Beruflich Dokumente
Kultur Dokumente
Statistical Molecular Thermodynamics: Christopher J. Cramer
Hochgeladen von
adiaz0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
15 Ansichten5 SeitenTermo videos
Originaltitel
ThermoVid_2_08
Copyright
© © All Rights Reserved
Verfügbare Formate
PDF, TXT oder online auf Scribd lesen
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenTermo videos
Copyright:
© All Rights Reserved
Verfügbare Formate
Als PDF, TXT herunterladen oder online auf Scribd lesen
0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
15 Ansichten5 SeitenStatistical Molecular Thermodynamics: Christopher J. Cramer
Hochgeladen von
adiazTermo videos
Copyright:
© All Rights Reserved
Verfügbare Formate
Als PDF, TXT herunterladen oder online auf Scribd lesen
Sie sind auf Seite 1von 5
Statistical Molecular
Thermodynamics
Christopher J. Cramer
Video 2.8
Review of Week 2
Critical Concepts from Week 2
The ideal gas equa-on of state is PV=nRT
The compressibility Z is dened as PV / RT
Gases are rarely ideal (only at very low pressure)
The
van
der
Waals
equa-on
of
state
be?er
predicts
non-ideal
gas
behavior,
as
do
the
Redlich-Kwong
and
Peng-Robinson
equa-ons
All cubic equa-ons of state are capable of describing the liquid
and gaseous regions of PV isotherms
At
the
cri-cal
point,
the
vdW
EOS
has
only
one
root,
which
permits
the
direct
rela-on
of
the
cri-cal
volume,
pressure
and
temperature
in
terms
of
the
vdW
constants
a
and
b.
This
is
how
a
and
b
are
determined
experimentally
Critical Concepts from Week 2
The
Law
of
Corresponding
States
says
that
all
gases
have
the
same
proper-es
if
compared
at
corresponding
condi-ons
Corresponding condi-ons means rela-ve to their respec-ve
cri-cal proper-es
The virial expansion is an equa-on of state whose coecients
relate directly to intermolecular interac-ons
The second virial coecient B2v measures the devia-on of the
volume of a real gas compared to an ideal one under the same
temperature and pressure condi-ons
Critical Concepts from Week 2
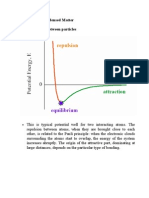
Lennard-Jones proposed an expression for the intermolecular
poten-al u(r) in terms of the strength of molecular interac-ons ()
and the size of the molecules ()
The Lennard-Jones poten-al has an r6 a?rac-ve term and an r12
repulsive term
Lennard-Jones parameters (,) can be determined from
experimental B2v values
London developed a quantum mechanical explana-on for
dispersion and derived an equa-on predic-ng its magnitude
based on atomic (or molecular) ioniza-on poten-als and
polarizabili-es
Critical Concepts from Week 2
A square-well poten-al permits analy-c solu-on of the integral
that determines B2V(T) as does a hard-sphere poten-al
The hard-sphere poten-al is appropriate only for gases at high
temperatures (where B2V(T) is posi-ve and eec-vely constant)
The square-well poten-al, with its addi-onal free parameter
that dictates the length over which a constant poten-al is
a?rac-ve, leads to fairly good es-mates for values over a broader
range of temperatures and pressures
Das könnte Ihnen auch gefallen
- Equation of State PDFDokument84 SeitenEquation of State PDFAnubhav SinghNoch keine Bewertungen
- Chapter 1Dokument5 SeitenChapter 1nisa_121babypinkNoch keine Bewertungen
- Virial Equation of StateDokument9 SeitenVirial Equation of StateSaba ArifNoch keine Bewertungen
- Properties of Gases: Kinetic Molecular Model to Van der Waals EquationDokument38 SeitenProperties of Gases: Kinetic Molecular Model to Van der Waals Equationmahmoud50% (2)
- Ideal and Real GasesDokument4 SeitenIdeal and Real GasesT Hari PrasadNoch keine Bewertungen
- Equations of State for Real Gases: Van der Waals and Virial ModelsDokument19 SeitenEquations of State for Real Gases: Van der Waals and Virial ModelsShamim khanNoch keine Bewertungen
