Beruflich Dokumente
Kultur Dokumente
Febrile Neutropenia Care Guideline
Hochgeladen von
denokayuMRCopyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Febrile Neutropenia Care Guideline
Hochgeladen von
denokayuMRCopyright:
Verfügbare Formate
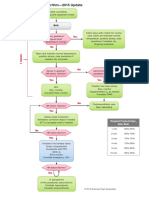
Febrile Neutropenia
Oncology Care Guideline
Inclusion Criteria:
Temp >38.3 C orally or > 38.0 C for longer than 1hr,
ANC < 500 cells/mm OR
ANC < 1000 cells/mm with a predict ed decline to
500 cells/mm or less over the next 48 hrs
Exclusion Criteria: BMT recipient
Assessment
Comprehensive H & P for subtle signs/symptoms, including
pain at sites most commonly infected
Vital signs, continuous pulse oximetry if respiratory signs/
symptoms
Interventions
CBC with differential, CMP
Blood cultures from each CVAD lumen/port, urinalysis & urine
c/s (no cath) for UTI symptoms, stool for C. difficile for GI
symptoms, VRP if URI signs/symptoms
Blood culture q 24 hours while febrile
CXR if respiratory signs/symptoms; chest CT if abnormal
Abd ultrasound or CT for abdominal pain
Heparin flush CVAD per protocol
Assess CVAD site for presence of infection & perform dressing
change within 4 hours of admission
Antibiotics Hemodynamically Stable
cefepime 50 mg/kg/dose IV q8hr (<40kg) (Max:
2 gm/dose) OR aztreonam 50 mg/kg/dose IV q
6 hrs (Max: 2 gm/dose) if allergic to
cephalosporins
IF indications for empiric vancomycin
present - ADD
vancomycin 15 mg/kg/dose IV q 6 hrs x 72 hrs (
if < 50kg) OR 1000 mg IV q 8h x 72 hrs (if > 50
kg)
IF typhlitis or C. difficile is suspected ADD
metronidazole 7.5 mg/kg/dose IV or PO q 6hrs
(Max: 2 gm/day)
Antibiotics Hemodynamically Unstable
(requires fluid boluses or pressors)
meropenem 40mg/kg/dose IV q
8hrs (Max: 2 gm/dose)
AND
vancomycin (x 72hrs) 15 mg/kg/
dose IV q 6 hrs if < 50kg or
1000 mg IV q 8h if > 50 kg
IF C. difficile is suspected
ADD metronidazole 7.5 mg/kg/
dose IV or PO q 6hrs (Max: 2
gm/day)
Continued Considerations
Adjust antibiotics based on culture results, clinical
course and serum levels.
Consider Vancomycin levels after 48-72 hours
Perform daily site specific exam, review of lab tests &
cultures, response to therapy (fever trends & signs/
symptoms of infection)
Evaluate drug toxicity including end-organ toxicity
(LFTs/renal function tests 2x/wk)
For follow up therapy, duration algorithms &
discharge criteria, see page 2.
Care Guidelines Committee Revision Approval 11-18-09
Previous version 7-06, revised 9-27-11 and 05-15-13
Recommendations/Considerations
Thoroughly assess common sites
of infection: GI tract, groin, skin,
lungs, sinuses, ears, perineum,
perirectum, intravascular access
sites.
Consider stress doses of IV
steroids for hypotension if
currently receiving steroids or
was recently tapered off steroids.
Administer antibiotics within 1
hour of arrival.
Central vascular access device
care should be performed per
CHOC protocol (Mosby CVAD).
Indications for Empiric
Vancomycin Use:
Blood culture positive for
Gram positive bacteria prior
to final ID & susceptibility
testing
Known colonization with
penicillin/cephalosporin
resistant pneumococci or
MRSA
Hypotension or septic shock
w/o an identified pathogen
Received high dose
cytarabine recently
AML
Soft tissue infection
Mucositis
Suspected meningitis
Cephalosporin allergic
Vancomycin Trough
Targets
Staph aureus: 15 20
mCg/mL
Staph epi: 10 15 mCg/
mL
No Vancomycin levels in
the first 48 to 72 hours
unless clinically indicated
Patient/Family Education
Review fever
guidelines &
temperature monitoring
Review S&S infection
Review handwashing
Reassess the appropriateness of Care Guidelines as condition changes and 24 hrs after admission. This guideline is a tool to aid in clinical decision making. It is
not a standard of care. The physician should deviate from the guideline when clinical judgment so indicates.
