Beruflich Dokumente
Kultur Dokumente
1985 Psy Rev
Hochgeladen von
PspduntanDuaribusebelasCopyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
1985 Psy Rev
Hochgeladen von
PspduntanDuaribusebelasCopyright:
Verfügbare Formate
See
discussions, stats, and author profiles for this publication at:
http://www.researchgate.net/publication/232499193
Prime Theory. An Integrated View
of Motivation and Emotion
ARTICLE in PSYCHOLOGICAL REVIEW JUNE 1985
Impact Factor: 7.97 DOI: 10.1037/0033-295X.92.3.389
CITATIONS
READS
165
1,168
1 AUTHOR:
Ross Buck
University of Connecticut
104 PUBLICATIONS 3,188 CITATIONS
SEE PROFILE
Available from: Ross Buck
Retrieved on: 20 December 2015
>85 b> the American Psychological Association, Inc.
0033-205X/85/M0.75
Psychological Review
1985. Vol. 92. No. 3. 389-413
Prime Theory: An Integrated View of Motivation and Emotion
Ross Buck
University of Connecticut
Motivation and emotion are viewed as different aspects of a single process in
which emotion involves the "readout" of motivational potential inherent in
hierarchically organized primary motivational/emotional systems (primes). The
most basic readout, Emotion 7, involves adaptive-homeostatic functions. In
species where communication about the state of certain primes became important,
Emotion II, involving their outward expression, evolved. With cognition, a third
type of readout evolved, Emotion III, involving the direct experience of certain
primes. A model of the interaction between primes and cognition is presented,
and the unique role of language in human motivation-emotion is discussed.
In recent years there has been a virtual
explosion of new information relevant to the
analysis of motivation and emotion that has
left attempts at theoretical integration far
behind. In the realm usually termed motivation, there has been increasing realization
that behavior can be initiated by external
stimuli and cognitive processes as well as by
the biological deficits that have served as the
cornerstone of the drive-reduction model.
Mogenson and Phillips (1976) pointed out
that any contemporary analysis of motivation
must account for behavior based on the expectation of reward (incentive motivation:
Bindra, 1968; Bolles, 1972) and for adaptive
behavior that anticipates homeostatic deficits
before they occur. The apparent ability of
animals to make responses that anticipate
their needs suggests that they must use processes that "represent within the brain the
nature of the outside world" (Oatley, 1973,
p. 12). In this article I argue that such
processes involve phenomena that have been
traditionally relegated to the realm of emotion.
The major physiological models of motivation have generally avoided the concept of
emotion, with its taint of subjectivism. These
I would like to thank a number of colleagues including
Reuben Baron, Paul Ekman, Phoebe Ellsworth, several
anonymous reviewers, and particularly, Carroll Izard and
the Editor for their valuable comments and suggestions
on this article.
Requests for reprints should be sent to Ross Buck,
Department of Communication Sciences, Box U-85, 850
Bolton Road, University of Connecticut, Starrs, Connecticut 06268.
389
models include that of Stellar (1954), which
posited specific drive systems for hunger,
thirst, and sex, based on excitatory and inhibitory centers within the hypothalamus;
arousal theory (Hebb, 1955; Lindsley, 1957),
based on the brainstem reticular formation;
and the analyses of reward-punishment
mechanisms, beginning with J. Olds and Milner (1954). Each of these models has proved
useful and has gained some degree of empirical support, but none is sufficient to constitute a general physiological model of motivation.
At the same time, there have been events
that have made the concept of emotion
more acceptable to behaviorally oriented psychologists. Darwin's (1872/1965) theory in
Expression of the Emotions in Man and
Animals formed the basis of several propositions that have gained considerable support:
(a) Emotion is based on activity in neurochemical systems in the central nervous system, (b) these systems are the product of
evolution and reflect survival requirements
within each species, and (c) activity in these
systems can be modified by learning. These
basic elements appear in Tomkins's (1962,
1963) pioneering theory of affect, in
Izard's (1971, 1977) evolutionary-developmental view of emotion, and in Ekman and
Friesen's (1969, 1975) neurocultural theory,
and they are consistent with much recent
work in ethology (Eibl-Eibesfeldt, 1970, 1972).