- 5 - CH 5 - EOSDokument70 Seiten5 - CH 5 - EOSAsshole100% (1)
- Experiment 4Dokument21 SeitenExperiment 4Krizz AstorgaNoch keine Bewertungen
- Gas Laws Explained: PV=nRT and MoreDokument25 SeitenGas Laws Explained: PV=nRT and MoreAbhishek ChakrabartiNoch keine Bewertungen
- Phys Chem II Gas Laws Lecture Notes - 230727 - 114428Dokument71 SeitenPhys Chem II Gas Laws Lecture Notes - 230727 - 114428Tshiamo MotaungNoch keine Bewertungen
- Review of Simple Forms of Equations of State: Ali Kh. Al-MatarDokument24 SeitenReview of Simple Forms of Equations of State: Ali Kh. Al-MatarAriel100% (1)
- Equations of State PDFDokument3 SeitenEquations of State PDFRoozbeh PNoch keine Bewertungen
- Unit 1Dokument17 SeitenUnit 1sumirNoch keine Bewertungen
- Unit II Phase Behaviour of HydrocarbonsDokument68 SeitenUnit II Phase Behaviour of HydrocarbonsVishal JadhavNoch keine Bewertungen
- Non-Ideal Gases 3Dokument26 SeitenNon-Ideal Gases 3AlanNoch keine Bewertungen
- Satish Chandra: Unit - I, Real GasesDokument6 SeitenSatish Chandra: Unit - I, Real GasesSanchari BiswasNoch keine Bewertungen
- Chemistry Chapter5 Class 11Dokument43 SeitenChemistry Chapter5 Class 11Ravinder singhNoch keine Bewertungen
- 5 - Behaviour of GasesDokument37 Seiten5 - Behaviour of Gasessiaskel100% (1)
- Dr. J. VenkatesanDokument56 SeitenDr. J. VenkatesanRajesh KumarNoch keine Bewertungen
- Class 11 Chemistry Chapter 5 Study MaterialDokument33 SeitenClass 11 Chemistry Chapter 5 Study MaterialmeghaNoch keine Bewertungen
- Ideal and Real GasesDokument90 SeitenIdeal and Real GasesShubham KanungoNoch keine Bewertungen
- Kinetic Molecular Theory of Gases ExplainedDokument12 SeitenKinetic Molecular Theory of Gases ExplainedMd Al AminNoch keine Bewertungen
- Equations of StateDokument33 SeitenEquations of StateDevika BharathanNoch keine Bewertungen
- Equations of State Explained in 7 ConceptsDokument26 SeitenEquations of State Explained in 7 ConceptsErlangga AriaNoch keine Bewertungen
- Wa0001.Dokument31 SeitenWa0001.Stefani KavangoNoch keine Bewertungen
- Real GasesDokument23 SeitenReal GasesfrankizztaNoch keine Bewertungen
- Basic Introduction To Gas-Condensate ReservoirDokument27 SeitenBasic Introduction To Gas-Condensate ReservoirMyatNoch keine Bewertungen
- Equation of State - WikipediaDokument78 SeitenEquation of State - WikipediaPrecious OluwadahunsiNoch keine Bewertungen
- Single Phase Diagram - Real Gases - 003Dokument36 SeitenSingle Phase Diagram - Real Gases - 003JatskinesisNoch keine Bewertungen
- Chapter 9 Gases After-Lecture-NoteDokument30 SeitenChapter 9 Gases After-Lecture-NoteElvis ChanNoch keine Bewertungen
- Gaseous State: Ideal GasesDokument13 SeitenGaseous State: Ideal GasesM.S.A. Mobashwer HossenNoch keine Bewertungen
- Behaviour of GasesDokument29 SeitenBehaviour of GasesAli RazaNoch keine Bewertungen
- Physical Chemistry Chapter 1 Properties of GasesDokument14 SeitenPhysical Chemistry Chapter 1 Properties of GasesTiara ElsinitaNoch keine Bewertungen
- Development of Eos For Vapours & Gases: Models For Highly Bountiful Phase .. .Dokument28 SeitenDevelopment of Eos For Vapours & Gases: Models For Highly Bountiful Phase .. .Muket AgmasNoch keine Bewertungen
- Equation of State: From Wikipedia, The Free EncyclopediaDokument11 SeitenEquation of State: From Wikipedia, The Free EncyclopediaPatricia de LeonNoch keine Bewertungen
- States of Matter (Gas)Dokument27 SeitenStates of Matter (Gas)Siti NuraqidahNoch keine Bewertungen
- Fundamentals of Fluid MechanicsDokument45 SeitenFundamentals of Fluid MechanicsRN Builder IpohNoch keine Bewertungen
- EOS For Real Gas FinalDokument17 SeitenEOS For Real Gas FinalrutvikNoch keine Bewertungen