2011 Childrens Hospital of Orange County
Page 1
Febrile Neutropenia Oncology Care Guideline
Responding
Afebrile
Signs/symptoms
stable or improving
Evaluate overall
response to empiric
therapy in 2-5 days
Not Responding/
Persistent Fever
Documented
infection
Documented
infection
Adjust antibiotics
based on sensitivities
D/C vancomycin if no
positive culture for
Gram positive
organisms
No positive
culture
Continue same
antibiotic course until
neutropenia resolving
(ANC 250-500)
OR
Discontinue antibiotics
after 7-14 days if
remains stable (ANC
<250)
Reassess indication for
continued use of
vancomycin
Receiving
biologicals likely
to cause fever
(i.e. ATG, Ara-C,
interferon
Discontinue
antibiotics when
afebrile x 48 hrs
Suggested Duration of Therapy
Bloodstream infection (uncomplicated)
Gram-negative: min 10 days after 1st neg culture
Gram-positive: min 10 days after 1st neg culture
S. aureus: min 14 days after 1st negative blood
culture
ID Consult & catheter removal for BSI with Candida, Pseudomonas
aeruginosa, atypical mycobacteria, molds, Stenotrophomonas
maltophilia
Consider catheter removal for BSI with S. aureus, Corynebacterium
jeikium, Acinetobacter, Bacillus organisms, and VRE
Sinusitis: 21 days
Bacterial pneumonia: minimum 14 days
Catheter removal for septic phlebitis, tunnel infection, or port pocket
infection
Adjust antibiotics
based on
sensitivities
D/C vancomycin
if no positive
culture for Gram
positive
organisms
No positive
culture
Unstable
Consider
ID Consult
Daily
reassessment
of signs/
symptoms and
culture results
Consider
imaging
Add empiric
fluconazole
If fever > 5 days,
consider
addition of mold
active agent
Stable
Continue current
therapy
OR
Consider
broadening
coverage for
anaerobes, i.e.
add
metronidazole to
cefepime
If continued
fever > 5 days,
consider addition
of mold active
agent
Consider
imaging
Discharge Criteria
Afebrile, vital signs stable x 24-48 hrs
Hemodynamically & clinically stable
Cultures negative
ANC 250 - 500 and increasing
Follow up care planned
Antibiotics prescribed for appropriate
duration.
Page 2
Reassess the appropriateness of Care Guidelines as condition changes and 24 hrs after admission. This guideline is a tool to aid in clinical
decision making. It is not a standard of care. The physician should deviate from the guideline when clinical judgment so indicates.
Das könnte Ihnen auch gefallen
- Part C: Questions and Answers Regarding Assessing The Appropriateness and Effectiveness of Methadone Maintenance TreatmentDokument5 SeitenPart C: Questions and Answers Regarding Assessing The Appropriateness and Effectiveness of Methadone Maintenance TreatmentYogesh ShindeNoch keine Bewertungen
- Fibromyalgia Fam Resilience PDFDokument19 SeitenFibromyalgia Fam Resilience PDFirimes carmelaNoch keine Bewertungen
- Hypertension Protocol PDFDokument2 SeitenHypertension Protocol PDFHitesh PednekarNoch keine Bewertungen
- Diffi Cult To Treat Asthma: Sandhya Khurana Fernando Holguin EditorsDokument339 SeitenDiffi Cult To Treat Asthma: Sandhya Khurana Fernando Holguin Editorsneumologia.intervencionNoch keine Bewertungen
- Template Soap NeuroDokument2 SeitenTemplate Soap NeuroJohan SitepuanNoch keine Bewertungen
- DR - Muhammad Aasam Maan: Consultant Pain SpecialistDokument23 SeitenDR - Muhammad Aasam Maan: Consultant Pain SpecialistMuhammad Aasim MaanNoch keine Bewertungen
- Humanism and Resilience in Residency TrainingDokument623 SeitenHumanism and Resilience in Residency TrainingBurner AccountNoch keine Bewertungen
- Kaplan's Clinical HypertensionDokument10 SeitenKaplan's Clinical HypertensionWerdiantero Firdho IchsannyNoch keine Bewertungen