These investigators have demonstrated that
the facial and gestural expressions associated
with certain basic emotional/motivational
states or primary affects are widely generalized
390
ROSS BUCK
within the human species, thus supporting a
major thesis of Darwin's. However, these
investigators have not considered the physiological bases of emotion in great detail, tending to emphasize facial expression and other
expressive movements in their studies of
emotion. Also, they have typically emphasized
specific primary emotions or affects (such as
Ekman's happiness, sadness, fear, anger, surprise, disgust) as distinct from drives (hunger,
thirst, sex).
Another major type of approach to emotion
emphasizes the role of cognition in interaction
with physiological factors. This approach has
ties to the classical James-Lange theory of
emotion (James, 1884), which posited that
the subjective experience of emotion involves
the cognitive awareness of bodily responses.
Schachter's (1964) self-attribution theory and
Lazarus's (1966) theory of coping have analyzed emotion in terms of the cognitive appraisal and labeling of external and internal
stimuli. Mandler (1975) and Averill (1980),
among others, have expanded on this theme.
This approach is powerful and is able to
address a wide range of phenomena. However,
as we shall see, it cannot easily deal with
emotional phenomena that seem to be a
product of brain activity alone.
A third type of theory of emotion, emphasizing central nervous system mechanisms,
derives from the classic position of W. B.
Cannon (1927). In contrast with James, Cannon argued that emotional stimuli cause brain
changes that, among other things, are a direct
source of subjective emotional experience.
Cannon suggested that emotional stimuli
cause subcortical systems to simultaneously
and independently inform the cortex (resulting in emotional experience) and the relevant
peripheral bodily systems (resulting in bodily
responses). Papez (1937) suggested that the
bodily response involves the hypothalamus,
which controls the autonomic and endocrine
systems, whereas the experience of emotion
involves the limbic system. MacLean (1968,
1970, 1973, 1981) later proposed the triune
theory, which views the brain as a hierarchy
that has appeared successively over the course
of evolution: a reptilian portion (brain-stem,
midbrain, and basal ganglia), a paleomammalian portion (including the limbic system),
and a neomammalian portion (including the
neocortex). More recent analyses of central
nervous system mechanisms of emotion, such
as those of Gray (1982) and Panksepp (1981,
1982), can be viewed as progressive developments of this general line of reasoning.
This article suggests a basis for integrating
these various views of motivation and emotion
in terms of a readout of information relevant
to bodily adaptation and homeostasis (Buck,
1980, 198la, 1984a, 1984b). It is not argued
that any of these views is incorrect, rather
that they are substantially correct as far as
they go, but that they are incomplete and
that it is possible to arrive at a comprehensive
model of motivation and emotion by taking
aspects of each of these views into account.
The goal of this article is to present an
integrated way of thinking about motivation
and emotion in their various physiological,
expressive, and cognitive aspects. It is not my
intention to suggest specific hypotheses that
derive from the present position or data that
would disconfirm it. I would hope instead
that this position is judged by its ability to
serve as a metatheory, an integrative mechanism to show the relation between theories
that are designed to handle more specific
ranges of phenomena. No new information
is being presented; rather, this is an attempt
to organize concisely and efficiently what is
known and, in so doing, to present a new
and coherent way of looking at the phenomena of motivation and emotion.
This article is structured in a series of
propositions with their associated reasoning
and documentation being relatively brief and
often limited to a few explanatory sentences
with references. The intent is to present a
general viewpoint without becoming involved
with extended discussions of specific points.
General Nature of Primary Motivational/
Emotional Systems
Biologically based primary motivational/
emotional systems (primes) have evolved
within each species with the basic role of
bodily adaptation and maintenance of homeostasis. The common characteristics of
primes may be summarized as follows: (a)
They evolve according to requirements for
MOTIVATION AND EMOTION
bodily adaptation and homeostasis, (b) they
involve active internal processes, (c) they
generally require internal or external stimuli
to become activated, (d) they are based on
innate mechanisms organized in the subcortical and paleocortical regions of the brain,
and (e) they are special-purpose systems that
serve a specific function in the species. These
characteristics are discussed in this section
and in the next two sections.
The term prime is suggested both because
it serves as an acronym for the phrase primary
motivational/emotional system and because
prime has the proper connotations in that it
suggests both an irreducible minimum that
is the biological basis of the systems and an
active internal process that requires external
stimuli to reach expression (i.e., "he or she
was primed to respond"). The unity of the
concept comes from (a) the common evolutionary origin in bodily adaptation and homeostasis, (b) the notion of a special-purpose
processing system based on a physiological
substrate, and (c) the fact that, whatever their
level of organization, the primes involve both
motivational and emotional phenomena.
