Cancer 27:1415, 1971
2006 WebMD, Inc. All rights reserved.
13. Batsakis JG, Bautina E: Metastases to major salivary
glands. Ann Otol Rhinol Laryngol 99:501, 1990
14. OBrien CJ, Fracs MS, Adams JR: Surgical management of the facial nerve in the presence of malignancy about the face. Curr Opin Otolaryngol Head
Neck Surg 9:90, 2001
15. Rabinov JD: Imaging of salivary gland pathology.
Radiol Clin North Am 38:1047, 2000
16. Higashi T, Murahashi H, Ikuta H, et al: Identification of Warthins tumor with technetium-99m pertechnetate. Clin Nucl Med 12:796, 1987
17. Gundlach P, Hopf J, Linnarz M: Introduction of a
new diagnostic procedure: salivary duct endoscopy
(sialendoscopy) clinical evaluation of sialendoscopy,
sialography, and x-ray imaging. Endosc Surg Allied
Technol 2:294, 1994
18. Qizilbash AH, Sianos J,Young JE, et al: Fine needle
aspiration biopsy cytology of major salivary glands.
Acta Cytol 29:503, 1985
19. Stewart CJ, MacKenzie K, McGarry GW, et al:
Fine-needle aspiration cytology of salivary gland: a
review of 341 cases. Diagn Cytopathol 22:139,
2000
20. Shaha AR, Webber C, DiMaio T, et al: Needle aspiration biopsy in salivary gland lesions. Am J Surg
160:373, 1990
21. Kesse KW, Manjaly G,Violaris N, et al: Ultrasoundguided biopsy in the evaluation of focal lesions and
diffuse swelling of the parotid gland. Br J Oral
Maxillofac Surg 40:384, 2002
22. Gross M, Ben-Yaacov A, Rund D, et al: Role of
open incisional biopsy in parotid tumors. Acta Otolaryngol 124:758, 2004
23. AJCC Cancer Staging Manual, 6th edition.
Springer-Verlag, New York, 2002
24. Vander Poorten VL, Balm AJ, Hilgers FJ, et al: The
development of a prognostic score for patients with
parotid carcinoma. Cancer 85:2057, 1999
25. Vander Poorten VL, Hart AA, van der Laan BF, et
al: Prognostic index for patients with parotid carci-
ACS Surgery: Principles and Practice
2 PAROTID MASS 9
2 HEAD AND NECK
noma: external validation using the nationwide
19851994 Dutch Head and Neck Oncology
Cooperative Group database. Cancer 97:1453,
2003
26. Nussbaum M, Bortikner D: Facial nerve preservation in parotid carcinoma. Bull NY Acad Med
62:862, 1986
27. Heller KS, Attie JN, Dubner S: Accuracy of frozen
section in the evaluation of salivary tumors. Am J
Surg 166:424, 1993
28. Armstrong JG, Harrison LB, Thaler HT, et al: The
indications for elective treatment of the neck in cancer of the major salivary glands. Cancer 69:615,
1992
29. Ferlito A, Shaha AR, Rinaldo A, et al: Management
of clinically negative cervical lymph nodes in patients with malignant neoplasms of the parotid
gland. ORL J Otorhinolaryngol Relat Spec 63:123,
2001
30. Regis De Brito Santos I, Kowalski LP, Cavalcante
De Araujo V, et al: Multivariate analysis of risk factors for neck metastases in surgically treated parotid
carcinomas. Arch Otolaryngol Head Neck Surg
127:56, 2001
31. Tullio A, Marchetti C, Sesenna E, et al: Treatment
of carcinoma of the parotid gland: the results of a
multicenter study. J Oral Maxillofac Surg 59:263,
2001
Oncol Biol Phys 27:235, 1993
35. Ellis GL, Auclair PL: Tumors of the Salivary
Glands. Atlas of Tumor Pathology, series 3, fascicle
17. Armed Forces Institute of Pathology, Washington, DC, 1996
36. Seifert G, Sobin LH: The World Health Organizations histological classification of salivary gland
tumors: a commentary on the second edition. Cancer 70:379, 1992
37. Shemen LJ, Huvos AG, Spiro RH: Squamous cell