- Ferri's Clinical Advisor 2021: 5 Books in 1Dokument2 SeitenFerri's Clinical Advisor 2021: 5 Books in 1Bosko Kecojevic0% (2)
- Oxford Psychiatry Library David Nutt David Castle Psychedelics As Psychiatric Medications Oxford UDokument180 SeitenOxford Psychiatry Library David Nutt David Castle Psychedelics As Psychiatric Medications Oxford URosa Elena Marquez ANoch keine Bewertungen
- Serving Mental Health Clients in Your LibraryDokument47 SeitenServing Mental Health Clients in Your LibraryRaiyan DipNoch keine Bewertungen
- Social Pharmacy Notes - 1Dokument5 SeitenSocial Pharmacy Notes - 1Gerald Limo Arap ChebiiNoch keine Bewertungen
- Monitoring The Progress of LabourDokument9 SeitenMonitoring The Progress of LabourMemMed Administrator0% (1)
- Psikogero B11Dokument90 SeitenPsikogero B11Eva Yeni RustianaNoch keine Bewertungen
- Acute COPD ExacerbationDokument65 SeitenAcute COPD ExacerbationMaryam FadahNoch keine Bewertungen
- Neonatal Resuscitation AlgorithmDokument1 SeiteNeonatal Resuscitation AlgorithmDedy Tesna Amijaya100% (1)
- Methods For Assessing Leg Length DiscrepancyDokument13 SeitenMethods For Assessing Leg Length DiscrepancyKanika SinhaNoch keine Bewertungen
- Fibromyalgia A Clinical ReviewDokument9 SeitenFibromyalgia A Clinical ReviewastrogliaNoch keine Bewertungen
- COPD Follow UpDokument1 SeiteCOPD Follow Upe-MedTools100% (4)
- Skeletal Muscle RelaxantsDokument34 SeitenSkeletal Muscle RelaxantsLohithNoch keine Bewertungen
- 3 ❤ Smart Sheet for History Taking in Pediatrics خلفية صفراءDokument14 Seiten3 ❤ Smart Sheet for History Taking in Pediatrics خلفية صفراءsalah almozahemNoch keine Bewertungen
- Phmse 140717231529 Phpapp02Dokument56 SeitenPhmse 140717231529 Phpapp02Hardeep KaurNoch keine Bewertungen
- 2023 - A Patient's Guide To Obstructive Sleep Apnea Syndrome (Arnav Shetty, Peter M Baptista Jardín)Dokument81 Seiten2023 - A Patient's Guide To Obstructive Sleep Apnea Syndrome (Arnav Shetty, Peter M Baptista Jardín)César EscobarNoch keine Bewertungen
- Care of The Patient With An Addictive PersonalityDokument11 SeitenCare of The Patient With An Addictive PersonalityShawn McMahonNoch keine Bewertungen
- Your Health, Your Decisions: How to Work with Your Doctor to Become a Knowledge-Powered PatientVon EverandYour Health, Your Decisions: How to Work with Your Doctor to Become a Knowledge-Powered PatientNoch keine Bewertungen
- Temporomandibular Disorders: EditorsDokument218 SeitenTemporomandibular Disorders: EditorsDra.lupita LoMaNoch keine Bewertungen
- Pain BookletDokument67 SeitenPain BookletRahul Goma PhuloreNoch keine Bewertungen
- CAMH Referral Form PDFDokument3 SeitenCAMH Referral Form PDFDeisel AssisNoch keine Bewertungen
- Common Issues in Breast Cancer SurvivorsDokument350 SeitenCommon Issues in Breast Cancer SurvivorsJoana JohnNoch keine Bewertungen
- ADHD in Adolescents PDFDokument72 SeitenADHD in Adolescents PDFAi SanNoch keine Bewertungen
- McCarberg, Bill and Clauw, Daniel J., Eds. - FibromyalgiaDokument175 SeitenMcCarberg, Bill and Clauw, Daniel J., Eds. - FibromyalgiaJuan José García GarcíaNoch keine Bewertungen
- Preventive Care Checklist Form ExplanationsDokument3 SeitenPreventive Care Checklist Form ExplanationsKak KfgaNoch keine Bewertungen
- UntitledDokument301 SeitenUntitledS.R.GNoch keine Bewertungen
- Diabetes in Clinical Practice: Questions and Answers from Case StudiesVon EverandDiabetes in Clinical Practice: Questions and Answers from Case StudiesNoch keine Bewertungen
- A Clinician’s Guide to Discussing Obesity with PatientsVon EverandA Clinician’s Guide to Discussing Obesity with PatientsNoch keine Bewertungen
