Beruflich Dokumente
Kultur Dokumente
Breast Reconstruction With SGAP and IGAP Flaps
Hochgeladen von
Jean-Philippe BinderCopyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Breast Reconstruction With SGAP and IGAP Flaps
Hochgeladen von
Jean-Philippe BinderCopyright:
Verfügbare Formate
BREAST
Breast Reconstruction with SGAP and IGAP Flaps
Maria M. LoTempio, M.D.
Robert J. Allen, M.D.
Charleston, S.C.; and Metairie, La.
Background: Perforator flaps represent the latest in the evolution of soft-tissue
flaps. They allow the transfer of the patients own skin and fat in a reliable
manner with minimal donor-site morbidity. The powerful perforator flap concept allows transfer of tissue from numerous, well-described donor sites to
almost any distant site with suitable recipient vessels. Large-volume flaps can be
reliably supported with perforators from areas such as the buttock and transferred microsurgically for breast reconstruction.
Indications: The ideal tissue for breast reconstruction is fat with or without skin,
not implants or muscle. Absolute contraindications specific to perforator flaps
in our practice include history of previous liposuction of the donor site, some
previous donor-site surgery, or active smoking (within 1 month before surgery).
Methods: Perforator flaps are supplied by blood vessels that arise from named,
axial vessels and perforate through or around overlying muscles and septa to
vascularize the overlying skin and fat. During flap harvest, these perforators are
meticulously dissected free from the surrounding muscle, which is spread in the
direction of the muscle fibers and preserved intact. The pedicle is anastomosed
to recipient vessels in the chest, and the donor site is closed without the use of
synthetic mesh.
Conclusion: Perforator flaps allow for safe, reliable tissue transfer from a variety
of sites and provide ideal tissue for breast reconstruction, with minimal donorsite morbidity. (Plast. Reconstr. Surg. 126: 393, 2010.)
n breast reconstruction, plastic surgeons commonly use silicone or saline implants. This technique has the advantages of minimal morbidity,
including immediate reconstruction, absence of a
donor site, and technical simplicity. Aesthetic results can range from acceptable to excellent with
implant placement, although these patients report that their result never feels natural. Approximately 25 percent of the women who present to
our group for breast reconstruction have had previous attempted implant reconstruction with failure. This accounts for approximately 600 breast
reconstructions over the past 17 years.
Breast reconstruction with perforator flaps has
allowed the transfer of the patients own skin and
fat in a reliable manner, with minimal donor-site
morbidity since 1992.1 This is the most recent development in the evolution of flaps for breast reconstruction. Flaps that relied on a random pattern blood supply were soon replaced by pedicled,
From the Division of Plastic Surgery, Medical University of
South Carolina, and the Section of Plastic Surgery, Omega
Hospital, Louisiana State University Health Sciences Center.
Received for publication June 5, 2007; accepted February 10,
2010.
Copyright 2010 by the American Society of Plastic Surgeons
DOI: 10.1097/PRS.0b013e3181de236a
axial pattern flaps that could reliably transfer greater
amounts of tissue. The inception of free tissue transfer allowed an infinite range of possibilities to appropriately match donor and recipient sites.2
Perforator flaps are not without their challenges, including variability of vascular anatomy.
Judgment as to how many, what size, and the location of perforators affect factors such as length
of surgery and incidence of postoperative fat necrosis. Flap insetting and vascular territory depend
on the above factors affecting flap circulation.
Fujino et al. first described the superior gluteal
myocutaneous free flap in 1975 for breast reconstruction. Shaw popularized the myocutaneous superior gluteal artery free flap; however, a short
vascular pedicle often led to additional vein grafting, thus limiting its popularity.37 In 1978, LeQuang performed the first breast reconstruction
with an inferior gluteal myocutaneous free flap.8
The inferior gluteal myocutaneous flap championed by Paletta et al. was mostly abandoned, pre-
Disclosure: The authors have no financial interest
in this research project or in any of the techniques or
equipment used in this study.
www.PRSJournal.com
393
Plastic and Reconstructive Surgery August 2010
sumably because of sciatic nerve injury exposure
and pain when sitting.9
The superior gluteal artery perforator flap
(SGAP) and the inferior gluteal artery perforator
(IGAP) flap were first introduced by our group in
1993. The advantages of the gluteal artery perforator flap include preservation of the gluteus
maximus muscle and elongation of the pedicle. In
our group, bilateral simultaneous SGAP/IGAP
flaps are performed but require two skilled microsurgeons to harvest the flaps.10,11 With preoperative use of computed tomographic/magnetic
resonance imaging angiograms, septocutaneous
SGAP/IGAP flaps for breast reconstruction are
now being performed.12 The angiograms allow us
to visualize the key perforators being musculocutaneous or septocutaneous, and the caliber, location, and course. As with other perforator flaps,
donor-site morbidity is minimal and no sacrifice of
muscle is required. Overall, we have used the
SGAP flap more than the IGAP flap, but the upper
buttock donor site may have a scooped-out appearance. The IGAP flap is a good option when
buttock tissue is used and the patient has a saddlebag deformity because of an improved donor-site
contour and the scar is hidden in the crease.1315
However, these techniques have brought new difficulties and problems that must be addressed. First
and foremost, these techniques require microsurgical expertise. Dr. Bill Shaw expressed that a superspecialist might perform certain types of free flaps
beyond the realm of the occasional microsurgeon
such as gluteal artery perforator flap breast reconstruction. The learning curve for perforator flap
breast reconstruction is estimated to be approximately 50 to 100 procedures.
The buttock is a good choice for breast reconstruction when the abdomen is not a viable option,
as is the medial thigh free flap. In our patient
population, the buttock is the donor site in 15
percent, the abdomen in 7 percent, and the medial thigh in 15 percent. Donor-site morbidity is
minimal, and no sacrifice of muscle is required.
Various locations, orientations, and dimensions of
the skin island have been attempted over the years.
Each has advantages and disadvantages. Initially,
we used an oblique ellipse totally over the muscle
oriented in the direction of the muscle fibers. This
gave the greatest chance of finding an adequate
perforator under the flap. With better appreciation of the vascular anatomy and confidence in
preoperative computed tomography and magnetic resonance imaging, there is more freedom is
designing the skin island. An oblique ellipse extending in the upper buttock superior from me-
394
dial to lateral has the advantage of concealing the
scar in swimwear and undergarments. By beveling
superiorly, a nicely shaped flap with less contour
deformity can be obtained. In 2004, we began
designing the IGAP flap so that the scar would be
in the natural inferior crease. By harvesting tissue
from the lowest part of the buttock and beveling
inferiorly, the shape of the rounded upper buttock was preserved. The pedicle length was often
longer than that of the SGAP flap, making the
anastomosis easier and negating the need to remove
rib cartilage because less length was required on the
recipient vessels. However, sitting directly on the
healing incision causes more pain than the SGAP
flap in the early postoperative period, and the rate
of dehiscence increases. This is particularly true in
bilateral simultaneous reconstructions where the patient cannot shift weight bearing to the nonoperated
side. The sciatic nerve has never been a problem in
our experience with approximately 120 IGAP flaps.