carcinoma of salivary gland origin. Head Neck Surg
9:235, 1987
38. Batsakis JG, Luna MA: Histopathologic grading of
salivary gland neoplasms: I. Mucoepidermoid carcinomas. Ann Otol Rhinol Laryngol 99:835, 1990
39. Vrielinck LJ, Ostyn F, van Damme B, et al:The significance of perineural spread in adenoid cystic carcinoma of the major and minor salivary glands. Int
J Oral Maxillofac Surg 17:190, 1988
40. Vikram B, Strong EW, Shah JP, et al: Radiation
therapy in adenoid-cystic carcinoma. Int J Radiat
Oncol Biol Phys 10:221, 1984
41. Spiro RH: Distant metastasis in adenoid cystic carcinoma of salivary origin. Am J Surg 174:495, 1997
42. Reddy PG, Arden RL, Mathog RH: Facial nerve
rehabilitation after radical parotidectomy. Laryngoscope 109:894, 1999
32. Harrison LB, Armstrong JG, Spiro RH, et al:
Postoperative radiation therapy for major salivary
gland malignancies. J Surg Oncol 45:52, 1990
43. Pillsbury HC, Fisch U: Extratemporal facial nerve
grafting and radiotherapy. Arch Otolaryngol 105:
441, 1979
33. Armstrong JG, Harrison LB, Spiro RH, et al:
Malignant tumors of major salivary gland origin: a
matched-pair analysis of the role of combined surgery and postoperative radiotherapy. Arch Otolaryngol Head Neck Surg 116:290, 1990
44. McGuirt WF, McCabe BF: Effect of radiation therapy on facial nerve cable autografts. Laryngoscope
87:415, 1977
34. Laramore GE, Krall JM, Griffin TW, et al: Neutron
versus photon irradiation for unresectable salivary
gland tumors: final report of an RTOG-MRC randomized clinical trial. Radiation Therapy Oncology
Group. Medical Research Council. Int J Radiat
45. Conley J, Baker DC: Hypoglossal-facial nerve anastomosis for reinnervation of the paralyzed face. Plast
Reconstr Surg 63:63, 1979
46. Levine RE, Shapiro JP: Reanimation of the paralyzed eyelid with the enhanced palpebral spring or
the gold weight: modern replacements for tarsorrhaphy. Facial Plast Surg 16:325, 2000
Das könnte Ihnen auch gefallen
- English Through 1 PDFDokument282 SeitenEnglish Through 1 PDFLeodan100% (3)
- Advanced Guide For Professional Homeopaths Luc de Schepper.04253 1Dokument24 SeitenAdvanced Guide For Professional Homeopaths Luc de Schepper.04253 1bshowalter_1Noch keine Bewertungen
- Project NewDokument33 SeitenProject Newceeyem83% (12)
- 404 Veterinary Referral Hospital - BrochureDokument11 Seiten404 Veterinary Referral Hospital - BrochureJoanne FagnouNoch keine Bewertungen
- Related NCPDokument5 SeitenRelated NCPLara GatbontonNoch keine Bewertungen
- Malignant Parotid Tumors: Introduction and AnatomyDokument1 SeiteMalignant Parotid Tumors: Introduction and AnatomyCeriaindriasariNoch keine Bewertungen
- Neck Mass ProtocolDokument8 SeitenNeck Mass ProtocolCharlene FernándezNoch keine Bewertungen
- Childhood Malignancies PP - Copy 2Dokument46 SeitenChildhood Malignancies PP - Copy 2ugonna nwokeNoch keine Bewertungen
- Differential Diagnosis of A Neck Mass - UpToDateDokument16 SeitenDifferential Diagnosis of A Neck Mass - UpToDatezzellowknifeNoch keine Bewertungen
- Cervicofacial LymphangiomasDokument11 SeitenCervicofacial LymphangiomasCharmila Sari100% (1)
- The Evaluation and Management of Neck Masses of Unknown EtiologyDokument38 SeitenThe Evaluation and Management of Neck Masses of Unknown EtiologyShaxawan Mahmood AliNoch keine Bewertungen
- Mucosal LesionDokument16 SeitenMucosal LesionMita PrasetyoNoch keine Bewertungen
