Beruflich Dokumente
Kultur Dokumente
ASME JHT Enthalpy 001605jhr
Hochgeladen von
Charlie MartinezCopyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
ASME JHT Enthalpy 001605jhr
Hochgeladen von
Charlie MartinezCopyright:
Verfügbare Formate
Analysis of Enthalpy
Approximation for Compressed
Liquid Water
Milivoje M. Kostic
e-mail: kostic@niu.edu
Northern Illinois University,
DeKalb, IL 60115-2854
It is custom to approximate solid and liquid thermodynamic properties as being a function of temperature only, since they are virtually incompressible, and Pdv boundary work
may be neglected. Furthermore, in classical literature, for isothermal compression processes, a general improvement and correction for liquid enthalpy approximation is
given by adding the pressure correction, vdP, to the corresponding saturation value. It
is shown that such correction given for isothermal processes is generally valid for isentropic processes only. Analysis of water real properties, over the saturation temperature
range and a wide pressure range up to 100 MPa, shows that the recommended corrections are only beneficial for higher pressures at smaller temperatures (below 200 C),
insignificant for smaller pressures at most of the temperatures, about the same but opposite sign (thus unnecessary) for intermediate temperatures and pressures, and more
erroneous (thus counterproductive and misleading) for higher temperatures and pressures, than the corresponding saturation values without any correction. The misconception in the literature is a result of the erroneous assumption, that due to incompressibility
for liquids in general, the internal energy is less dependent on pressure than
enthalpy. DOI: 10.1115/1.2175090
Keywords: enthalpy, water, thermodynamic properties, thermodynamic analysis,
isothermal, isentropic
Introduction
Since solids and liquids are virtually but not exactly incompressible, then the compression work, Pdv, could be neglected and
thus many properties virtually will not be a function of pressure
but temperature only, such as specific internal energy, u, etc. Furthermore, any process is also at the same time an isochoric,
constant-volume process. Namely, the isobaric, constant-pressure
process will be a simultaneously constant-volume process for an
incompressible substance, so that specific heat at constant pressure, c p, and constant volume, cv, are the same, or approximately
the same for virtually incompressible real solids and liquids, particularly when compared to vapors and gases, i.e.:
u uT usatT and c p cv cT
4
Furthermore, for isothermal processes dT = 0 and du 0, then
dh vdP, and finally, for finite pressure difference change from
saturated pressure, Psat, corresponding to the given temperature,
T, the specific enthalpy with correction, hcorrT , P at that temperature, T, and any pressure, P, will be 1,2:
5a
Even the specific enthalpy for a liquid from here on word specific will be assumed and omitted for brevity, can be approximated to be independent from pressure and conveniently taken to
be equal to the corresponding saturated liquid value at the given
temperature:
hP,T hT hsatT
However, enthalpy is unique, since it is explicitly defined as a
function of pressure, namely:
hu+Pv
thus,
hT, P = uT, P + P v usatT + P v
3
Therefore, it is common in most engineering references, including
classical and widely used thermodynamics textbooks 1,2, to
evaluate the change of enthalpy, assuming incompressibility dv
= 0, but taking correction for pressure increase as:
Contributed by the Heat Transfer Division of ASME for publication in the JOURHEAT TRANSFER. Manuscript received December 13, 2004; final manuscript
received November 1, 2005. Review conducted by John H. Lienhard V. Paper presented at the 2004 ASME International Mechanical Engineering Congress IMECE2004, November 1319, 2004, Anaheim, California, USA.
NAL OF
Journal of Heat Transfer
5b
where, hsatT and vsatT are liquid saturation enthalpy and liquid
saturation specific volume at given temperature, T, respectively. It
is stated in many references, including 1,2, that the above equations 5a and 5b are recommended as the correction for isothermal, liquid enthalpy dependence on pressure, and that it is more
accurate than a simple, approximation without correction, hsat Eq.
2.
It is the objective of this paper to point out the erroneous general recommendations in the literature. The correction Eq. 5, as
recommended in many references, is only useful for higher pressures at smaller temperatures, but is actually more erroneous thus
counterproductive and misleading for higher temperatures and
pressures, and is about the same but opposite sign, thus not necessary for intermediate temperatures, than the simple approximation Eq. 2 without any correction. Corresponding analysis using real water data 3 and physical justification are presented
below.