- Kinetic Molecular Theory and Maxwell's Distribution of VelocitiesDokument40 SeitenKinetic Molecular Theory and Maxwell's Distribution of VelocitiesNandan PomalNoch keine Bewertungen
- Experimental determination of gas constant R (R=0.08656Dokument3 SeitenExperimental determination of gas constant R (R=0.08656Janiel Arlan OmboyNoch keine Bewertungen
- 5.states of Matter: Some Important Points and Terms of The ChapterDokument6 Seiten5.states of Matter: Some Important Points and Terms of The ChapterMUSTAFA DOSANINoch keine Bewertungen
- Multiphase Systems - Part IDokument20 SeitenMultiphase Systems - Part I랄뚜기Noch keine Bewertungen
- Thermodynamic Property RelationsDokument26 SeitenThermodynamic Property RelationsSec CNoch keine Bewertungen
- Real Gases October 2022Dokument15 SeitenReal Gases October 2022Victor MbowuraNoch keine Bewertungen
- Kinetic Theory of Gases ExplainedDokument14 SeitenKinetic Theory of Gases ExplainedAnu Radha100% (2)
- 5.1 Pressure: Chapter 5: GasesDokument4 Seiten5.1 Pressure: Chapter 5: GasesSam ChungNoch keine Bewertungen
- Gases: Theodore L. Brown H. Eugene Lemay, Jr. and Bruce E. BurstenDokument28 SeitenGases: Theodore L. Brown H. Eugene Lemay, Jr. and Bruce E. BurstenAmalia AnggreiniNoch keine Bewertungen
- States of MatterDokument39 SeitenStates of MatterKRISH RAWATNoch keine Bewertungen
- Mechnotes: Unit - 1 ObjectiveDokument25 SeitenMechnotes: Unit - 1 ObjectiveKaran SelvaNoch keine Bewertungen
- Classical Mechanics FormulaDokument20 SeitenClassical Mechanics FormulaMehdi KhouadriNoch keine Bewertungen
- 9.pertemuan 6. GasesDokument28 Seiten9.pertemuan 6. GasesMUHAMMAD HEFFIQRI RIADYNoch keine Bewertungen
- Chem 14 Laboratory Report Van Der Waals Isotherms 5 PDF FreeDokument6 SeitenChem 14 Laboratory Report Van Der Waals Isotherms 5 PDF FreePedro Ian QuintanillaNoch keine Bewertungen
- 11) Gas Laws - Second Edition_1551343848Dokument9 Seiten11) Gas Laws - Second Edition_1551343848ungaranigundla sachivalayamNoch keine Bewertungen
- REN5415 Y19 Lec11&12Dokument69 SeitenREN5415 Y19 Lec11&12Abdulla MohammadNoch keine Bewertungen
- CH 353 Problem Set 1Dokument3 SeitenCH 353 Problem Set 1jon jonNoch keine Bewertungen
- Lecture 11 BD in GasesDokument24 SeitenLecture 11 BD in GasesSyed KumailNoch keine Bewertungen
- Working Guide to Vapor-Liquid Phase Equilibria CalculationsVon EverandWorking Guide to Vapor-Liquid Phase Equilibria CalculationsBewertung: 5 von 5 Sternen5/5 (1)
- Electrochemical Kinetics: Theoretical AspectsVon EverandElectrochemical Kinetics: Theoretical AspectsBewertung: 5 von 5 Sternen5/5 (1)
- Manual Aspen Hysys - Part40 PDFDokument1 SeiteManual Aspen Hysys - Part40 PDFadiazNoch keine Bewertungen
- Statistical Molecular Thermodynamics: Christopher J. CramerDokument7 SeitenStatistical Molecular Thermodynamics: Christopher J. CrameradiazNoch keine Bewertungen
- Statistical Molecular Thermodynamics: Christopher J. CramerDokument8 SeitenStatistical Molecular Thermodynamics: Christopher J. CrameradiazNoch keine Bewertungen
- Statistical Molecular Thermodynamics: Christopher J. CramerDokument5 SeitenStatistical Molecular Thermodynamics: Christopher J. CrameradiazNoch keine Bewertungen
- Statistical Molecular Thermodynamics - The Partition Function and Boltzmann PopulationDokument8 SeitenStatistical Molecular Thermodynamics - The Partition Function and Boltzmann PopulationadiazNoch keine Bewertungen
- Statistical Molecular Thermodynamics: Christopher J. CramerDokument4 SeitenStatistical Molecular Thermodynamics: Christopher J. CrameradiazNoch keine Bewertungen
- Statistical Molecular Thermodynamics: Christopher J. CramerDokument7 SeitenStatistical Molecular Thermodynamics: Christopher J. CrameradiazNoch keine Bewertungen