- An Approach To The Neck Masses: BY: Hardi H. QaderDokument49 SeitenAn Approach To The Neck Masses: BY: Hardi H. QaderEmad AlryashiNoch keine Bewertungen
- Salivary Gland Tumors: 1. AdenomasDokument18 SeitenSalivary Gland Tumors: 1. AdenomasMahammed Ahmed BadrNoch keine Bewertungen
- 6 Neck DissectionDokument9 Seiten6 Neck DissectionAnne MarieNoch keine Bewertungen
- Differential Diagnosis of A Neck Mass - UpToDate PDFDokument16 SeitenDifferential Diagnosis of A Neck Mass - UpToDate PDFDr.Anurag FursuleNoch keine Bewertungen
- ABC - Oral CancerDokument4 SeitenABC - Oral Cancerdewishinta12Noch keine Bewertungen
- Dermatologic Manifestations of Paraneoplastic Syndromes - emedICINEDokument34 SeitenDermatologic Manifestations of Paraneoplastic Syndromes - emedICINEAbdul QuyyumNoch keine Bewertungen
- Jia WeiDokument10 SeitenJia WeiYipno Wanhar El MawardiNoch keine Bewertungen
- Oropharyngeal TumoursDokument8 SeitenOropharyngeal Tumourseldoc13Noch keine Bewertungen
- SGL Case 31 - Study GuideDokument20 SeitenSGL Case 31 - Study GuidebangityNoch keine Bewertungen
- Aruns PL - Ade FinalDokument9 SeitenAruns PL - Ade FinaldrarunsinghNoch keine Bewertungen
- Normal Mediastinal Anatomy, Pathologies and Diagnostic MethodsDokument9 SeitenNormal Mediastinal Anatomy, Pathologies and Diagnostic MethodsJuma AwarNoch keine Bewertungen
- SurgeryDokument47 SeitenSurgerymohamed muhsinNoch keine Bewertungen
- Orphanet Journal of Rare Diseases: CraniopharyngiomaDokument7 SeitenOrphanet Journal of Rare Diseases: CraniopharyngiomaRiri KumalaNoch keine Bewertungen
- Parotid Gland NeoplasmDokument107 SeitenParotid Gland NeoplasmigorNoch keine Bewertungen
- Tumours of Thyroid GlandDokument28 SeitenTumours of Thyroid Glandjenny girlNoch keine Bewertungen
- Vascular TumorsDokument10 SeitenVascular Tumorscata2082Noch keine Bewertungen
- Extra Oral ExaminationDokument19 SeitenExtra Oral ExaminationmarwanNoch keine Bewertungen
- An Approach To Diagnosis: Neck LumpDokument36 SeitenAn Approach To Diagnosis: Neck LumpTracy WheelerNoch keine Bewertungen
- Juvenile Nasopharyngeal Angiofibroma (JNA)Dokument19 SeitenJuvenile Nasopharyngeal Angiofibroma (JNA)YogiHadityaNoch keine Bewertungen
- Jurnal Patologi AnatomiDokument5 SeitenJurnal Patologi Anatomiafiqzakieilhami11Noch keine Bewertungen
- Practice Essentials: View Media GalleryDokument8 SeitenPractice Essentials: View Media GallerywzlNoch keine Bewertungen
- Lateral Neck SwellingDokument62 SeitenLateral Neck Swellingசுபத்ரா சந்திரசேகர்Noch keine Bewertungen
- Solitary Nodules Are Most Likely To Be Malignant in Patients Older Than 60 Years and in Patients Younger Than 30 YearsDokument5 SeitenSolitary Nodules Are Most Likely To Be Malignant in Patients Older Than 60 Years and in Patients Younger Than 30 YearsAbdurrahman Afa HaridhiNoch keine Bewertungen
- Adult Neck MassesDokument7 SeitenAdult Neck MassesHanhan90Noch keine Bewertungen
- Paraneoplastic DermatosesDokument57 SeitenParaneoplastic DermatosesMohamed Riyaz100% (1)
- Lecture 21 Epithelial PathoDokument10 SeitenLecture 21 Epithelial PathoSRO oONoch keine Bewertungen
- Case Study in OrthoDokument15 SeitenCase Study in OrthoDaryl Rojas Gabriel QuindiaganNoch keine Bewertungen
- Background MED SCAPEDokument13 SeitenBackground MED SCAPEEva Marini SimbolonNoch keine Bewertungen
- Cervical Lymph NodeDokument4 SeitenCervical Lymph NodeHermawan HmnNoch keine Bewertungen