Copyright 2006 by ASME
MAY 2006, Vol. 128 / 421
422 / Vol. 128, MAY 2006
Table 1 Compressed liquid water property data at 260 C 3 and different enthalpy corrections
P
MPa
v
m3 / kg
u
kJ/kg
h
kJ/kg
s
kJ/ kg K
Cv
kJ/ kg K
Cp
kJ/ kg K
csound
m/s
JT
K/MPa
hu
kJ/kg
hv
kJ/kg
hP
kJ/kg
hcorr
kJ/kg
h or
CpdP
kJ/kg
4.69sat
5
10
15
20
25
30
35
40
45
50
55
60
65
70
75
80
85
90
95
100
0.001276
0.001276
0.001265
0.001256
0.001247
0.001239
0.001231
0.001224
0.001217
0.001211
0.001204
0.001199
0.001193
0.001187
0.001182
0.001177
0.001172
0.001167
0.001163
0.001158
0.001154
1129.0
1128.5
1121.6
1115.1
1109.0
1103.2
1097.8
1092.5
1087.6
1082.8
1078.2
1073.8
1069.6
1065.5
1061.6
1057.7
1054.0
1050.5
1047.0
1043.6
1040.3
1135.0
1134.9
1134.3
1134.0
1134.0
1134.2
1134.7
1135.4
1136.3
1137.3
1138.4
1139.7
1141.1
1142.7
1144.3
1146.0
1147.8
1149.7
1151.6
1153.7
1155.8
2.8849
2.8841
2.8710
2.8586
2.8469
2.8357
2.8250
2.8148
2.8050
2.7955
2.7864
2.7775
2.7690
2.7607
2.7526
2.7447
2.7371
2.7297
2.7224
2.7153
2.7084
3.1301
3.1299
3.1257
3.1220
3.1187
3.1158
3.1131
3.1107
3.1084
3.1063
3.1043
3.1024
3.1006
3.0989
3.0973
3.0957
3.0943
3.0928
3.0914
3.0901
3.0888
4.9856
4.9804
4.9019
4.8336
4.7733
4.7195
4.6711
4.6272
4.5871
4.5502
4.5162
4.4847
4.4553
4.4279
4.4022
4.3781
4.3553
4.3338
4.3134
4.2940
4.2756
1105.3
1107.2
1135.8
1162.6
1187.8
1211.7
1234.3
1256.0
1276.7
1296.6
1315.8
1334.3
1352.2
1369.5
1386.3
1402.6
1418.5
1433.9
1449.0
1463.7
1478.1
0.03472
0.03373
0.01884
0.00582
0.00
0.50
7.40
13.90
20.00
25.80
31.20
36.50
41.40
46.20
50.80
55.20
59.40
63.50
67.40
71.30
75.00
78.50
82.00
85.40
88.70
0.00
0.00
0.00
0.39
6.74
13.05
19.31
25.52
31.70
37.84
43.94
50.01
56.05
62.05
68.03
73.98
79.91
85.80
91.68
97.53
103.35
109.15
114.93
0.00
0.39
6.77
13.15
19.53
25.91
32.30
38.68
45.06
51.44
57.82
64.20
70.58
76.96
83.34
89.72
96.10
102.48
108.86
115.24
121.62
0.00
0.05
0.70
1.00
1.00
0.75
0.26
0.42
1.28
2.30
3.46
4.76
6.17
7.70
9.32
11.03
12.83
14.71
16.67
18.69
20.77
0.00568
0.01595
0.02519
0.03355
0.04117
0.04816
0.05458
0.06051
0.06601
0.07113
0.07590
0.08037
0.08456
0.08850
0.09220
0.09570
0.09901
0.08
0.20
0.35
0.53
0.74
0.98
1.24
1.52
1.82
2.12
2.45
2.80
3.15
3.52
3.90
4.29
4.69
5.09
5.51
hsat-h
kJ/kg
hcorr-h
kJ/kg
0.00
0.10
0.70
1.00
1.00
0.80
0.30
0.00
0.49
7.47
14.15
20.53
26.71
32.60
38.28
43.76
49.14
54.42
59.50
64.48
69.26
74.04
78.72
83.30
87.78
92.26
96.54
100.82
0.40
1.30
2.30
3.40
4.70
6.10
7.70
9.30
11.00
12.80
14.70
16.60
18.70
20.80
Transactions of the ASME
Journal of Heat Transfer
Table 2 Compressed liquid water property data at different temperatures and pressures 3
T=4C
T = 20 C
P
MPa
v
m3 / kg
u
kJ/kg
h
kJ/kg
v
m3 / kg
Sat
5
10
15
20
25
30
35
40
45
50
55
60
65
70
75
80
85
90
95
100
0.001000
0.000998
0.000995
0.000993
0.000990
0.000988
0.000986
0.000984
0.000981
0.000979
0.000977
0.000975
0.000973
0.000971
0.000969
0.000967
0.000965
0.000963
0.000961
0.000959
0.000958
16.81
16.81
16.79
16.77
16.74
16.70
16.65
16.59
16.53
16.46
16.38
16.30
16.21
16.12
16.01
15.91
15.80
15.68
15.56
15.44
15.31
16.81
21.80
26.75
31.66
36.55
41.40
46.23
51.02
55.79
60.53
65.24
69.93
74.59
79.23
83.84
88.43
93.00
97.55
102.07
106.58
111.06
0.001002
0.001000
0.000997
0.000995
0.000993
0.000991
0.000989
0.000987
0.000985
0.000982
0.000980
0.000978
0.000977
0.000975
0.000973
0.000971
0.000969
0.000967
0.000965
0.000964
0.000962
T = 200 C
u
kJ/ kg
83.912
83.609
83.308
83.007
82.708
82.409
82.112
81.815
81.520
81.225
80.931
80.637
80.345
80.053
79.762
79.471
79.182
78.893
78.604
78.317
78.031
T = 50 C
MAY 2006, Vol. 128 / 423
v
m3 / kg
u
kJ/kg
h
kJ/kg
v
m3 / kg
u
kJ/ kg
Sat
5
10
15
20
25
30
35
40
45
50
55
60
65
70
75
80
85
90
95
100
0.001157
0.001153
0.001148
0.001144
0.001139
0.001135
0.00113
0.001126
0.001122
0.001119
0.001115
0.001111
0.001108