- Statistical Molecular Thermodynamics: Molecular InteractionsDokument8 SeitenStatistical Molecular Thermodynamics: Molecular InteractionsadiazNoch keine Bewertungen
- Molecular Partition Function and Relationship Between Q and qDokument6 SeitenMolecular Partition Function and Relationship Between Q and qadiazNoch keine Bewertungen
- Statistical Molecular Thermodynamics Ideal Gas Internal EnergyDokument7 SeitenStatistical Molecular Thermodynamics Ideal Gas Internal EnergyadiazNoch keine Bewertungen
- Statistical Molecular Thermodynamics - Monatomic Ideal Gas Heat Capacity (39Dokument7 SeitenStatistical Molecular Thermodynamics - Monatomic Ideal Gas Heat Capacity (39adiazNoch keine Bewertungen
- Statistical Molecular Thermodynamics: Christopher J. CramerDokument7 SeitenStatistical Molecular Thermodynamics: Christopher J. CrameradiazNoch keine Bewertungen
- Statistical Molecular Thermodynamics - Virial ExpansionDokument6 SeitenStatistical Molecular Thermodynamics - Virial ExpansionadiazNoch keine Bewertungen
- Statistical Molecular Thermodynamics: Christopher J. CramerDokument8 SeitenStatistical Molecular Thermodynamics: Christopher J. CrameradiazNoch keine Bewertungen
- Statistical Molecular Thermodynamics: Christopher J. CramerDokument7 SeitenStatistical Molecular Thermodynamics: Christopher J. CrameradiazNoch keine Bewertungen
- Statistical Molecular Thermodynamics: Christopher J. CramerDokument5 SeitenStatistical Molecular Thermodynamics: Christopher J. CrameradiazNoch keine Bewertungen
- Statistical Molecular Thermodynamics: Christopher J. CramerDokument5 SeitenStatistical Molecular Thermodynamics: Christopher J. CrameradiazNoch keine Bewertungen
- Statistical Molecular Thermodynamics: Christopher J. CramerDokument5 SeitenStatistical Molecular Thermodynamics: Christopher J. CrameradiazNoch keine Bewertungen
- Statistical Molecular Thermodynamics: Christopher J. CramerDokument6 SeitenStatistical Molecular Thermodynamics: Christopher J. CrameradiazNoch keine Bewertungen
- Statistical Molecular Thermodynamics - Ideal Gas Equation of StateDokument6 SeitenStatistical Molecular Thermodynamics - Ideal Gas Equation of StateadiazNoch keine Bewertungen
- Statistical Molecular Thermodynamics: Christopher J. CramerDokument5 SeitenStatistical Molecular Thermodynamics: Christopher J. CrameradiazNoch keine Bewertungen
- Statistical Molecular Thermodynamics: Christopher J. CramerDokument6 SeitenStatistical Molecular Thermodynamics: Christopher J. CrameradiazNoch keine Bewertungen
- Statistical Molecular Thermodynamics: Christopher J. CramerDokument7 SeitenStatistical Molecular Thermodynamics: Christopher J. CrameradiazNoch keine Bewertungen
- ThermoVid 1 07 PDFDokument7 SeitenThermoVid 1 07 PDFadiazNoch keine Bewertungen
- Statistical Molecular Thermodynamics: Christopher J. CramerDokument7 SeitenStatistical Molecular Thermodynamics: Christopher J. CrameradiazNoch keine Bewertungen
- Statistical Molecular Thermodynamics: Christopher J. CramerDokument5 SeitenStatistical Molecular Thermodynamics: Christopher J. CrameradiazNoch keine Bewertungen
- Statistical Molecular Thermodynamics: Christopher J. CramerDokument7 SeitenStatistical Molecular Thermodynamics: Christopher J. CrameradiazNoch keine Bewertungen
- Statistical Molecular Thermodynamics: Christopher J. CramerDokument6 SeitenStatistical Molecular Thermodynamics: Christopher J. CrameradiazNoch keine Bewertungen
- IntroductionDokument34 SeitenIntroductionmarranNoch keine Bewertungen
- CL 5313 17021-1 Requirements Matrix-1458-5Dokument6 SeitenCL 5313 17021-1 Requirements Matrix-1458-5Ana AnaNoch keine Bewertungen
- Russian Tea Market Growth and Brand PreferenceDokument6 SeitenRussian Tea Market Growth and Brand PreferenceKing KhanNoch keine Bewertungen