- Kenya Treatment GuidelinesDokument230 SeitenKenya Treatment GuidelinesKevin ChapleyNoch keine Bewertungen
- Tips - Anaphylaxis and Hypersensitivity Reactions PDFDokument376 SeitenTips - Anaphylaxis and Hypersensitivity Reactions PDFsorinproiecteNoch keine Bewertungen
- Tobacco Dependence - A Comprehensive Guide To Prevention and TreatmentDokument302 SeitenTobacco Dependence - A Comprehensive Guide To Prevention and TreatmentRania ANoch keine Bewertungen
- By Ethel Maureen B. Pagaddu, MDDokument49 SeitenBy Ethel Maureen B. Pagaddu, MDRyan James Lorenzo MiguelNoch keine Bewertungen
- Spirometry: Performance and Interpretation A Guide For General PractitionersDokument15 SeitenSpirometry: Performance and Interpretation A Guide For General PractitionersAgi AngloSaxonNoch keine Bewertungen
- CNS Depressants and Muscle RelaxantsDokument23 SeitenCNS Depressants and Muscle RelaxantsSV SagarNoch keine Bewertungen
- PocketGuide FINAL6 PDFDokument2 SeitenPocketGuide FINAL6 PDFSolomon Seth SallforsNoch keine Bewertungen
- MMP Handy Chart October 2011 V2Dokument69 SeitenMMP Handy Chart October 2011 V2Icha IchaNoch keine Bewertungen
- 4 - Endocrinology Tiki TakaDokument32 Seiten4 - Endocrinology Tiki TakaHemaNoch keine Bewertungen
- ONDOC Format & Guide To Oral PresentationsDokument10 SeitenONDOC Format & Guide To Oral PresentationsZainNoch keine Bewertungen
- Secondary Fracture Prevention: An International PerspectiveVon EverandSecondary Fracture Prevention: An International PerspectiveMarkus J. SeibelNoch keine Bewertungen
- Professional ResumeDokument1 SeiteProfessional ResumeDouglas RutledgeNoch keine Bewertungen
- IRMA Pediatric Palliative CommunicationDokument40 SeitenIRMA Pediatric Palliative CommunicationHelmi IrmawantyNoch keine Bewertungen
- Clinical Guideline DepressionDokument2 SeitenClinical Guideline DepressionAllan DiasNoch keine Bewertungen
- Chronic Pain Clinic Patient History SheetDokument4 SeitenChronic Pain Clinic Patient History SheetBelal N. MahfouzNoch keine Bewertungen
- Yale - Abnormal Uterine Bleeding Preceptor - 389620 - 284 - 45546 - v1Dokument12 SeitenYale - Abnormal Uterine Bleeding Preceptor - 389620 - 284 - 45546 - v1omegasauron0gmailcomNoch keine Bewertungen
- High Dose Methadone To Buprenorphine Transfer NHS Scottland PDFDokument1 SeiteHigh Dose Methadone To Buprenorphine Transfer NHS Scottland PDFohenriquezNoch keine Bewertungen
- Hepatorenal Syndrome, A Simple Guide To The Condition, Diagnosis, Treatment And Related ConditionsVon EverandHepatorenal Syndrome, A Simple Guide To The Condition, Diagnosis, Treatment And Related ConditionsNoch keine Bewertungen
- ACute Pain Management Transforming Evidence Into PracticeDokument60 SeitenACute Pain Management Transforming Evidence Into PracticeSurjya UpadhyayNoch keine Bewertungen
- Substance AbuseDokument10 SeitenSubstance AbuseAdriano SonnyNoch keine Bewertungen
- Wound Care Patient Assessment ChartDokument2 SeitenWound Care Patient Assessment ChartBrian HarrisNoch keine Bewertungen
- Case 36 AscitesDokument4 SeitenCase 36 AscitesMichaelNoch keine Bewertungen
- Cardiology Clinic 2006Dokument620 SeitenCardiology Clinic 2006JoNykolasNoch keine Bewertungen
- 2011 09 Medical HistoryDokument52 Seiten2011 09 Medical HistoryGurpreet CharaNoch keine Bewertungen
- COVID-19 Preventive Behaviors and Influencing Factors in The Iranian Population A Web-Based SurveyDokument7 SeitenCOVID-19 Preventive Behaviors and Influencing Factors in The Iranian Population A Web-Based SurveyAsti YumnaNoch keine Bewertungen