0.001104
0.001101
0.001098
0.001095
0.001091
0.001088
0.001086
0.001083
850.47
847.91
844.31
840.84
837.49
834.24
831.10
828.06
825.10
822.23
819.45
816.73
814.09
811.51
809.00
806.56
804.17
801.83
799.55
797.32
795.14
852.27
853.68
855.80
857.99
860.27
862.61
865.02
867.48
870.00
872.57
875.19
877.85
880.55
883.29
886.07
888.89
891.73
894.61
897.51
900.44
903.40
0.001252
0.00125
0.001241
0.001233
0.001225
0.001218
0.001211
0.001205
0.001199
0.001193
0.001187
0.001182
0.001176
0.001171
0.001167
0.001162
0.001157
0.001153
0.001149
0.001145
0.001141
1080.8
1079.5
1073.4
1067.6
1062.2
1057.0
1052.0
1047.3
1042.7
1038.4
1034.2
1030.1
1026.2
1022.5
1018.8
1015.3
1011.8
1008.5
1005.3
1002.1
999.06
T = 150 C
h
kJ/ kg
v
m3 / kg
u
kJ/ kg
h
kJ/ kg
v
m3 / kg
u
kJ/kg
h
kJ/kg
v
m3 / kg
u
kJ/kg
h
kJ/kg
83.914
88.607
93.281
97.934
102.57
107.18
111.77
116.34
120.90
125.44
129.95
134.45
138.94
143.40
147.85
152.29
156.70
161.10
165.49
169.86
174.22
0.001012
0.001010
0.001008
0.001006
0.001004
0.001001
0.000999
0.000997
0.000995
0.000993
0.000991
0.000989
0.000988
0.000986
0.000984
0.000982
0.000980
0.000978
0.000977
0.000975
0.000973
209.33
208.59
207.86
207.15
206.44
205.75
205.07
204.40
203.75
203.10
202.46
201.84
201.22
200.61
200.01
199.42
198.84
198.26
197.70
197.14
196.59
209.34
213.64
217.94
222.23
226.51
230.79
235.05
239.31
243.56
247.80
252.03
256.26
260.47
264.68
268.88
273.07
277.26
281.44
285.61
289.77
293.92
0.001044
0.001041
0.001039
0.001036
0.001034
0.001031
0.001029
0.001027
0.001025
0.001022
0.001020
0.001018
0.001016
0.001014
0.001012
0.001010
0.001008
0.001006
0.001004
0.001002
0.001000
419.06
417.64
416.23
414.85
413.50
412.17
410.87
409.60
408.35
407.13
405.93
404.76
403.61
402.47
401.36
400.27
399.20
398.14
397.11
396.09
395.09
419.17
422.85
426.62
430.39
434.17
437.95
441.74
445.54
449.33
453.14
456.94
460.75
464.56
468.37
472.19
476.00
479.82
483.64
487.46
491.29
495.11
0.001091
0.001088
0.001084
0.001081
0.001078
0.001075
0.001072
0.001069
0.001066
0.001064
0.001061
0.001058
0.001056
0.001053
0.001051
0.001048
0.001046
0.001043
0.001041
0.001039
0.001036
631.66
629.55
627.27
625.05
622.89
620.78
618.73
616.72
614.77
612.85
610.98
609.15
607.36
605.61
603.89
602.21
600.56
598.95
597.36
595.81
594.29
632.18
634.98
638.11
641.27
644.45
647.66
650.89
654.14
657.42
660.71
664.02
667.35
670.69
674.05
677.43
680.81
684.21
687.63
691.05
694.48
697.93
T = 250 C
P
MPa
T = 100 C
T = 300 C
h
kJ/ kg
1085.8
1085.7
1085.8
1086.1
1086.7
1087.4
1088.4
1089.4
1090.7
1092.0
1093.5
1095.1
1096.8
1098.6
1100.5
1102.4
1104.4
1106.5
1108.7
1110.9
1113.1
v
m3 / kg
u
kJ/ kg
T = 350 C
h
kJ/ kg
0.001404
1332.9
1345.0
Psat = 8.5879 MPa
0.001398
1329.4
1343.3
0.001378
1317.6
1338.3
0.001361
1307.1
1334.4
0.001346
1297.6
1331.3
0.001332
1288.9
1328.9
0.001320
1280.8
1327.0
0.001308
1273.3
1325.6
0.001298
1266.2
1324.6
0.001288
1259.6
1324.0
0.001279
1253.3
1323.6
0.001270
1247.3
1323.5
0.001262
1241.6
1323.6
0.001254
1236.1
1323.9
0.001247
1230.9
1324.4
0.001240
1225.9
1325.1
0.001233
1221.1
1325.9
0.001227
1216.4
1326.8
0.001221
1212.0
1327.9
0.001215
1207.6
1329.1
v
m3 / kg
0.00174
u
kJ/kg
h
kJ/kg
1642.1
1670.9
Psat = 16.529 MPa
0.001665
0.001599
0.001553
0.001517
0.001488
0.001464
0.001443
0.001424
0.001407
0.001391
0.001377
0.001364
0.001353
0.001341
0.001331
0.001321
0.001312
1612.7
1583.9
1562.2
1544.5
1529.3
1515.9
1503.9
1493.1
1483.1
1473.8
1465.2
1457.1
1449.5
1442.3
1435.5
1429.0
1422.8
1646.0
1623.9
1608.8
1597.6
1588.8
1581.8
1576.1
1571.4
1567.5
1564.3
1561.6
1559.5
1557.7
1556.3
1555.3
1554.5
1554.0
424 / Vol. 128, MAY 2006
Table 3 Compressed liquid water enthalpies, h, their approximation differences, hsat h Eq. 2, and hcorr h Eq. 5, and related percentages Note: More erroneous approximations are indicated in bold.