- Esophageal Cancer Esophageal Cancer (Or Oesophageal Cancer) Is Malignancy of The Esophagus. There Are Various SubtypesDokument6 SeitenEsophageal Cancer Esophageal Cancer (Or Oesophageal Cancer) Is Malignancy of The Esophagus. There Are Various Subtypesphvega06Noch keine Bewertungen
- Sheet 11 PDFDokument16 SeitenSheet 11 PDFHanin AbukhiaraNoch keine Bewertungen
- ODMR Short NotesDokument117 SeitenODMR Short NotessamikshaNoch keine Bewertungen
- PROPTOSISDokument26 SeitenPROPTOSISAhmad fayazNoch keine Bewertungen
- Cystic HygromaDokument5 SeitenCystic HygromaAde Triansyah EmsilNoch keine Bewertungen
- Why Does My Patient Limphadenopathy or Splenomegaly?Dokument14 SeitenWhy Does My Patient Limphadenopathy or Splenomegaly?lacmftcNoch keine Bewertungen
- Grand Rounds Index UTMB Otolaryngology Home PageDokument9 SeitenGrand Rounds Index UTMB Otolaryngology Home PageandiNoch keine Bewertungen
- Oral Cancer MADE EASYDokument19 SeitenOral Cancer MADE EASYMohammedNoch keine Bewertungen
- NCP For Laryngeal CancerDokument5 SeitenNCP For Laryngeal CancerMădălina PinciucNoch keine Bewertungen
- Approach To Patients With LymphadenopathyDokument5 SeitenApproach To Patients With LymphadenopathyAngela Mitchelle NyanganNoch keine Bewertungen
- Testicular TumorsDokument6 SeitenTesticular Tumorsyoussef.aziz2020Noch keine Bewertungen
- 1868-Article Text-6804-1-10-20180620Dokument3 Seiten1868-Article Text-6804-1-10-20180620Wina ViqaNoch keine Bewertungen
- Radiology Case Report - Splenic AbscessDokument6 SeitenRadiology Case Report - Splenic AbscessAbeebNoch keine Bewertungen
- CASE ANALYSIS - Nasopharyngeal CancerDokument8 SeitenCASE ANALYSIS - Nasopharyngeal CancerTerry Mae Atilazal SarciaNoch keine Bewertungen
- Benign Extrapleural Solitary Fibrous Tumorofthe Headand NeckDokument7 SeitenBenign Extrapleural Solitary Fibrous Tumorofthe Headand NeckCara Danielle PabellanoNoch keine Bewertungen
- EARLY RECOGNITION TO DETECT THE MALIGNANCY IN CHILDREN - Dr. ElmiDokument8 SeitenEARLY RECOGNITION TO DETECT THE MALIGNANCY IN CHILDREN - Dr. ElmiElmi AstrabelNoch keine Bewertungen
- Brain Abscess MimicsDokument16 SeitenBrain Abscess MimicsRikizu HobbiesNoch keine Bewertungen
- Nonsquamous Cell Malignant Tumours of The Oral Cavity: An OverviewDokument6 SeitenNonsquamous Cell Malignant Tumours of The Oral Cavity: An OverviewranggadrNoch keine Bewertungen
- US Clínica de Glándulas Salivales 2009Dokument28 SeitenUS Clínica de Glándulas Salivales 2009Lucila BrañaNoch keine Bewertungen
- Nasopharyngeal CarcinomaDokument25 SeitenNasopharyngeal Carcinomananda surastyo100% (1)
- (Fig. 17.3, A) : 3. Adenoma-Carcinoma Sequence Evolves From Pre-Existing Adenomas, Referred To AsDokument31 Seiten(Fig. 17.3, A) : 3. Adenoma-Carcinoma Sequence Evolves From Pre-Existing Adenomas, Referred To AsdystoNoch keine Bewertungen
- Salivary Gland Cancer: From Diagnosis to Tailored TreatmentVon EverandSalivary Gland Cancer: From Diagnosis to Tailored TreatmentLisa LicitraNoch keine Bewertungen
- Atlas of Diagnostically Challenging Melanocytic NeoplasmsVon EverandAtlas of Diagnostically Challenging Melanocytic NeoplasmsNoch keine Bewertungen
- Hypertension in 2017-What Is The Right Target JAMA 2017Dokument2 SeitenHypertension in 2017-What Is The Right Target JAMA 2017JulianNoch keine Bewertungen
- Interventional Procedures and Future Drug Therapy For HypertensionDokument14 SeitenInterventional Procedures and Future Drug Therapy For HypertensionAndre Eka Putra PrakosaNoch keine Bewertungen