However, some small sensory nerves may have to be
divided with flap harvest. The ideal candidate is
someone with a large buttock (pear shape) and a B
size breast. In the right candidate, the in-the-crease
IGAP flap can give an excellent breast reconstruction with a hardly noticeable donor site. After initial
enthusiasm with the in-the-crease IGAP flap, we are
now using the SGAP flap in slightly more than 50
percent of our patients. Ultimately, the women do
their research and come with their opinions about
which donor site they prefer. Advantages and disadvantages of SGAP and IGAP donor sites are compared in Table 1).
The gluteal artery perforator is an excellent
option for breast reconstruction. This flap can also
be used as a pedicled flap for coverage of other
areas, in particular, pressure sores.1518
INDICATIONS
Women who have undergone mastectomies
and wish to undergo reconstruction with autologous tissue are potential candidates for SGAP or
IGAP flaps. Those in whom the abdomen cannot
be used as a donor site either because of previous
Table 1. Advantages and Disadvantages of SGAP
and IGAP Donor Sites
Donor-Site Comparison
Scar concealed with swimsuit
Upper buttock fullness maintained
Saddlebag correction
Possible hip roll improvement
Tenderness sitting early postoperatively
Longer pedicle
SGAP
IGAP
Yes
No
No
Yes
No
No
No
Yes
Yes
No
Yes
Yes
Volume 126, Number 2 Reconstruction with SGAP and IGAP Flaps
abdominoplasty or liposuction or who have more
excess tissue in the buttock area than in the abdomen are the best candidates. The buttock has a
high fat-to-skin ratio, whereas the abdomen has a
high skin-to-fat ratio. Patients who require mostly
fat and little skin may be candidates for SGAP/
IGAPS flaps. A significant amount of tissue may be
harvested and, in our experience, the average final
inset weights of our SGAP and IGAP flaps were
slightly greater than weights of the mastectomy
specimens removed.
Absolute contraindications specific to SGAP/
IGAP flap breast reconstruction include previous
liposuction at the donor site or active smoking within
1 month before surgery. Liposuction of the upper
buttock is rare and does not often affect harvesting
of the SGAP flap; however, liposuction of the saddlebag area can affect the IGAP flap viability.
ANATOMY
The superior gluteal artery is a continuation of
the posterior division of the internal iliac artery. It
is a short artery, which runs dorsally between the
lumbosacral trunk and the first sacral nerve. It emanates from the pelvis above the upper border of the
piriformis muscle, where it soon divides into both
superficial and deep branches. The deep branch
travels between the iliac bone and gluteus medius
muscle. The superficial branch continues to give off
contributions to the upper portion of the gluteus
muscle and overlying fat and skin. Anatomical location is planned when the femur is slightly flexed and
rotated inward; a line is drawn from the posterior
superior iliac spine to the posterior superior angle of
the greater trochanter. The point of entrance of the
superior gluteal artery from the upper part of the
greater sciatic foramen corresponds to the junction
of the upper and middle thirds of this line. Perforating vessels are found off the superior branch of
the superior gluteal artery.19,20
The inferior gluteal artery is a terminal branch
of the anterior division of the internal iliac artery
and exits the pelvis through the greater sciatic
foramen.21,22 Landmarks can also be used to identify the location of the emergence of the inferior
gluteal artery outside the pelvis. A line is drawn
from the posterior superior iliac spine to the outer
part of the ischial tuberosity; the junction of its lower
with its middle third marks the point of emergence
of the inferior gluteal and its surrounding vessels
from the lower part of the greater sciatic foramen.
The artery accompanies the greater sciatic nerve,
internal pudendal vessels, and the posterior femoral
cutaneous nerve. In this subfascial recess, the inferior gluteal vein will receive tributaries from other
pelvic veins. The inferior gluteal vasculature continues toward the surface by perforating the sacral fascia. It exits the pelvis caudal to the piriformis muscle.
Once under the inferior portion of the gluteus maximus, perforating vessels are seen branching out
through the substance of the muscle to feed the
overlying skin and fat. The course of the inferior
gluteal artery perforating vessels is more oblique
through the substance of the gluteus maximus muscle than the course of the superior gluteal artery
perforators, which tend to travel more directly to the
superficial tissue up through the muscle. Thus, the
length of the inferior gluteal artery perforator and
the resultant pedicle length for the IGAP flap is 7 to
10 cm. The SGAP pedicle is 5 to 8 cm in length.
Because the skin island is placed inferior to the origin of the inferior gluteal vessels, a longer pedicle
is usually obtained.
The direction of the perforating vessels can be
superior, lateral, or inferior. Perforating vessels
that nourish the medial and inferior portions of
the buttock have relatively short intramuscular
lengths, between 5 and 7 cm, depending on the
thickness of the muscle. Perforators that nourish
the lateral portions of the overlying skin paddle
are observed traveling through the muscle substance
in an oblique manner 4 to 6 cm before turning
upward toward the skin surface. By traveling through
the muscle for relatively long distances, these vessels
are longer than their medially based counterparts.
The perforating vessels can be separated from the
underlying gluteus maximus muscle and fascia and
traced down to the parent vessel, forming the basis
for the inferior gluteal artery perforator flap. Between two and four perforating vessels originating
from the inferior gluteal artery will be located in the
lower half of the gluteus maximus.12
After giving off perforators in the buttocks, the
inferior gluteal artery then descends into the thigh
accompanied by the posterior femoral cutaneous
nerve and follows a long course, eventually surfacing to supply the skin of the posterior thigh.15
The branches of the inferior gluteal nerve (fifth
lumbar and first and second sacral nerves) supply
the skin of the inferior buttock. A neurosensory
flap can be elevated if these nerves are preserved
in the dissection of the flap.16,17
The superior gluteal nerve arises from the dorsal divisions of the fourth and fifth lumbar and first
sacral nerves. It exits the pelvis through the greater
sciatic foramen above the piriformis muscle, accompanied by the superior gluteal vessels, and
divides into both superior and inferior branches.