TC
P
MPa
4C
h kJ/kg
hsat h
hcorr h
kJ/kg
kJ/kg
%
%
Sat.
20 C
h kJ/kg
hsat h
hcorr h
kJ/kg
kJ/kg
%
%
16.81
0
0%
50 C
h kJ/kg
hsat h
hcorr h
kJ/kg
kJ/kg
%
%
83.91
0
0%
0
0%
100 C
h kJ/kg
hsat h
hcorr h
kJ/kg
kJ/kg
%
%
209.34
0
0%
0
0%
150 C
h kJ/kg
hsat h
hcorr h
kJ/kg
kJ/kg
%
%
419.17
0
0%
0
0%
200 C
h kJ/kg
hsat h
hcorr h
kJ/kg
kJ/kg
%
%
632.18
0
0%
0
0%
250 C
h kJ/kg
hsat h
hcorr h
kJ/kg
kJ/kg
%
%
852.27
0
0%
0
0%
300 C
h kJ/kg
hsat h
hcorr h
kJ/kg
kJ/kg
%
%
1085.80
0
0%
0
0%
0
0%
350 C
h kJ/kg
hsat h
hcorr h
kJ/kg
kJ/kg
%
%
1345.00
0
0%
0
0%
1670.90
0
0%
0
0%
Transactions of the ASME
21.80
0.02
4.98
0.1%
22.9%
88.61
0.31
4.69
0.4%
5.3%
213.64
0.75
4.30
0.4%
2.0%
422.85
1.43
3.68
0.3%
0.9%
634.98
2.13
2.80
0.3%
0.4%
853.68
2.57
1.41
0.3%
0.2%
1085.70
0.10
1.38
0.0%
0.1%
Vapor
Psat=
8.5879 MPa
10
26.75
0.07
9.93
0.3%
37.1%
93.28
0.65
9.37
0.7%
10.0%
217.94
1.51
8.60
0.7%
3.9%
426.62
2.88
7.45
0.7%
1.7%
638.11
4.46
5.93
0.7%
0.9%
855.80
6.24
3.53
0.7%
0.4%
1085.80
0.00
7.54
0.0%
0.7%
1343.30
1.70
3.68
0.1%
0.3%
15
31.66
0.15
14.85
0.5%
46.9%
97.93
1.00
14.02
1.0%
14.3%
222.23
2.28
12.89
1.0%
5.8%
430.39
4.33
11.22
1.0%
2.6%
641.27
6.75
9.09
1.1%
1.4%
857.99
9.83
5.72
1.1%
0.7%
1086.10
13.50
0.30
0.0%
1.2%
1338.30
6.70
15.70
0.5%
1.2%
20
36.55
0.27
19.74
0.7%
54.0%
102.57
1.38
18.66
1.3%
18.2%
226.51
3.06
17.17
1.4%
7.6%
434.17
5.76
15.00
1.3%
3.5%
644.45
9.02
12.27
1.4%
1.9%
860.27
13.33
8.00
1.5%
0.9%
1086.70
19.16
0.90
1.8%
0.1%
1334.40
10.60
26.62
0.8%
2.0%
1646.00
24.90
30.94
1.5%
1.9%
30
46.23
0.59
29.41
1.3%
63.6%
111.77
2.20
27.86
2.0%
24.9%
235.05
4.64
25.71
2.0%
10.9%
441.74
8.63
22.57
2.0%
5.1%
650.89
13.49
18.71
2.1%
2.9%
865.02
20.15
12.75
2.3%
1.5%
1088.40
29.97
2.60
2.8%
0.2%
1328.90
16.10
46.17
1.2%
3.5%
1608.80
62.10
85.54
3.9%
5.3%
40
55.79
1.03
38.98
1.8%
69.9%
120.90
3.08
36.99
2.6%
30.6%
243.56
6.25
34.22
2.6%
14.0%
449.33
11.47
30.16
2.6%
6.7%
657.42
17.86
25.24
2.7%
3.8%
870.00
26.73
17.73
3.1%
2.0%
1090.70
40.19
4.90
3.7%
0.4%
1325.60
19.40
63.51
1.5%
4.8%
1588.80
82.10
122.94
5.2%
7.7%
50
65.24
1.58
48.43
2.4%
74.2%
129.95
4.05
46.04
3.1%
35.4%
252.03
7.90
42.69
3.1%
16.9%
456.94
14.30
37.77
3.1%
8.3%
664.02
22.17
31.84
3.3%
4.8%
875.19
33.11
22.92
3.8%
2.6%
1093.50
49.91
7.70
4.6%
0.7%
1324.00
21.00
79.15
1.6%
6.0%
1576.10
94.80
153.04
6.0%
9.7%
60
74.59
2.23
57.78
3.0%
77.5%
138.94
5.08
55.03
3.7%
39.6%
260.47
9.58
51.13
3.7%
19.6%
464.56
17.11
45.39
3.7%
9.8%
670.69
26.40
38.51
3.9%
5.7%
880.55
39.31
28.28
4.5%
3.2%
1096.80
59.12
11.00
5.4%
1.0%
1323.50
21.50
93.69
1.6%
7.1%
1567.50
103.40
179.04
6.6%
11.4%
80
93.00
3.82
76.19
4.1%
81.9%
156.70
7.36
72.79
4.7%
46.4%
277.26
13.04
67.92
4.7%
24.5%
479.82
22.72
60.65
4.7%
12.6%
684.21
34.69
52.03
5.1%
7.6%
891.73
51.26
39.46
5.7%
4.4%
1104.40
76.56
18.60
6.9%
1.7%
1325.10
19.90
120.18
1.5%
9.1%
1557.70
113.20
223.64
7.3%
14.4%
100
111.06
5.76
94.25
5.2%
84.9%
174.22
9.87
90.31
5.7%
51.8%
293.92
16.62
84.58
5.7%
28.8%
495.11
28.30
75.94
5.7%
15.3%
697.93
42.78
65.75
6.1%
9.4%
903.40
62.72
51.13
6.9%
5.7%
1113.10
92.89
27.30
8.3%
2.5%
1329.10
15.90
144.26
1.2%
10.9%
1554.00
116.90
262.14
7.5%
16.9%
Vapor
Psat=
6.529 MPa
Analysis
Compressed liquid water properties 3, for different pressure at
260 C, are presented in Table 1 and selected properties for different temperatures in Table 2. In addition, the corresponding corrections hu, hv, and hP, see Eqs. 48 are tabulated
along with differences of the approximated enthalpies, without
hsat h and with correction hcorr h from the real enthalpy values, h, in the last two columns in Table 1, respectively, for saturated liquid water and compressed liquid water up to 100 MPa.
Cumulative water enthalpy data with the corresponding differences and related percentages are presented in Table 3 for a wide