- The Use of Vasopressor and Inotropes in The Emergency Departement For The Treatment of ShockDokument36 SeitenThe Use of Vasopressor and Inotropes in The Emergency Departement For The Treatment of ShockAndre Eka Putra PrakosaNoch keine Bewertungen
- Hypertension in 2017-What Is The Right Target JAMA 2017Dokument2 SeitenHypertension in 2017-What Is The Right Target JAMA 2017JulianNoch keine Bewertungen
- Sepsis-Induced Myocardial Dysfunction Pathophysiology and Management PDFDokument10 SeitenSepsis-Induced Myocardial Dysfunction Pathophysiology and Management PDFAndre Eka Putra PrakosaNoch keine Bewertungen
- Rhythm Control Versus Rate Control For Atrial Fibrillation and Heart FailureDokument11 SeitenRhythm Control Versus Rate Control For Atrial Fibrillation and Heart FailureAndre Eka Putra PrakosaNoch keine Bewertungen
- Burnout SyndromeDokument2 SeitenBurnout SyndromeAlina NeagoeNoch keine Bewertungen
- Hepatorenal Syndrome Current Diagnostic and Therapeutic ConceptsDokument3 SeitenHepatorenal Syndrome Current Diagnostic and Therapeutic ConceptsAndre Eka Putra PrakosaNoch keine Bewertungen
- 5 - Population and SampleDokument13 Seiten5 - Population and SampleAndre Eka Putra Prakosa100% (1)
- Hormones Drive in Normal LaborDokument6 SeitenHormones Drive in Normal LaborAndre Eka Putra PrakosaNoch keine Bewertungen
- A Review of Nebivolol Pharmacology and Clinical Evidence: Key PointsDokument23 SeitenA Review of Nebivolol Pharmacology and Clinical Evidence: Key PointsAndre Eka Putra PrakosaNoch keine Bewertungen
- Dabigatran Versus Warfarin in Patients With Atrial Fibrillation PDFDokument13 SeitenDabigatran Versus Warfarin in Patients With Atrial Fibrillation PDFAndre Eka Putra PrakosaNoch keine Bewertungen
- Coronary Heart Disease and Sexual ActivityDokument6 SeitenCoronary Heart Disease and Sexual ActivityAndre Eka Putra PrakosaNoch keine Bewertungen
- Drugs That May Cause or Exacerbate Heart Failure A Scientific Statement From The American Heart AssociationDokument39 SeitenDrugs That May Cause or Exacerbate Heart Failure A Scientific Statement From The American Heart AssociationAndre Eka Putra PrakosaNoch keine Bewertungen
- Partisipan Dan Saluran Bisnis PemasaranDokument4 SeitenPartisipan Dan Saluran Bisnis PemasaranAndre Eka Putra PrakosaNoch keine Bewertungen
- Referat PediatriDokument2 SeitenReferat PediatriRestik Anggada PratamaNoch keine Bewertungen
- Jadwal Jaga IGDDokument3 SeitenJadwal Jaga IGDAndre Eka Putra PrakosaNoch keine Bewertungen
- Cancer GovDokument47 SeitenCancer GovAndre Eka Putra PrakosaNoch keine Bewertungen
- Daftar Pustaka Tumor ParotisDokument2 SeitenDaftar Pustaka Tumor ParotisAndre Eka Putra PrakosaNoch keine Bewertungen
- Practical 12. ReuploadDokument13 SeitenPractical 12. Reuploadhawaalia534Noch keine Bewertungen
- Usg Fast 3Dokument12 SeitenUsg Fast 3Andre Eka Putra PrakosaNoch keine Bewertungen
- Usg Fast 3Dokument12 SeitenUsg Fast 3Andre Eka Putra PrakosaNoch keine Bewertungen
- DR - Sapta FarmasiDokument1 SeiteDR - Sapta FarmasiAndre Eka Putra PrakosaNoch keine Bewertungen
- Physics, Physiology and Medicine of Diving PDFDokument30 SeitenPhysics, Physiology and Medicine of Diving PDFAndre Eka Putra Prakosa100% (1)
- Physics, Physiology and Medicine of Diving PDFDokument30 SeitenPhysics, Physiology and Medicine of Diving PDFAndre Eka Putra Prakosa100% (1)
- Eula Microsoft Visual StudioDokument3 SeitenEula Microsoft Visual StudioqwwerttyyNoch keine Bewertungen