The superior and inferior branches of the nerves
travel with their corresponding arterial branches
395
Plastic and Reconstructive Surgery August 2010
to end up in the gluteus medius, gluteus minimus,
and tensor fasciae latae, respectively.
The inferior gluteal nerve arises from the dorsal divisions of the fifth lumbar and first and second sacral nerves. It exits the pelvis through the
greater sciatic foramen, below the piriformis muscle, and divides into branches that enter the deep
surface of the gluteus maximus.
The posterior femoral cutaneous nerve innervates the skin of the perineum and posterior surface
of the thigh and leg. It arises partly from the dorsal
divisions of the first and second and from the ventral
divisions of the second and third sacral nerves, and
issues from the pelvis through the greater sciatic
foramen below the piriformis muscle, along with the
inferior gluteal artery. It then descends beneath the
gluteus maximus, the fascia lata, and travels over
the long head of the biceps femoris to the posterior
knee. Finally, it pierces the deep fascia and accompanies the lesser saphenous vein to the middle of the
posterior leg. Some terminal branches communicate with the sural nerve. All its branches are cutaneous and distributed to the gluteal region, the perineum, and the posterior thigh and leg.
SURGICAL TECHNIQUE
The patient usually is seen in our office 1 day before surgery. The surgical plan again is reviewed with
the patient, and any remaining questions are answered.
The chest is marked in the sitting position.
The midline and the inframammary crease on
both sides are marked. For patients undergoing
immediate breast reconstruction, suggested skin
markings are drawn on the breast, which include
marks around the nipple-areola complex and previous biopsy site. In patients who are undergoing
a nipple-sparing mastectomy, a vertical, lateral, or
inframammary incision is marked.
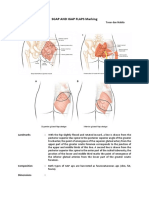
For unilateral SGAP flap markings, the patient is
placed in the lateral decubitus position. Preoperative computed tomography, magnetic resonance angiography, and/or a Doppler probe is used to locate
perforating vessels from the superior gluteal artery.
These are usually located approximately one-third of
the distance on a line from the posterior superior
iliac crest to the greater trochanter. Additional perforators may be found slightly more lateral from
above. It should be noted that perforators located
laterally would produce longer pedicles. Septocutaneous perforators are the most lateral and course
between the gluteal maximus and medius. The skin
paddle is marked in an oblique pattern from inferomedial to superolateral to include these perforators.
On average, the flap height is 7 to 10 cm and the flap
396
length is 18 to 22 cm. For bilateral SGAP planning,
the patient is marked in the prone position.
For the IGAP flap, the gluteal fold is noted with
the patient in the standing position. The inferior
limit of the flap is marked 1 cm inferior and parallel
to the gluteal fold. The patient is then placed in the
lateral position for unilateral reconstruction and the
prone position for bilateral reconstruction. Computed tomography or magnetic resonance angiography and the Doppler probe are used to locate
perforating vessels from the inferior gluteal artery.
An ellipse is drawn for the skin paddle to include
these perforators, which roughly parallels the gluteal
fold with dimensions of approximately 7 18 cm.
For correction of a saddlebag deformity, the skin
pattern is shifted laterally. This also prevents harvesting the fat pad over the ischial tuberosity medial
to the gluteus maximus muscle.
For unilateral procedures, the patient is placed
in the lateral decubitus position and a two-team approach is used. The recipient vessels are prepared
while the SGAP/IGAP flap is harvested. For breast
reconstruction, the internal mammary vessels or
internal mammary perforators are preferred, as
anastomosis to these vessels allows easier medialization of the flap when it is inset. The IGAP flap
often has a long enough pedicle that will reach to
the thoracodorsal vessels; however, the SGAP flap
may be challenging because of a shorter pedicle.
For bilateral simultaneous gluteal artery perforator flap reconstruction, the procedure is started
supine. After mastectomy and recipient vessel
preparation, the patient is positioned for flap harvest. Then, the patient is repositioned supine for
anastomosis and insetting.
The skin incisions are made and Bovie electrocautery is used to divide the flap down to the
muscle of the gluteus maximus. Significant beveling is used as needed, particularly lateral to the
muscle superior and inferior to harvest enough
tissue for width and volume to create a natural
breast shape. The flap is elevated from the muscle
in the subfascial plane and the perforators approached beginning from lateral to medial or medial to lateral. Use of a single large perforator is
preferred, if it is present, but several perforators,
which lie in the same plane and the direction of
the gluteus maximus muscle fibers, can be taken
together as well. The muscle is then spread in the
direction of the muscle fibers and the perforators
followed through the muscle. The dissection continues until both the artery and the vein are of
sufficient size to be anastomosed to the recipient
vessels in the chest. The artery usually is the limiting factor in this dissection. The arterial perfo-
Volume 126, Number 2 Reconstruction with SGAP and IGAP Flaps
Fig. 1. Case 1. (Above, left) Preoperative view of a patient with ductal carcinoma in situ of the left breast who underwent a
mastectomy with SGAP flap reconstruction and a symmetrical procedure of the right breast with SGAP flap reconstruction.
(Below, left) Preoperative view of the donor SGAP flap site. (Above, right) Appearance after the patient had undergone reconstruction with bilateral SGAP flap and second-stage nipple reconstruction. (Below, right) Postoperative view of the healed
bilateral SGAP flap donor sites.
rator is visualized and preserved as it enters the
main ascending superior gluteal artery or the descending inferior gluteal artery. The preferable
artery and vein diameter for anastomosis is 2.0 to
2.5 mm and 3.0 to 4.0 mm, respectively. When
using the internal mammary vein perforators as
recipient, a shorter pedicle and smaller artery will
suffice, thereby simplifying flap harvest.
Harvesting the in-the-crease IGAP flap allows
more beveling superiorly and inferiorly because
soft-tissue deficiency in the crease is normal. Laterally thicker fat from the trochanteric area can be
taken, increasing flap volume and decreasing the
saddlebag deformity. When harvesting the IGAP
flap, care must be taken to preserve the lighter
colored medial fat pad, which overlies the ischium
medial to the gluteus maximus muscle. Preservation of this specialized fat pad will prevent possible
donor-site discomfort when sitting in the future.
When the recipient vessels are ready, the
gluteal artery and vein are divided and the flap
harvested and weighed. The skin and fat overlying the gluteus maximus muscle and posterior
thigh with the IGAP flap are elevated superiorly
and inferiorly to allow layered approximation of
the fat of the donor site to prevent a contour
deformity. The donor site is closed in layers over
a suction drain with absorbable suture. Adding
a permanent removable skin suture increases
the strength of the skin closure.