The reasoning behind the notion of primes
proceeds as follows. Each species has evolved
systems of behavior that respond to challenging stimuli in ways that are adaptive to that
particular species. Adaptive responses are
"those which bring the animal into contact
with stimuli relevant to its survival (approach)
and those which remove it from stimuli which
are threatening to its survival (withdrawal)"
(Glickman & Schiff, 1967, p. 102). Because
of general similarities in species requirements
within the ecosystem of the Earth, and relations between species during the course of
evolution, some of these systems are relatively
universal (i.e., the needs for food, water, and
oxygen among animals). However, others are
quite specific, reflecting the unique requirements of a given species. Examples include
the systems underlying the migratory patterns
in many birds, the resistance to pain in the
camel, the gnawing and burrowing behavior
of small rodents, and the long period of
attachment to the young in primates.
As these examples imply, primes exist at
various levels of organization. At one extreme
there are automatic unconditioned actions
that are released by specific external or inter-
391
nal stimuli, including what have been termed
tmpisms, taxes, endogenous automatic movements, and reflexes (cf. Cofer & Appley,
1964). These are based on relatively simple
neural systems and perform highly specific
functions vital to the organism. At a more
complex level of organization, there are the
instincts or fixed action patterns, which involve innate tendencies to perform specific
actions. These involve a number of coordinating neural mechanisms culminating in an
orderly series of behaviors. These built-in
sequences of behavior are characteristic of a
given species and may be quite complex, but
they are highly stereotyped when examined
closely. At the next level are primary drives,
which involve internal bodily disturbances
resulting from natural tissue needs, such as
the need for food, water, sleep, sex, air,
temperature regulation, and pain avoidance.
Drives do not result in a built-in behavior
sequence, but rather energize behavior, causing the organism to search the environment
for stimuli (food, water) that satisfy the need.
This satisfaction or drive reduction serves to
reinforce the responses that preceded it and
thus encourages learning that is relevant to
the satisfaction of the need. Thus the goal of
the drive is fixed, but sequence of behavior
leading to the goal is learned.'
At the next level are affects, which are the
motivational systems most commonly associated with emotion. The primary
affects
include happiness, sadness, fear, anger, surprise, and disgust (cf. Ekman & Friesen,
1975; Tomkins, 1962, 1963). Primary affects
are based upon specific hard-wired systems
in the brain, but instead of serving highly
specific functions, they involve general response tendencies vis-a-vis the environment.
The capacity to experience these affects is
innate and unlearned, and each has been
shown to be associated with highly generalized
expressive displays (i.e., facial expressions,
postures; cf. Ekman, Sorenson, & Friesen,
1969). However, the circumstances under
1
In the case of acquired or secondary drives, the
organism learns not only the sequence of behavior needed
to satisfy the goal, but also the value of the goal itself:
Initially neutral stimuli in the environment that are
associated with the reinforcement of a biological drive
come to be reinforcing (incentives) in themselves.
392
ROSS BUCK
which they are experienced and the ways in
which they are expressed involve learning.
At another level is a kind of prime system,
analogous in many respects to drives, but in
which the natural tissue need is difficult to
specify. Some have suggested that it involves
a need for stimulation on the part of the
nervous system itself. Thus, R. White (1959)
discussed the inability of the concepts of
instinct and drive to explain the tendencies
of animals and humans to explore, seek
stimulation, and manipulate the environment,
and suggested that the common property of
these behaviors is that they have an effect
upon the environment. He proposed that
there is an intrinsic need, termed effectance
motivation, for an effective interaction with
the environment, which makes exploration,
stimulus seeking, and a wide variety of related
behaviors intrinsically rewarding. The result
of effectance motivation is competence in
dealing with the environment in effective
ways. White suggested that effectance motivation is the motivational force that underlies
cognitive development, noting Piaget's (1971)
views regarding the development of a cognitive
representation of oneself and the external
world.