range of temperatures between triple and critical points, and, a
wide range of pressures from saturation up to 100 MPa. Note that
the tabulated enthalpy differences with correction is based on Eq.
5, i.e., using correction hcorr hP only, as generally recommended in the literature, while corrections hu, and hv are
neglected 1,2.
The corrections in Tables 1 and 3 are calculated using the following equations:
hu = u = uP,T usatT
hv =
hP =
N1
P dv
i=1sat
N1
v dP
i=1sat
1
Pi + Pi+1 vi+1 vi
2
1
vi + vi+1 Pi+1 Pi hcorr
2
Note that in Eq. 5 the fluid is assumed to be incompressible,
while in Eqs. 68 variability in v, although small, is taken into
account, thus the differences in hcorr and hP values in Table 1.
Furthermore, for the isothermal compression, the correction hv
is much smaller than the corrections hu and hP, and thus it
may be neglected. However, corrections hu negative and corrections hP positive are comparable in magnitude but opposite in sign, so it is better not to take the corrections, as in Eq. 2,
than to take only the correction hcorr hP, as in Eq. 5. It will
be shown below that the recommended enthalpy correction for the
isothermal compression 1,2, is actually valid for isentropic
compression.
It appears from real data values in Tables 1 and 2 that the
magnitude of the negative correction hu i.e., u increases
with both pressure and temperature, while positive correction
hP i.e., vdP depends mostly on pressure since the specific
volume, v, does not change significantly. As is evident from data
in Table 3, the correction, Eq. 5 1,2, as a general improvement
for compressed liquid enthalpy calculation, is not justified in general, but for smaller temperatures only less than 200 C. However, it is more erroneous and thus counterproductive and misleading for higher temperatures above 200 C and particularly
at higher pressures, where a simple approximation Eq. 2, without any correction, is more accurate.
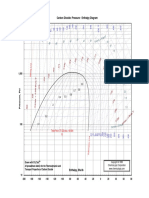
In Fig. 1, the compression of saturated liquid water, state f sat
at 260 C, is presented for isothermal compression to state T50
at 50 MPa, and for isentropic compression to state s50 to the
same pressure of 50 MPa see Table 1 for property data.
Since du = q w where w = Pdv, and real liquids in this
case water are not exactly incompressible, then during isentropic
compression q = Tds = 0 there will be some negative work Pdv
and an increase of internal energy and temperature in this case for
11.7 C, from 260 C to 271.7 C 3. However, during the isothermal compression, to cool and maintain constant water temperature, there must be some heat transfer out, q, and in the process the internal energy, u, will be decreased with pressure
increase at constant temperature see the corresponding data in
Tables 1 and 2; note hu = u 0 if P 0. At high temperatures, 300 C and above, even specific enthalpy is decreasing with
Journal of Heat Transfer
Fig. 1 Isothermal and isentropic compression of saturated liquid water
pressure increase due to a strong decrease of internal energy, making the recommended positive correction, hcorr, to be erroneous
and thus counterproductive and misleading, see Tables 2 and 3.
The hu correction is not included in Eq. 5 even though its
magnitude may be, and sometimes is, larger than the included
hcorr hP correction. Therefore, the recommended enthalpy
correction for isothermal compression in the literature is appropriate for the isentropic processes see additional justification below, but not appropriate for isothermal processes, although it
may sometimes be beneficial, due to an erroneous assumption that
internal energy is not, and enthalpy is, dependent on pressure. It is
quite the opposite in Table 1, see how the corresponding values
u and h change with pressure at constant temperature of 260 C.