- Seed QualityDokument17 SeitenSeed Qualityroisatunnisa'Noch keine Bewertungen
- Submitted By: Group 6 MT 3BDokument49 SeitenSubmitted By: Group 6 MT 3BChristine Joy TanglaoNoch keine Bewertungen
- Renukaprasad Ys - Medicine - Thesis NewDokument110 SeitenRenukaprasad Ys - Medicine - Thesis NewLokesh ChopraNoch keine Bewertungen
- Yellow Fever Virus PDFDokument8 SeitenYellow Fever Virus PDFmanoj_rkl_07Noch keine Bewertungen
- Real Time PCR: ICMR No. SUPRA001fDokument2 SeitenReal Time PCR: ICMR No. SUPRA001fPatel AayushiNoch keine Bewertungen
- Test # 1 Microbiology: You Can Not Use Notes or Books To Take This Test - Read Your Questions CarefullyDokument44 SeitenTest # 1 Microbiology: You Can Not Use Notes or Books To Take This Test - Read Your Questions CarefullyEddie Allen100% (2)
- Medicine Lec.12 - Protozoal InfectionDokument60 SeitenMedicine Lec.12 - Protozoal Infection7fefdfbea1Noch keine Bewertungen
- AQA Immunity BookletDokument6 SeitenAQA Immunity Bookletnchauhan212Noch keine Bewertungen
- BotrytisDokument6 SeitenBotrytisjelenaaa1987Noch keine Bewertungen
- SEPTICARTHRITISDokument2 SeitenSEPTICARTHRITISapi-3822433Noch keine Bewertungen
- Kode ICD 10Dokument19 SeitenKode ICD 10Sri RachmanidewiNoch keine Bewertungen
- AbscessDokument5 SeitenAbscessJarotbemnkompinNoch keine Bewertungen
- Medical Virology Dr. Saif AL-Mayah: Rabies Virus Disease:This Virus Causes Rabies. Important PropertiesDokument9 SeitenMedical Virology Dr. Saif AL-Mayah: Rabies Virus Disease:This Virus Causes Rabies. Important PropertiesAtheer AlabdyNoch keine Bewertungen
- Health ReportingDokument48 SeitenHealth ReportinggaoherNoch keine Bewertungen
- STOMATITISDokument17 SeitenSTOMATITISTeguh Adi PartamaNoch keine Bewertungen
- Pestilence: Pest Pestis Plague Epidemic DiseaseDokument2 SeitenPestilence: Pest Pestis Plague Epidemic Diseasepuskesmas beloNoch keine Bewertungen
- Biology Chapter 40-1 Infectious DiseasesDokument23 SeitenBiology Chapter 40-1 Infectious Diseasesapi-239353579Noch keine Bewertungen
- The Analisys of Loperamide For DiarrheaDokument26 SeitenThe Analisys of Loperamide For DiarrheaGaluh ChanNoch keine Bewertungen
- Enteric FeverDokument51 SeitenEnteric FeverBinayaNoch keine Bewertungen
- BCH PresentationDokument35 SeitenBCH PresentationKoshy GeorgeNoch keine Bewertungen
- This Is Conversation I Had With A Job Interview Process On Google HangoutsDokument16 SeitenThis Is Conversation I Had With A Job Interview Process On Google Hangoutsapi-302442088Noch keine Bewertungen
- Virology (Harr, BOC, Apollon, Elsevier)Dokument2 SeitenVirology (Harr, BOC, Apollon, Elsevier)Sophia Elinor MenesesNoch keine Bewertungen
- COVID-19 Pandemic Dental Treatment Consent Form: Clinic Name: AddressDokument1 SeiteCOVID-19 Pandemic Dental Treatment Consent Form: Clinic Name: AddressisteNoch keine Bewertungen
- IMMUNITYDokument58 SeitenIMMUNITYkamala 123Noch keine Bewertungen
- Rheumatic FeverDokument16 SeitenRheumatic Feversteve benNoch keine Bewertungen
- Tomato Pests DiseasesDokument3 SeitenTomato Pests DiseasesBashar DaoudNoch keine Bewertungen
- In Vitro Antibiofilm Activity Of: Pomegranate (Punica Granatum) Juice On Oral PathogensDokument6 SeitenIn Vitro Antibiofilm Activity Of: Pomegranate (Punica Granatum) Juice On Oral PathogensYosivak Maulisa BlangidikoNoch keine Bewertungen
- Cell Basics Microbial DiversityDokument33 SeitenCell Basics Microbial DiversityKevin WilliamNoch keine Bewertungen
- Catastrophic Neurologic Disorders in The Emergency Department 2nd Ed PDFDokument344 SeitenCatastrophic Neurologic Disorders in The Emergency Department 2nd Ed PDFTarmidi MidziNoch keine Bewertungen