The anastomosis is performed to the recipient
vessels under the operating microscope. The flap
is inset over a suction drain into the breast pocket,
with care taken not to twist or kink the pedicle. To
create a spherical flap, the ends of the ellipse are
excised or approximated. The flap may be inset
horizontally, vertically, or obliquely, depending
on the situation.
397
Plastic and Reconstructive Surgery August 2010
CASE REPORTS
Case 1
A 52-year-old woman presented with a history of ductal carcinoma of the left breast. She underwent a left skin-sparing
mastectomy with an SGAP flap reconstruction. On the contralateral side, she underwent a right prophylactic nipple-sparring mastectomy with breast reconstruction using an SGAP flap
(Fig. 1). Her gluteal region shows adequate adiposity to make
a full B-cup size (Fig. 1). Her scars were symmetrical, preserving
the natural buttock contour (Fig. 1).
Case 2
A 44-year-old patient presented who had undergone a right
mastectomy for invasive ductal carcinoma (Fig. 2). In Figure 2,
the preoperative markings are shown. She has more inferior
gluteal adiposity versus superior gluteal fat with which to reconstruct her right breast to match her opposite breast. Patients
will often have an opinion regarding the location from which
they would like the gluteal tissue to be taken. This patient
preferred her lower buttock to be used. The marks are where
the best Doppler signals were heard. The skin pattern is drawn
in relations to these perforators. At 6-month follow-up, the
patient has a right reconstructed breast matching her left side
The buttock scar is in the crease (Fig. 2).
Case 3
A 42-year-old patient presented who had bilateral mastectomies for ductal carcinoma with tissue expander placement (Fig.
3). In Figure 3, the preoperative markings for a bilateral septocutaneous gluteal artery perforator flap are drawn based on
a computed tomographic angiogram depicting the septocutaneous gluteal artery perforators. Figure 3 shows the computed
tomographic angiogram and the patient 6 months after bilateral septocutaneous SGAP flap reconstruction.
POSTOPERATIVE CARE
Currently in our unit, patients have a 1- to
2-hour stay in the recovery room and then are
transferred to their private room, where they
have monitoring of the flap circulation every 2
hours for the night and then every 4 hours.
The intensive care unit is not needed. Patients
typically go home on the third or fourth postoperative day. The drain at the donor site usually will be left in place for at least 10 days.
Breast drains are usually removed on postoperative day 3.
Fig. 2. Case 2. (Above, left) Preoperative view of a woman who had undergone a right mastectomy secondary to breast cancer.
(Below, left) Preoperative view of the right IGAP flap donor site. (Above, right) Postoperative view of the patient after a right IGAP flap
breast reconstruction. (Below, right) Postoperative view of the healed right donor sites of the IGAP flap.
398
Volume 126, Number 2 Reconstruction with SGAP and IGAP Flaps
Fig. 3. Case 3. (Above, left) Preoperative view. (Above, right) Computed tomographic angiogram showing the septocutaneous gluteal artery perforators. (Center, left and center, right) Preoperative markings of the septocutaneous
gluteal artery perforator flap. (Below, left and below, right) Appearance 6 months postoperatively.
ADVANTAGES
Both the SGAP flap and the IGAP flap are excellent for breast reconstruction, especially when
200 to 600 g is needed. The SGAP flap is a thick flap,
with an adequate pedicle (5 to 7 cm) for breast
reconstruction. It has a better donor site with the
superior lateral flap design, and the scar is concealed
by a swimsuit.
The IGAP flap is a thick flap, with longer pedicle (7 to 10 cm). The scar is hidden if done in the
399
Plastic and Reconstructive Surgery August 2010
crease, there are fewer contour defects compared
with the SGAP flap, and there is a long pedicle that
can be anastomosed to the thoracodorsal system
for breast reconstruction if the internal mammary
vessels are not available.
DISADVANTAGES
Disadvantages of the SGAP flap include contour defects, visible scar, and loss of padding on
thin patients. Disadvantages of the IGAP flap include the fact that the donor site may be painful
to sit on for 3 to 6 weeks. Injury to the small
cutaneous nerves during pedicle dissection is a
possibility. There is also donor scar show with
French cut swimsuits.
COMPLICATIONS
In a review of 492 gluteal artery perforator
flaps performed by our unit for breast reconstruction, the incidence of complications was
low. The overall take-back rate for vascular complications was 6 percent, with the most common
being venous (4 percent) and arterial (2 percent). The total flap failure rate was approximately 2 percent. Donor-site seroma occurred in
15 percent of patients, requiring aspiration. Approximately 20 percent of patients required revision of the donor site at the second stage of
breast reconstruction.19,21
The most common reason for donor-site revisions of the SGAP flap is contour deformity of
the upper buttock. The most common revision for
the donor site IGAP flap is liposuction of the lateral trochanter fat for contouring. Dog-ear revisions are often performed at the time of secondstage breast reconstruction for both SGAP and
IGAP flaps.
Recipient-site complications include a fat necrosis rate of 8 percent, with both SGAP and IGAP
flaps requiring revision. Breast flap contour asymmetry requires fat grafting or revision in approximately 10 percent of cases.
CONCLUSIONS
Perforator flaps have raised the standard in
breast reconstruction. By replacing like with like,
we can achieve permanent natural results with
minimal donor-site deformities. Being able to
choose from many donor-site options makes virtually all patients candidates for this method of
autogenous reconstruction. To make this option
more available and desirable, there is plenty of
room for improvement. The length of the procedure needs to be decreased, scars and buttock
contour need to be improved, and complications
400
need to be decreased. With improvements in technology and technique, these goals can be realized.
The in-the-crease IGAP flap offers preservation of
buttock shape, a scar hidden in a natural crease,
and adequate thickness fat for a youthful, attractive breast. The SGAP flap has little or no
postoperative pain and leaves a scar easily concealed with swimwear. The septocutaneous
SGAP flap allows a more favorable donor site
more superolateral in the hip roll area. The
contour can be quite good without taking a flap
that is too large and performing a buttock lift
with proper-layered closure.
Maria M. LoTempio, M.D.
55 East 87th Street
New York, N.Y. 10128
mlotempio@yahoo.com
REFERENCES
1. Allen RJ, Treece P. Deep inferior epigastric perforator flap
for breast reconstruction. Ann Plast Surg. 1994;32:
3238.
2. Taylor GI, Daniel RK. The anatomy of several free flap donor
sites. Plast Reconstr Surg. 1975;56:243253.
3. Shaw WW. Breast reconstruction by superior gluteal microvascular free flaps without silicone implants. Plast Reconstr
Surg. 1983;72:490501.
4. Shaw WW. Microvascular free flap breast reconstruction. Clin
Plast Surg. 1984;11:333341.