- Nitrox Physiology OHP PDFDokument25 SeitenNitrox Physiology OHP PDFAndre Eka Putra PrakosaNoch keine Bewertungen
- Nitrox Physiology OHP PDFDokument25 SeitenNitrox Physiology OHP PDFAndre Eka Putra PrakosaNoch keine Bewertungen
- 301 Full PDFDokument7 Seiten301 Full PDFAndre Eka Putra PrakosaNoch keine Bewertungen
- Pharmacology MCQsDokument88 SeitenPharmacology MCQsDr.Tawheed86% (7)
- 20IB314 Bayer Corporation Vs Union of IndiaDokument12 Seiten20IB314 Bayer Corporation Vs Union of IndiaApoorv TyagiNoch keine Bewertungen
- Underground Clinical Vignettes BiochemistryDokument128 SeitenUnderground Clinical Vignettes Biochemistrymartha2112Noch keine Bewertungen
- 2017 Written Mock Exam - Questions and AnswersDokument86 Seiten2017 Written Mock Exam - Questions and AnswersVictoria V. Dela CruzNoch keine Bewertungen
- PI MaltoferDokument15 SeitenPI MaltoferrazhossenNoch keine Bewertungen
- (Leon Gordis) Epidemiology (4th Edition)Dokument26 Seiten(Leon Gordis) Epidemiology (4th Edition)Anna Dixon100% (1)
- 2nd Announcement 18th ASNO Bali 2023Dokument21 Seiten2nd Announcement 18th ASNO Bali 2023Dayu Triana RahmawatiNoch keine Bewertungen
- m146 PDFDokument697 Seitenm146 PDFspgoniNoch keine Bewertungen
- Cancer and Oncology - NCLEX Quiz 152 - ProProfs QuizDokument6 SeitenCancer and Oncology - NCLEX Quiz 152 - ProProfs QuizKyla Mae JumaritoNoch keine Bewertungen
- Monoclonal Antibodies: Molecular Pathology June 2000Dokument8 SeitenMonoclonal Antibodies: Molecular Pathology June 2000AnasNoch keine Bewertungen
- Gynecology 1.1 - History-Taking, Physical Examination, and Office GynecologyDokument9 SeitenGynecology 1.1 - History-Taking, Physical Examination, and Office GynecologyZaza100% (2)
- Cancer Prediction and Prognosis Using Machine Learning TechniquesDokument5 SeitenCancer Prediction and Prognosis Using Machine Learning TechniquesInternational Journal of Innovative Science and Research TechnologyNoch keine Bewertungen
- Chemical Peels in Aesthetic Dermatology - An Update 2009 PDFDokument12 SeitenChemical Peels in Aesthetic Dermatology - An Update 2009 PDFjujuNoch keine Bewertungen
- Yang ShengDokument62 SeitenYang ShengRedbaron88Noch keine Bewertungen
- 1 s2.0 S1040842801002141 MainDokument22 Seiten1 s2.0 S1040842801002141 MainHayat BouchoumNoch keine Bewertungen
- Air PollutionDokument15 SeitenAir PollutionLusineNoch keine Bewertungen
- MeningiomaDokument7 SeitenMeningiomadrhendyjuniorNoch keine Bewertungen
- Toxoplasma Pneumocystis Microsporidia BabesiaDokument40 SeitenToxoplasma Pneumocystis Microsporidia BabesiadhaineyNoch keine Bewertungen
- Liver PathologyDokument16 SeitenLiver PathologyMuhammad Salman100% (1)
- Ring Enhancing LesionsDokument50 SeitenRing Enhancing LesionsVivek GuptaNoch keine Bewertungen
- Microwaves and Children at School - Barrie Trower TestimonyDokument23 SeitenMicrowaves and Children at School - Barrie Trower Testimonyoinotnaissab100% (1)
- Melanoma: NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines)Dokument154 SeitenMelanoma: NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines)Ilham PermanaNoch keine Bewertungen
- Breast AssessmentDokument2 SeitenBreast AssessmentHNoch keine Bewertungen
- The Global Impact of Respiratory Disease ESDokument48 SeitenThe Global Impact of Respiratory Disease ESKevin100% (1)
- Final Year QN Papers (1990-2017)Dokument37 SeitenFinal Year QN Papers (1990-2017)vardhan hhh100% (4)