The above physical justifications could be confirmed using the
corresponding differential property correlations obtained using the
Maxwells relations 1:
10
From Eq. 9 it is evident that change in internal energy for the
isothermal process is zero du = 0 only for ideal incompressible
fluids dv = 0, but for real liquids dv 0 the bracketed term
with dv in Eq. 9 is not zero since real liquids are not exactly
incompressible.
Equation 10 confirms that pressure correction vdP is always
appropriate for isentropic processes dh = Tds + vdP = vdP for s
= const, but not for isothermal processes as given in many referMAY 2006, Vol. 128 / 425
ences 1,2. For example, for an isentropic compression of
liquid water from saturation at 260 C to 50 MPa, see Fig. 1
and data in Table 1 Psat = 4.69 MPa, hsat = 1135.0 kJ/ kg, ssat
= 2.8849 kJ/ kg K, the temperature and enthalpy will increase to
271.7 C and hs50 = 1191.7 kJ/ kg, respectively 3. The isentropic
enthalpy increase, hs50 hsat = 1191.7 1135.0 kJ/ kg= 56.7 kJ/
kg is virtually identical to the corresponding enthalpy correction
hP = vdP = 56.05 KJ/ kg hcorr = 57.82 kJ/ kg, where the
small differences in Table 1 are due to an integral approximation
with discrete summation Eq.8 or assuming v = vsat = const in
Eq. 5 during the process.
For the isothermal process the enthalpy pressure-correction
could be evaluated using the three corrections, Eqs. 68, or
integrating Eq. 9 or 10, or combined Eqs. 9 and 10, i.e.:
of the erroneous assumption that, due to incompressibility for liquids in general, the internal energy is less dependent on pressure
than enthalpy. The literature recommendations may be erroneous
and thus counterproductive and misleading, as is the case for liquid water at higher temperatures and pressures. For intermediate
pressures and temperatures, the enthalpy corrections recommended in the literature are unnecessary, since the errors are about
the same in magnitude but opposite in sign as if the corresponding saturated enthalpy values without any corrections were used.
The isothermal corrections are only beneficial for very high pressures at smaller temperatures below 200 C for liquid water.
In summary, the recommended pressure corrections in the classical reference textbooks for isothermal, liquid enthalpy approximation are not appropriate and often insignificant, unnecessary or
more erroneous than the simple approximation using the corresponding saturated values. Furthermore, it is shown here that the
recommended pressure correction for isothermal processes are
actually valid in general for enthalpy correction for isentropic
processes.
Nomenclature
11
Since the Joule-Thomson coefficient is defined as JT = T / Ph,
then Eq. 11 could be expressed as:
hT = hP,T hsatTT = hu + hv + hP
=
C PJTdP
Psat
N1
i=1sat
1
C PJT,i + C PJT,i+1 Pi+1 Pi
2
12
c , c p , cv specific heat, at constant pressure, or constant
volume
csound speed of sound
h enthalpy correction including all relevant corrections, Eq. 12
hcorr enthalpy correction due to the change of Psat
= P Psat, Eq. 5a
hu enthalpy correction due to the change of u,
Eq. 6
hv enthalpy correction due to the change of v,
Eq. 7
hP enthalpy correction due to the change of P,
Eq. 8
h liquid enthalpy at any P and T
hcorr liquid enthalpy approximation with vsatPsat
correction as recommended in the literature
hsat saturation liquid enthalpy
P pressure
q heat transfer per unit of mass
s entropy
T temperature
u internal thermal energy
v specific volume
w boundary work per unit of mass
JT The Joule-Thomson coefficient
Note that real data values for both, the JT and CP are available
3, and for the isothermal enthalpy correction, h, Eq. 12 was
used instead of Eqs. 68, see Table 1.
Compressed liquid water enthalpies h, different corrections,
their approximation differences hsat h, and hcorr h and the corresponding error percentages, with regard to the saturation value,
are presented in Table 3, without any correction Eq. 2 and the
correction recommended in the literature hcorr hP, Eq. 5,
for a wide range of temperatures and pressures. The corrections,
as recommended in 1,2 Eq. 5, are only beneficial for higher
pressures at smaller temperatures, insignificant for smaller pressures at most of the temperatures, about the same but opposite
sign thus unnecessary for intermediate temperatures and pressures, and more erroneous thus counterproductive and misleading for higher temperatures and pressures, than the simple approximation without any correction Eq. 2, as seen from Tables
1 and 3.
Subscripts
s50 isentropic compression from saturated to
50 MPa
sat for saturated liquid
T50 isothermal compression from saturated to
50 MPa
References
Conclusion
An analysis with physical justification, supported by water real
enthalpy data, regarding liquid enthalpy approximation, is presented here. The analysis shows, and a conclusion is drawn, that
the recommendation in the classical reference textbooks for improvement of enthalpy calculation of compressed liquids, by accounting for pressure dependence, is not generally valid for isothermal processes. The misconception in the literature is a result
426 / Vol. 128, MAY 2006
1 Cengel, Y. A., and Boles, M. A., 2002, Thermodynamics, An Engineering
Approach, 4th ed., McGraw-Hill, New York, Sec. 2.5.3, p. 84.