5. Shaw WW. Superior gluteal free flap breast reconstruction.
Clin Plast Surg. 1998;25:267274.
6. Codner MA, Nahai F. The gluteal free flap breast reconstruction: Making it work. Clin Plast Surg. 1994;24:289296.
7. Fujino T, Harashina T, Aoyagi F. Reconstruction for aplasia
of the breast and pectoral region by microvascular transfer
of a free flap from the buttock. Plast Reconstr Surg. 1975;56:
178181.
8. Le-Quang C. Secondary microsurgical reconstruction of the
breast and free inferior gluteal flap (in French). Ann Chir
Plast Esthet. 1992;37:723741.
9. Paletta CE, Bostwick J III, Nahai F. The inferior gluteal free
flap in breast reconstruction. Plast Reconstr Surg. 1989;84:
875883.
10. Guerra AB, Soueid N, Metzinger SE, et al. Simultaneous
bilateral breast reconstruction with superior gluteal artery
perforator (SGAP) flaps. Ann Plast Surg. 2004;53:305310.
11. DellaCroce FJ, Sullivan SK. Application and refinement of
the superior gluteal artery perforator free flap for bilateral
simultaneous breast reconstruction. Plast Reconstr Surg. 2005;
116:97103; discussion 104105.
12. Tuinder S, Van Der Hulst R, Lataster A, Boeckx W. Superior gluteal artery perforator flap based on septal perforators: Preliminary study. Plast Reconstr Surg. 2008;122:
146e148e.
13. Allen RJ, Tucker C Jr. Superior gluteal artery perforator free
flap for breast reconstruction. Plast Reconstr Surg. 1995;95:
12071212.
14. Allen RJ, Levine JL, Granzow JW. The in-the-crease inferior
gluteal artery perforator flap for breast reconstruction. Plast
Reconstr Surg. 2006;118:333339.
Volume 126, Number 2 Reconstruction with SGAP and IGAP Flaps
15. Granzow JW, Levine JL, Chiu ES, Allen RJ. Breast reconstruction with gluteal artery perforator flaps. J Plast Reconstr
Aesthet Surg. 2006;59:571579.
16. Koshima I, Moriguchi T, Soeda S, Kawata S, Ohta S, Ikeda A.
The gluteal perforator-based flap for repair of sacral pressure
sores. Plast Reconstr Surg. 1993;91:678683.
17. Windhofer C, Brenner E, Moriggl B, Papp C. Relationship
between the descending branch of the inferior gluteal artery
and the posterior femoral cutaneous nerve applicable to flap
surgery. Surg Radiol Anat. 2002;24:253257.
18. Blondeel PN. The sensate free superior gluteal artery perforator (S-GAP) flap: A valuable alternative in autologous
breast reconstruction. Br J Plast Surg. 1999;52:185193.
19. Guerra A, Metzinger S, Gill Bidros R, et al. Breast reconstruction with gluteal artery perforator (GAP) flaps: A
critical analysis of 142 cases. Ann Plast Surg. 2004;52:118
125.
20. Strauch B, Yu HL. Gluteal region. In: Strauch B, Yu HL, eds.
Atlas of Microvascular Surgery: Anatomy and Operative Approaches. New
York: Thieme Medical; 1993:102119.
21. Ao M, Mae O, Namba Y, Asagoe K. Perforator-based flap for
coverage of lumbosacral defects. Plast Reconstr Surg. 1998;
101:987991.
22. Roche NA, Van Landuyt K, Blondeel PN, Matton G, Monstrey
SJ. The use of pedicled perforator flaps for reconstruction of
lumbosacral defects. Ann Plast Surg. 2000;45:714.
Instructions for Authors: Update
Registering Clinical Trials
Beginning in July of 2007, PRS has required all articles reporting results of clinical trials to be registered in
a public trials registry that is in conformity with the International Committee of Medical Journal Editors
(ICMJE). All clinical trials, regardless of when they were completed, and secondary analyses of original clinical
trials must be registered before submission of a manuscript based on the trial. Phase I trials designed to study
pharmacokinetics or major toxicity are exempt.
Manuscripts reporting on clinical trials (as defined above) should indicate that the trial is registered and
include the registry information on a separate page, immediately following the authors financial disclosure
information. Required registry information includes trial registry name, registration identification number,
and the URL for the registry.
Trials should be registered in one of the following trial registries:
http://www.clinicaltrials.gov/ (Clinical Trials)
http://actr.org.au (Australian Clinical Trials Registry)
http://isrctn.org (ISRCTN Register)
http://www.trialregister.nl/trialreg/index.asp (Netherlands Trial Register)
http://www.umin.ac.jp/ctr (UMIN Clinical Trials Registry)
More information on registering clinical trials can be found in the following article: Rohrich, R. J., and
Longaker, M. T. Registering clinical trials in Plastic and Reconstructive Surgery. Plast. Reconstr. Surg. 119: 1097,
2007.