The primes are special-purpose systems
that have evolved to serve specific functions
and are associated with specific biological
structuresneurochemical systemsthat can
in principle be identified within the nervous
system. As one moves from reflexes and
fixed-action patterns to primary drives, acquired drives, primary affects, and effectance
motivation, the prime systems in question
interact to an increasing extent with generalpurpose systems of conditioning, learning,
and cognition, which have evolved to enable
the organism to construct an internal representation of certain important aspects of the
external and internal environment (including
the state of the special-purpose systems). The
nature and complexity of this representation
varies with different species. This interaction
allows the behavior in question to become
increasingly flexible and open to both environmental influence and processes of selfregulation.
This flexibility is not contrary to the notion
that the prime systems are themselves biologically built-in, special-purpose processing systems. Indeed, with the higher levels of primes,
flexibility and openness to influence from the
environment and self are hard-wired into the
system. For example, in the case of a socially
relevant affect such as anger, the affect per se
is a prime based on a specific neural system
that is universal to the species. The ways in
which this system is aroused is, however,
based on learning, and the social expression
of anger is channeled by learned display rules
(although it may leak out in expressive behaviors not closely monitored by the individual; cf. Ekman & Friesen, 1975). As we shall
see, even the experience of anger may be
affected by learning: Some individuals may
learn to ignore angry feelings or label them
as something other than anger. The aspect of
the angry response that is least affected by
learning is its impact on adaptive/homeostatic
systems. The presence of arousal in the anger
system may potentially place the body under
physical stressthat is, perhaps suppressing
the immune systemeven if it is not manifested in other ways.
To recap briefly, the prime systems are
seen to be organized in a hierarchical manner,
in which phylogenetically older and newer
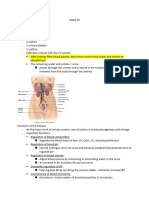
systems occur at different levels of the hierarchy. A visual representation of this hierarchy
is presented in Figure 1. At the lower levels
are systems designed to perform relatively
specific functions, whereas at the upper levels
are systems that interact with general-purpose
systems, reflecting the organism's experience
in the environment. Thus one can think of a
continuum in which learning and cognition
play a progressively larger role as one goes
up the hierarchy.
Each prime is associated with a physiological system or substrate. These substrates
are neurochemical circuits that may be anatomically distinct or intertwined with one
another. They may also appear at a number
of levels in the nervous system; neural mechanisms directly related to sex, for example,
have been identified from the lower spinal
cord to the limbic system. Such systems may
be characterized by their arousability or capacity to become active, and arousal or actual
state of activity (cf. Whalen, I966).2
2
Arousal is often used to refer to an undifferentiated
state of activation, but this is not the case here. Instead,
each motivational/emotional substrate is considered to
be associated with a particular sort of response.
393
MOTIVATION AND EMOTION
LINGUISTICALLY-BASED MOTIVES AND EMOTIONS.
EFFECTANCE MOTIVATION
"NEW" BRAIN
STRUCTURES
PRIMARY AFFECTS
i
ACQUIRED DRIVES
I
PRIMARY DRIVES
I
INSTINCTS
REFLEXES
'OLD" BRAIN
STRUCTURES
LEARNING
MORE
IMPORTANT
LEARNING
LESS
IMPORTANT
Figure I. Hierarchy of motivational/emotional systems.
Variations in arousability function to increase or decrease the range of environmental
stimuli that trigger psychophysiological, subjective, and/or overt responses. For example,
von Hoist and von Saint Paul (1962) demonstrated that a stuffed polecat elicited no
attack from a rooster until neural systems in
the rooster associated with aggression were
artificially stimulated. In the absence of the
stuffed polecat, the same brain stimulation
caused only motor restlessness in the rooster.
Spontaneous activity in similar circuits in
humans has been associated with subjective
feelings of anger and a heightened readiness
to react to aggression-eliciting stimuli (King,
1961). Cases of violence associated with disorders in the temporal lobe have been attributed to fextreme hyperactivity to very mild
aggression-eliciting stimuli caused by spontaneous activity in aggression circuits, which
is caused in turn by abnormal electrochemical
activity in the brain. Mark and Ervin (1970)
cited a number of cases of temporal lobe
disease in which mild stimuli (a baby crying,
a brush against the arm) have elicited vicious
attack. Studies of the electrical stimulation
of the brain in humans have demonstrated
that apparently complete subjective and objective emotional responses can be elicited
that vary with the region stimulated (cf.