2 Moran, M. J., and Shapiro, H. N., 2000, Fundamentals of Engineering Thermodynamics, 4th ed., Wiley, New York, Sec. 3.3.6, p. 109.
3 Lemmon, E. W., McLinden, M. O., and Friend, D. G., 2005, Thermophysical
Properties of Fluid Systems, NIST Chemistry WebBook, NIST Standard Reference Database No. 69, P. J. Linstrom, and W. G. Mallard, eds., National
Institute of Standards and Technology, Gaithersburg, MD http://
webbook.nist.gov/chemistry/fluid.
Transactions of the ASME
Das könnte Ihnen auch gefallen
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Von EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Bewertung: 4.5 von 5 Sternen4.5/5 (121)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryVon EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryBewertung: 3.5 von 5 Sternen3.5/5 (231)
- Grit: The Power of Passion and PerseveranceVon EverandGrit: The Power of Passion and PerseveranceBewertung: 4 von 5 Sternen4/5 (588)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaVon EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaBewertung: 4.5 von 5 Sternen4.5/5 (266)
- Never Split the Difference: Negotiating As If Your Life Depended On ItVon EverandNever Split the Difference: Negotiating As If Your Life Depended On ItBewertung: 4.5 von 5 Sternen4.5/5 (838)
- The Emperor of All Maladies: A Biography of CancerVon EverandThe Emperor of All Maladies: A Biography of CancerBewertung: 4.5 von 5 Sternen4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingVon EverandThe Little Book of Hygge: Danish Secrets to Happy LivingBewertung: 3.5 von 5 Sternen3.5/5 (400)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeVon EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeBewertung: 4 von 5 Sternen4/5 (5794)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyVon EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyBewertung: 3.5 von 5 Sternen3.5/5 (2259)
- Shoe Dog: A Memoir by the Creator of NikeVon EverandShoe Dog: A Memoir by the Creator of NikeBewertung: 4.5 von 5 Sternen4.5/5 (537)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreVon EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreBewertung: 4 von 5 Sternen4/5 (1090)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersVon EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersBewertung: 4.5 von 5 Sternen4.5/5 (345)
- Team of Rivals: The Political Genius of Abraham LincolnVon EverandTeam of Rivals: The Political Genius of Abraham LincolnBewertung: 4.5 von 5 Sternen4.5/5 (234)
- Her Body and Other Parties: StoriesVon EverandHer Body and Other Parties: StoriesBewertung: 4 von 5 Sternen4/5 (821)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceVon EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceBewertung: 4 von 5 Sternen4/5 (895)
- The Unwinding: An Inner History of the New AmericaVon EverandThe Unwinding: An Inner History of the New AmericaBewertung: 4 von 5 Sternen4/5 (45)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureVon EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureBewertung: 4.5 von 5 Sternen4.5/5 (474)
- On Fire: The (Burning) Case for a Green New DealVon EverandOn Fire: The (Burning) Case for a Green New DealBewertung: 4 von 5 Sternen4/5 (74)
- The Yellow House: A Memoir (2019 National Book Award Winner)Von EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Bewertung: 4 von 5 Sternen4/5 (98)
- Amisol Trio™: Skin Barrier and Hair ConditionerDokument18 SeitenAmisol Trio™: Skin Barrier and Hair ConditionerCharlie MartinezNoch keine Bewertungen
- Commissioning Report For Boiler Air and Flue Gas System Unit 1Dokument6 SeitenCommissioning Report For Boiler Air and Flue Gas System Unit 1Arif FebriantoNoch keine Bewertungen
- PPR Pipe Insulation GraphDokument10 SeitenPPR Pipe Insulation GraphJonNoch keine Bewertungen
- HVAC Design Basics Questions PDFDokument2 SeitenHVAC Design Basics Questions PDFAnbalagan Prabhu100% (1)
- Chapter 1 Fundamental Concept of ThermodynamicsDokument16 SeitenChapter 1 Fundamental Concept of ThermodynamicsZaza Rafali100% (1)
- Tanque STA RITEDokument3 SeitenTanque STA RITECharlie Martinez100% (1)
- Art. 44o NECDokument4 SeitenArt. 44o NECCharlie MartinezNoch keine Bewertungen
- Conductores ElectricosDokument10 SeitenConductores ElectricosCharlie MartinezNoch keine Bewertungen
- Catologo Lennox Xc20 - Elite SeriesDokument20 SeitenCatologo Lennox Xc20 - Elite SeriesCharlie MartinezNoch keine Bewertungen