401
Das könnte Ihnen auch gefallen
- Nipple ReconstructionDokument116 SeitenNipple ReconstructionMaxim PekarevNoch keine Bewertungen
- Secondary Deofrmities of Cleft Lip NoseDokument8 SeitenSecondary Deofrmities of Cleft Lip NoseahmedatefNoch keine Bewertungen
- Expanded Maternity Leave Law 2019Dokument3 SeitenExpanded Maternity Leave Law 2019Alliah Climaco ÜNoch keine Bewertungen
- Cleft Lip and Palate An Evidence Based Review PDFDokument16 SeitenCleft Lip and Palate An Evidence Based Review PDFariskaNoch keine Bewertungen
- SinulDokument86 SeitenSinulCucos NataliaNoch keine Bewertungen
- Conceptual Frameworks and Accounting Standard - ProblemsDokument3 SeitenConceptual Frameworks and Accounting Standard - ProblemsIris Mnemosyne100% (1)
- BJT Frequency ResponseDokument32 SeitenBJT Frequency ResponseBryan Owen Salcedo SantosNoch keine Bewertungen
- Cleft Lip Cleft Palate: Presented By: Ivan AvenadoDokument16 SeitenCleft Lip Cleft Palate: Presented By: Ivan AvenadoIvan Liquiran AvenadoNoch keine Bewertungen
- Apert Syndrome - Sultan BaroamaimDokument37 SeitenApert Syndrome - Sultan BaroamaimsultanNoch keine Bewertungen
- LAWDokument8 SeitenLAWNoha GuianodinNoch keine Bewertungen
- Ptosis Evaluation and ManagementDokument6 SeitenPtosis Evaluation and ManagementMitaInnanaNoch keine Bewertungen
- Maternity LeaveDokument4 SeitenMaternity LeaveFrances LorenzoNoch keine Bewertungen
- Common Craniofacial SyndromesDokument126 SeitenCommon Craniofacial Syndromesdr parveen bathlaNoch keine Bewertungen
- Analytic Techniques and Instrumentation Lecture FormatDokument15 SeitenAnalytic Techniques and Instrumentation Lecture FormatMandy A. DelfinNoch keine Bewertungen
- TMJ AnkylosisDokument41 SeitenTMJ AnkylosisSaritha DeviNoch keine Bewertungen
- Toyota Shaw V CA Digest For SalesDokument2 SeitenToyota Shaw V CA Digest For SaleschaynagirlNoch keine Bewertungen
- Introduction To General Ethics and Ethical Values2Dokument2 SeitenIntroduction To General Ethics and Ethical Values2Decim VirateNoch keine Bewertungen
- Wurgler Baker Investor SentimentDokument23 SeitenWurgler Baker Investor SentimentJohnathan WangNoch keine Bewertungen
- Carpal Tunnel SyndromeDokument7 SeitenCarpal Tunnel Syndromeclaudia100% (1)
- Atopic DermatitisDokument9 SeitenAtopic DermatitisJorge De VeraNoch keine Bewertungen
- Cat DissectionDokument63 SeitenCat DissectionPaul AsturbiarisNoch keine Bewertungen
- Climate Change IPCC Report Is 'Code Red For Humanity', UN...Dokument3 SeitenClimate Change IPCC Report Is 'Code Red For Humanity', UN...UtkuNoch keine Bewertungen
- Independent and Dependent Variables Operational Definitions Evaluating Operational Definitions Planning The Method SectionDokument31 SeitenIndependent and Dependent Variables Operational Definitions Evaluating Operational Definitions Planning The Method SectionShahid Ali DurraniNoch keine Bewertungen
- Latissimus Dorsi Flap To BreastDokument13 SeitenLatissimus Dorsi Flap To Breastokida192100% (1)
- Tessier CleftDokument37 SeitenTessier CleftJeel MohtaNoch keine Bewertungen
- SS Plastic - General Principles (2014)Dokument11 SeitenSS Plastic - General Principles (2014)ChethranNoch keine Bewertungen
- Willian Seguerra - Week 3 - Task 1Dokument4 SeitenWillian Seguerra - Week 3 - Task 1Willian SeguerraNoch keine Bewertungen
- E306 Analysis and ConclusionDokument1 SeiteE306 Analysis and ConclusionAlyssa AltreNoch keine Bewertungen
- Neck Lump HistoryDokument4 SeitenNeck Lump HistoryAlmomnbllah Ahmed100% (1)
- Sgap and Igap Flaps MarkingDokument6 SeitenSgap and Igap Flaps MarkingFeliciaDewiNoch keine Bewertungen
- RRLDokument96 SeitenRRLKristiene Kyle AquinoNoch keine Bewertungen
- Cleft Lip, Cleft Palate, and Velopharyngeal.36Dokument19 SeitenCleft Lip, Cleft Palate, and Velopharyngeal.36Jimmy Mejía Cirujano PlásticoNoch keine Bewertungen
- Final ArthridesDokument22 SeitenFinal ArthrideskizpirinNoch keine Bewertungen
- NSTP ReportDokument9 SeitenNSTP ReportCarmela CantalejoNoch keine Bewertungen
- SamplingDokument10 SeitenSamplingmichelle granado100% (1)
- VBACDokument12 SeitenVBACSakinah Roslan100% (1)
- Chapter 11 AnsDokument14 SeitenChapter 11 AnsZen TungpalanNoch keine Bewertungen
- Laboratory Exercise No 3BDokument6 SeitenLaboratory Exercise No 3BMichael AbeledaNoch keine Bewertungen
- Should A Country Adopt Fixed or Flexible Exchange Rate System?Dokument8 SeitenShould A Country Adopt Fixed or Flexible Exchange Rate System?Zakaria CassimNoch keine Bewertungen
- FAQs Solo Parent and Parental LeaveDokument3 SeitenFAQs Solo Parent and Parental Leavemolv22Noch keine Bewertungen
- Adjusting EntriesDokument3 SeitenAdjusting EntriesMarini HernandezNoch keine Bewertungen
- Body Dysmorphic TraynorDokument12 SeitenBody Dysmorphic TraynornoorgianilestariNoch keine Bewertungen
- Perineal RationaleDokument2 SeitenPerineal RationaleNikki GuisonNoch keine Bewertungen
- Gluteal Artery Perforator FlapsDokument8 SeitenGluteal Artery Perforator FlapsJose Mauricio Suarez BecerraNoch keine Bewertungen
- Sps 20079Dokument10 SeitenSps 20079Zouzou YuNoch keine Bewertungen
- Tips and Tricks For Diep Flap Breast Reconstruction in Patients With Previous Abdominal ScarDokument11 SeitenTips and Tricks For Diep Flap Breast Reconstruction in Patients With Previous Abdominal ScarKhalid AlmutairiNoch keine Bewertungen
- Journal Pre-Proof: Jpras OpenDokument28 SeitenJournal Pre-Proof: Jpras OpenDika CodNoch keine Bewertungen
- Updates in Aesthetic Surgery II 1112 Article.10Dokument14 SeitenUpdates in Aesthetic Surgery II 1112 Article.10Hải Dương MinhNoch keine Bewertungen
- Revision in Autologous Breast ReconstructionDokument24 SeitenRevision in Autologous Breast ReconstructionJose Mauricio Suarez BecerraNoch keine Bewertungen
- Near-Circumferential Lower Body Lift: A Review of 40 Outpatient ProceduresDokument8 SeitenNear-Circumferential Lower Body Lift: A Review of 40 Outpatient ProceduresYoussif KhachabaNoch keine Bewertungen