- Elmeasure Solenoid Ates CatalogDokument12 SeitenElmeasure Solenoid Ates CatalogSEO BDMNoch keine Bewertungen
- Timely characters and creatorsDokument4 SeitenTimely characters and creatorsnschober3Noch keine Bewertungen
- NetsimDokument18 SeitenNetsimArpitha HsNoch keine Bewertungen
- Modul-Document Control Training - Agus F - 12 Juli 2023 Rev1Dokument34 SeitenModul-Document Control Training - Agus F - 12 Juli 2023 Rev1vanesaNoch keine Bewertungen
- Put The Items From Exercise 1 in The Correct ColumnDokument8 SeitenPut The Items From Exercise 1 in The Correct ColumnDylan Alejandro Guzman Gomez100% (1)
- The Singular Mind of Terry Tao - The New York TimesDokument13 SeitenThe Singular Mind of Terry Tao - The New York TimesX FlaneurNoch keine Bewertungen
- Unit 1 - International Banking Meaning: Banking Transactions Crossing National Boundaries Are CalledDokument6 SeitenUnit 1 - International Banking Meaning: Banking Transactions Crossing National Boundaries Are CalledGanesh medisettiNoch keine Bewertungen
- Planview Innovation Management Maturity Model PDFDokument1 SeitePlanview Innovation Management Maturity Model PDFMiguel Alfonso Mercado GarcíaNoch keine Bewertungen
- Yanmar America publication listing for engine parts, service, and operation manualsDokument602 SeitenYanmar America publication listing for engine parts, service, and operation manualsEnrique Murgia50% (2)
- Paradigm Shift Essay 2Dokument17 SeitenParadigm Shift Essay 2api-607732716Noch keine Bewertungen
- Tygon S3 E-3603: The Only Choice For Phthalate-Free Flexible TubingDokument4 SeitenTygon S3 E-3603: The Only Choice For Phthalate-Free Flexible TubingAluizioNoch keine Bewertungen
- 14 15 XII Chem Organic ChaptDokument2 Seiten14 15 XII Chem Organic ChaptsubiNoch keine Bewertungen
- Lesson 2 Mathematics Curriculum in The Intermediate GradesDokument15 SeitenLesson 2 Mathematics Curriculum in The Intermediate GradesRose Angel Manaog100% (1)
- Lecture 1: Newton Forward and Backward Interpolation: M R Mishra May 9, 2022Dokument10 SeitenLecture 1: Newton Forward and Backward Interpolation: M R Mishra May 9, 2022MANAS RANJAN MISHRANoch keine Bewertungen
- Irctc Tour May 2023Dokument6 SeitenIrctc Tour May 2023Mysa ChakrapaniNoch keine Bewertungen
- Radio Theory: Frequency or AmplitudeDokument11 SeitenRadio Theory: Frequency or AmplitudeMoslem GrimaldiNoch keine Bewertungen
- ME6404 Thermal EngineeringDokument18 SeitenME6404 Thermal EngineeringAnonymous mRBbdopMKfNoch keine Bewertungen
- Spsi RDokument2 SeitenSpsi RBrandy ANoch keine Bewertungen
- Lanegan (Greg Prato)Dokument254 SeitenLanegan (Greg Prato)Maria LuisaNoch keine Bewertungen
- 2007 Bomet District Paper 2Dokument16 Seiten2007 Bomet District Paper 2Ednah WambuiNoch keine Bewertungen
- MSDS FluorouracilDokument3 SeitenMSDS FluorouracilRita NascimentoNoch keine Bewertungen
- Vidura College Marketing AnalysisDokument24 SeitenVidura College Marketing Analysiskingcoconut kingcoconutNoch keine Bewertungen
- NOTE CHAPTER 3 The Mole Concept, Chemical Formula and EquationDokument10 SeitenNOTE CHAPTER 3 The Mole Concept, Chemical Formula and EquationNur AfiqahNoch keine Bewertungen
- Daftar PustakaDokument4 SeitenDaftar PustakaRamli UsmanNoch keine Bewertungen
- Inventarisasi Data Kondisi Jalan Ke Dalam Aplikasi Sistem Informasi Geografis (Sig)Dokument10 SeitenInventarisasi Data Kondisi Jalan Ke Dalam Aplikasi Sistem Informasi Geografis (Sig)Wiro SablengNoch keine Bewertungen
- AVANTIZ 2021 LNR125 (B927) EngineDokument16 SeitenAVANTIZ 2021 LNR125 (B927) EngineNg Chor TeckNoch keine Bewertungen
- 13 Fashion Studies Textbook XIDokument158 Seiten13 Fashion Studies Textbook XIMeeta GawriNoch keine Bewertungen