- Catologo Lennox Cbx32mv - Elite SeriesDokument28 SeitenCatologo Lennox Cbx32mv - Elite SeriesCharlie MartinezNoch keine Bewertungen
- DUCT - Duct System Sizing Program: PurposeDokument7 SeitenDUCT - Duct System Sizing Program: PurposeCharlie MartinezNoch keine Bewertungen
- DOE 2.2 - Libraries & ReportsDokument286 SeitenDOE 2.2 - Libraries & ReportsCharlie MartinezNoch keine Bewertungen
- Hoja Tecnica de Motores WegDokument3 SeitenHoja Tecnica de Motores WegCharlie MartinezNoch keine Bewertungen
- Manometro Presion Diferencial PDFDokument1 SeiteManometro Presion Diferencial PDFCharlie MartinezNoch keine Bewertungen
- HvacLoadExplorer ManualDokument52 SeitenHvacLoadExplorer ManualCharlie MartinezNoch keine Bewertungen
- Clase 1Dokument9 SeitenClase 1Charlie MartinezNoch keine Bewertungen
- Brochure - Minisplit - Lennox (English)Dokument4 SeitenBrochure - Minisplit - Lennox (English)Charlie MartinezNoch keine Bewertungen
- Section 3 Basic OperationsDokument17 SeitenSection 3 Basic OperationsCharlie MartinezNoch keine Bewertungen
- Physical Properties of RefrigerantsDokument2 SeitenPhysical Properties of Refrigerantsioan_vNoch keine Bewertungen
- Experiment 3: Temperature Measurement MethodDokument12 SeitenExperiment 3: Temperature Measurement Methodmohamad munzirNoch keine Bewertungen
- Refrigeration: Vapor Compression Refrigeration SystemsDokument8 SeitenRefrigeration: Vapor Compression Refrigeration SystemsRita FaridaNoch keine Bewertungen
- Typical All Air System Explanation With SketchesDokument19 SeitenTypical All Air System Explanation With SketchesMADHU MITHANoch keine Bewertungen
- TC Fundamentals 2011 PsychrometricsDokument57 SeitenTC Fundamentals 2011 PsychrometricsTapas ChaudhuriNoch keine Bewertungen
- GCSE Physics For You 5th EditionDokument2 SeitenGCSE Physics For You 5th EditionAlina Starken100% (1)
- Irs qb1Dokument4 SeitenIrs qb1PachaiyappanRangaNathanNoch keine Bewertungen
- College Physics 2e-WEB 7zesafu RemovedDokument6 SeitenCollege Physics 2e-WEB 7zesafu RemovednantespieroNoch keine Bewertungen
- REHEAT-REGENERATIVE RANKINE CYCLE CalculatorDokument86 SeitenREHEAT-REGENERATIVE RANKINE CYCLE CalculatorJustine SomentacNoch keine Bewertungen
- T-S ChartDokument1 SeiteT-S ChartPhanhai KakaNoch keine Bewertungen
- Schema R717Dokument1 SeiteSchema R717KenosaNoch keine Bewertungen
- CO2 Mollier Chart PDFDokument1 SeiteCO2 Mollier Chart PDFmarko quirozNoch keine Bewertungen
- Gas Turbine Numerical PDFDokument5 SeitenGas Turbine Numerical PDFWaseem KhanNoch keine Bewertungen
- ThermodynamicsDokument3 SeitenThermodynamicsShyn GysawNoch keine Bewertungen
- TD 3 RD ModuleDokument35 SeitenTD 3 RD ModulemujeebNoch keine Bewertungen
- h64422 Suva410A Thermo Prop Eng PDFDokument24 Seitenh64422 Suva410A Thermo Prop Eng PDFMADhouse31Noch keine Bewertungen
- TERMOHIDRAULIKA CEVNIH SISTEMA KOTLOVA NA BLOKOVIMA B1 I B2 TERMOELEKTRANE „NIKOLA TESLA B” _ THERMALHYDRAULICS OF TUBE SYSTEMS IN STEAM BOILERS AT UNITS B1 AND B2 OF THERMAL POWER PLANT „NIKOLA TESLA B“Dokument10 SeitenTERMOHIDRAULIKA CEVNIH SISTEMA KOTLOVA NA BLOKOVIMA B1 I B2 TERMOELEKTRANE „NIKOLA TESLA B” _ THERMALHYDRAULICS OF TUBE SYSTEMS IN STEAM BOILERS AT UNITS B1 AND B2 OF THERMAL POWER PLANT „NIKOLA TESLA B“Nikola MilovanovićNoch keine Bewertungen
- Chapter 1Dokument27 SeitenChapter 1نزار الدهاميNoch keine Bewertungen
- Thermodynamic Properties of Fluids (Chap 3) SmithDokument70 SeitenThermodynamic Properties of Fluids (Chap 3) SmithS S S REDDY100% (1)
- ThermodynamicsDokument2 SeitenThermodynamicsbalusharma1212Noch keine Bewertungen
- Boris S. Bokstein, Mikhail I. Mendelev, David J. Srolovitz Thermodynamics and Kinetics in Materials Science A Short CourseDokument8 SeitenBoris S. Bokstein, Mikhail I. Mendelev, David J. Srolovitz Thermodynamics and Kinetics in Materials Science A Short CourseJuan Angel AlvaradoNoch keine Bewertungen
- XSteam Excel v2Dokument4 SeitenXSteam Excel v2asifbabuNoch keine Bewertungen
- Gardco ASTM ThermometersDokument15 SeitenGardco ASTM ThermometersmegacobNoch keine Bewertungen
- 11th Class Notes 2024 Physics CH 11Dokument22 Seiten11th Class Notes 2024 Physics CH 11abroasadali223Noch keine Bewertungen
- SCN1501 2024 A1Dokument4 SeitenSCN1501 2024 A1sizwegatsheni07Noch keine Bewertungen
- Catalog PT. WGTDokument22 SeitenCatalog PT. WGTRudy AriNoch keine Bewertungen