- Sozer 2018Dokument33 SeitenSozer 2018МаратNoch keine Bewertungen
- Breast Reconstruction With Microvascular MS-TRAM and DIEP FlapsDokument8 SeitenBreast Reconstruction With Microvascular MS-TRAM and DIEP FlapsisabelNoch keine Bewertungen
- Lipoplasty of The BackDokument7 SeitenLipoplasty of The BackAng BiNoch keine Bewertungen
- Surgical Correction of Gynecomastia With Minimal Scarring: Multimediamanuscript AestheticDokument5 SeitenSurgical Correction of Gynecomastia With Minimal Scarring: Multimediamanuscript AestheticAngga Putra Kusuma KusumaNoch keine Bewertungen
- Plastic Surgery: Understanding Abdominoplasty and LiposuctionDokument14 SeitenPlastic Surgery: Understanding Abdominoplasty and LiposuctiondoctorbanNoch keine Bewertungen
- Extended Abdominoplasty - Applications and A New Classification System For AbdominoplastyDokument7 SeitenExtended Abdominoplasty - Applications and A New Classification System For AbdominoplastyDaniel Alfonso Gonzalez MercadoNoch keine Bewertungen
- Sleeve-Gastrectomy 2011 BrethauerDokument15 SeitenSleeve-Gastrectomy 2011 BrethauerDavid Schnettler RodriguezNoch keine Bewertungen
- Umbilicalherniarepair: Overview of Approaches and Review of LiteratureDokument16 SeitenUmbilicalherniarepair: Overview of Approaches and Review of LiteratureVictor Matias BarriosNoch keine Bewertungen
- Anatomically Based Breast Augmentation: A 6-Plane Autologous ApproachDokument2 SeitenAnatomically Based Breast Augmentation: A 6-Plane Autologous Approachcusom34Noch keine Bewertungen
- Jurnal Breast Rekon Presentasi 10 Okt 18 RevisiDokument57 SeitenJurnal Breast Rekon Presentasi 10 Okt 18 RevisiFebriadi RNoch keine Bewertungen
- Science - 13 May 2022Dokument180 SeitenScience - 13 May 2022Jean-Philippe BinderNoch keine Bewertungen
- Encyclopedia of German Tanks of World War TwoDokument275 SeitenEncyclopedia of German Tanks of World War TwoJean-Philippe Binder100% (1)
- 2003 10 Liposuction and ComplicationsDokument7 Seiten2003 10 Liposuction and ComplicationsJean-Philippe BinderNoch keine Bewertungen
- Assimilkanji PDFDokument136 SeitenAssimilkanji PDFJean-Philippe BinderNoch keine Bewertungen
- How It Works - Book of Did You Know 3rd EditionDokument132 SeitenHow It Works - Book of Did You Know 3rd EditionJean-Philippe Binder100% (9)
- The Swift Programming Language in French Ohxpr832Dokument27 SeitenThe Swift Programming Language in French Ohxpr832Jean-Philippe BinderNoch keine Bewertungen
- Vascular ImagingDokument277 SeitenVascular ImagingJean-Philippe BinderNoch keine Bewertungen
- Section 1: Isometric Training Muscular Contraction The Resistance Band The Running ProcessDokument7 SeitenSection 1: Isometric Training Muscular Contraction The Resistance Band The Running ProcessHarold100% (1)
- Pemerintah Provinsi Maluku No - Rekam Medis:: Rsud Dr. H. Ishak UmarellaDokument3 SeitenPemerintah Provinsi Maluku No - Rekam Medis:: Rsud Dr. H. Ishak UmarellaEjha JackvegaNoch keine Bewertungen
- 12 Weeks of WorkoutsDokument7 Seiten12 Weeks of WorkoutsJumen TamayoNoch keine Bewertungen
- Oestoarthritis Oestoarthritis: Physiotherapy (Cardiff University) Physiotherapy (Cardiff University)Dokument3 SeitenOestoarthritis Oestoarthritis: Physiotherapy (Cardiff University) Physiotherapy (Cardiff University)Waseem Khan AfridiNoch keine Bewertungen
- Final Ortho Case YashikaDokument47 SeitenFinal Ortho Case YashikaYashika GuptaNoch keine Bewertungen
- Anatomical TerminologyDokument57 SeitenAnatomical TerminologyAlicina DaleNoch keine Bewertungen
- PT Rock Medical Company ProfileDokument10 SeitenPT Rock Medical Company ProfileRahmat AnwarNoch keine Bewertungen
- ANAT 215 Lecture NotesDokument2 SeitenANAT 215 Lecture Notesjchyeung20Noch keine Bewertungen
- 1500 Stretches The Complete Guide To Flexibility and MovementDokument1.949 Seiten1500 Stretches The Complete Guide To Flexibility and MovementDusadee Bualert67% (3)
- Watkins Et Al. (2022)Dokument9 SeitenWatkins Et Al. (2022)DANIA NAJUA BINTI ZAINALNoch keine Bewertungen
- Nouveau Texte OpenDocumentDokument8 SeitenNouveau Texte OpenDocumentClmentNoch keine Bewertungen
- Kista GanglionDokument7 SeitenKista GanglionMelissa KanggrianiNoch keine Bewertungen
- FitSimplifyResistanceTubeBandEbook PDFDokument35 SeitenFitSimplifyResistanceTubeBandEbook PDFAnthony DinicolantonioNoch keine Bewertungen
- Ortho MrcsDokument2 SeitenOrtho MrcsAnna SeeNoch keine Bewertungen
- 26 Material of Suspension TherapyDokument15 Seiten26 Material of Suspension TherapyM Farrukh shahzad0% (1)
- Penyuluhan Kesehatan Osteoporosis Pada Lansia Di Posyandu Lansia Anggrek Dusun Nitipuran, Sonosewu, BantulDokument5 SeitenPenyuluhan Kesehatan Osteoporosis Pada Lansia Di Posyandu Lansia Anggrek Dusun Nitipuran, Sonosewu, BantulRizky AnalystNoch keine Bewertungen
- Anatomy Midterm Study GuideDokument53 SeitenAnatomy Midterm Study Guidelovelyc95Noch keine Bewertungen
- Emergency in Musculoskeletal Trauma and Spinal Injuries - Dr. Asrafi Rizki Gatam SpOTDokument42 SeitenEmergency in Musculoskeletal Trauma and Spinal Injuries - Dr. Asrafi Rizki Gatam SpOTefancoolhand09Noch keine Bewertungen
- Cavovarus Foot in PediatricsDokument8 SeitenCavovarus Foot in PediatricshardboneNoch keine Bewertungen
- 12 Week Dumbbell Workout Plan PDFDokument9 Seiten12 Week Dumbbell Workout Plan PDFdewi kinderfunNoch keine Bewertungen
- Fractura Columnae VertebralisDokument4 SeitenFractura Columnae VertebralisTheo DapamedeNoch keine Bewertungen
- ICU Guide OneDokument318 SeitenICU Guide OneSamar DakkakNoch keine Bewertungen
- Human Anatomy 8th Edition Marieb Solutions Manual DownloadDokument14 SeitenHuman Anatomy 8th Edition Marieb Solutions Manual DownloadMary Brown100% (23)
- 4 Principles of Fracture Fixation - HandoutDokument12 Seiten4 Principles of Fracture Fixation - HandoutMuhammad Yasir Ahmad Muslim100% (1)
- Sacral Dysfunction Bilateral Flexed and Extended SacrumDokument22 SeitenSacral Dysfunction Bilateral Flexed and Extended SacrumTrina SiNoch keine Bewertungen
- Question-Bank With AnswerDokument107 SeitenQuestion-Bank With Answerkeithan96Noch keine Bewertungen
- 2nd PERIODICAL EXAMINATION SCIENCE 6Dokument2 Seiten2nd PERIODICAL EXAMINATION SCIENCE 6Hera S LinduganNoch keine Bewertungen
- PNF StretchingDokument6 SeitenPNF StretchingKrutika SarangpuriaNoch keine Bewertungen
- Wrist & Hand ComplexDokument7 SeitenWrist & Hand ComplexMike de MesaNoch keine Bewertungen
- Effect of Static Knee Joint Flexion On Vastus Medialis Obliquus Fiber Angle in Patellofemoral Pain Syndrome - An Ultrasonographic StudyDokument9 SeitenEffect of Static Knee Joint Flexion On Vastus Medialis Obliquus Fiber Angle in Patellofemoral Pain Syndrome - An Ultrasonographic Studycris weeNoch keine Bewertungen
- The Age of Magical Overthinking: Notes on Modern IrrationalityVon EverandThe Age of Magical Overthinking: Notes on Modern IrrationalityBewertung: 4 von 5 Sternen4/5 (32)
- ADHD is Awesome: A Guide to (Mostly) Thriving with ADHDVon EverandADHD is Awesome: A Guide to (Mostly) Thriving with ADHDBewertung: 5 von 5 Sternen5/5 (3)
- Summary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedVon EverandSummary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedBewertung: 4.5 von 5 Sternen4.5/5 (82)
- Love Life: How to Raise Your Standards, Find Your Person, and Live Happily (No Matter What)Von EverandLove Life: How to Raise Your Standards, Find Your Person, and Live Happily (No Matter What)Bewertung: 3 von 5 Sternen3/5 (1)
- LIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionVon EverandLIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionBewertung: 4 von 5 Sternen4/5 (404)
- By the Time You Read This: The Space between Cheslie's Smile and Mental Illness—Her Story in Her Own WordsVon EverandBy the Time You Read This: The Space between Cheslie's Smile and Mental Illness—Her Story in Her Own WordsNoch keine Bewertungen
- The Comfort of Crows: A Backyard YearVon EverandThe Comfort of Crows: A Backyard YearBewertung: 4.5 von 5 Sternen4.5/5 (23)
- The Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaVon EverandThe Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaBewertung: 4.5 von 5 Sternen4.5/5 (266)
- To Explain the World: The Discovery of Modern ScienceVon EverandTo Explain the World: The Discovery of Modern ScienceBewertung: 3.5 von 5 Sternen3.5/5 (51)
- Think This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeVon EverandThink This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeBewertung: 2 von 5 Sternen2/5 (1)
- The Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsVon EverandThe Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsBewertung: 4 von 5 Sternen4/5 (4)
- The Twentysomething Treatment: A Revolutionary Remedy for an Uncertain AgeVon EverandThe Twentysomething Treatment: A Revolutionary Remedy for an Uncertain AgeBewertung: 4.5 von 5 Sternen4.5/5 (2)
- The Obesity Code: Unlocking the Secrets of Weight LossVon EverandThe Obesity Code: Unlocking the Secrets of Weight LossBewertung: 4 von 5 Sternen4/5 (6)
- Summary: Outlive: The Science and Art of Longevity by Peter Attia MD, With Bill Gifford: Key Takeaways, Summary & AnalysisVon EverandSummary: Outlive: The Science and Art of Longevity by Peter Attia MD, With Bill Gifford: Key Takeaways, Summary & AnalysisBewertung: 4.5 von 5 Sternen4.5/5 (44)
- Dark Psychology & Manipulation: Discover How To Analyze People and Master Human Behaviour Using Emotional Influence Techniques, Body Language Secrets, Covert NLP, Speed Reading, and Hypnosis.Von EverandDark Psychology & Manipulation: Discover How To Analyze People and Master Human Behaviour Using Emotional Influence Techniques, Body Language Secrets, Covert NLP, Speed Reading, and Hypnosis.Bewertung: 4.5 von 5 Sternen4.5/5 (110)
- Manipulation: The Ultimate Guide To Influence People with Persuasion, Mind Control and NLP With Highly Effective Manipulation TechniquesVon EverandManipulation: The Ultimate Guide To Influence People with Persuasion, Mind Control and NLP With Highly Effective Manipulation TechniquesBewertung: 4.5 von 5 Sternen4.5/5 (1412)
- Raising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsVon EverandRaising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsBewertung: 5 von 5 Sternen5/5 (1)
- Critical Thinking: How to Effectively Reason, Understand Irrationality, and Make Better DecisionsVon EverandCritical Thinking: How to Effectively Reason, Understand Irrationality, and Make Better DecisionsBewertung: 4.5 von 5 Sternen4.5/5 (39)
- The Courage Habit: How to Accept Your Fears, Release the Past, and Live Your Courageous LifeVon EverandThe Courage Habit: How to Accept Your Fears, Release the Past, and Live Your Courageous LifeBewertung: 4.5 von 5 Sternen4.5/5 (254)
- Raising Good Humans: A Mindful Guide to Breaking the Cycle of Reactive Parenting and Raising Kind, Confident KidsVon EverandRaising Good Humans: A Mindful Guide to Breaking the Cycle of Reactive Parenting and Raising Kind, Confident KidsBewertung: 4.5 von 5 Sternen4.5/5 (170)
- Cult, A Love Story: Ten Years Inside a Canadian Cult and the Subsequent Long Road of RecoveryVon EverandCult, A Love Story: Ten Years Inside a Canadian Cult and the Subsequent Long Road of RecoveryBewertung: 4 von 5 Sternen4/5 (46)
- The Marshmallow Test: Mastering Self-ControlVon EverandThe Marshmallow Test: Mastering Self-ControlBewertung: 4.5 von 5 Sternen4.5/5 (60)
- The Garden Within: Where the War with Your Emotions Ends and Your Most Powerful Life BeginsVon EverandThe Garden Within: Where the War with Your Emotions Ends and Your Most Powerful Life BeginsNoch keine Bewertungen
- Hearts of Darkness: Serial Killers, The Behavioral Science Unit, and My Life as a Woman in the FBIVon EverandHearts of Darkness: Serial Killers, The Behavioral Science Unit, and My Life as a Woman in the FBIBewertung: 4 von 5 Sternen4/5 (20)