Buck, 1976).
The arousability of a neurochemical system
is dependent on a large number of factors,
including long-term influences (heredity, disease, early experience), short-term influences
(blood chemistry, drugs), and interactions
with other neurochemical systems (Moyer,
1971).
Much has been learned about the nature
of the neurochemical mechanisms underlying
motivation and emotion, and new techniques
for studying the nervous system promise revolutionary advances in the near future. For
example, the brainstem reticular formation
contains arousal mechanisms involved in attention and sleep-wakefulness, as described
by Lindsley (1951, 1957) and others, and the
specific regions and chemical substances involved are being actively explored (cf. Vanderwolf & Robinson, 1981). Also, fiber tracts
coursing through the hypothalamus are involved in reward-punishment systems, where
a reward system is one in which activity is
associated with reinforcement and a punishment system is one in which activity is associated with frustration, punishment, or
nonreward (M. Olds & Fobes, 1981; Routtenberg, 1978). The details of how these systems
work are not yet clear, but there is evidence
that the reward-punishment system contains
neurochemical systems that affect intracranial
self-stimulation, mood, learning, and memory
in systematic ways (cf. Panksepp, 1981, 1982).
Also within the brainstem and hypothalamus are a variety of neurochemical substrates
that underlie species-specific behavior patterns. Valenstein, Cox, and Kakolewski (1970)
suggested that electrical stimulation of the
hypothalamus activates a wired-in hierarchy
of behaviors specific to the species. The response that is elicited is the prepotent response
for that species (i.e., eating behavior). However, if the goal object for that response is
removed, the second response in the hierarchy
often appears: Rats show gnawing and hoarding, roosters crow, gerbils thump their feet,
and so forth. Other authors (Glickman &
Schiff, 1967; Wise, 1968) have pointed out
394
ROSS BUCK
that these results could be due to independent
neurochemical circuits in the closely packed
hypothalamus being activated to differing degrees by the stimulating electrode. The occurrence of these species-specific responses
may be internally reinforced, perhaps via the
species-specific mechanism itself, perhaps via
more general reward-punishment systems, or
both (cf. Buck, 1976, pp. 76-77; Panksepp,
1981).
The limbic system includes paleocortical
structures (old cortical structures with 3-5
layers) plus the amygdala (Grossman, 1967).
MacLean (1968, 1969, 1970) suggested that
the limbic system includes neurochemical
circuits involving aggression and fear (particularly involving the amygdala) and circuits
involving sociability and sex (particularly involving the septal area). Details of the evidence relating to these systems is summarized
in Buck (1976, pp. 79-92). Briefly, it is well
established in both humans and animals that
electrical stimulation of the amygdala often
produces rage, whereas bilateral lesioning of
the same region lessens aggression (cf. Mark
& Ervin, 1970, and Terzian & Dalle Ore,
1955, for studies relating to humans). Conversely, lesions of the septal area in animals
is associated at least initially with heightened
aggression, whereas stimulation of that area
in humans elicits pleasurable reactions, often
with sexual content (Heath, 1964a, 1964b).
This consideration of the neurochemical
systems underlying motivation and emotion
is relevant to understanding the differences
between typological approaches to the classification of emotion such as those of
Tomkins, Ekman, and Izard, which assume
fundamentally different emotion types (fear,
anger, surprise, etc.), versus dimensional approaches, which assume that emotions vary
quantitatively from each other along bipolar
dimensions (strong-weak, pleasant-unpleasant; cf. Russell, 1979, 1983; Russell & Mehrabian, 1977; Schlosberg, 1952). A consideration of the neurochemical structures suggests
that both kinds of system occur. The reticular
system appears to vary along an arousal or
intensity dimension (strong-weak) and the
reward-punishment system may vary along
an evaluation dimension (pleasant-unpleasant). The hypothalamus and limbic system,
in contrast, contain neurochemical systems
associated with relatively distinct types of
emotional/motivational states (sex, fear, anger,
etc.). Thus it is not surprising that emotion
reflects both kinds of structure.
Motivation is a potential inherent in the
structure of the primes. Motivation is a
general term for the forces controllingactivating and directingbehavior. It is defined
here as a potential that is inherent in the
structure of systems of behavior controlin
this instance, inherent in the primes. This
conception of motivation as a potential is
analogous to the notion of potential energy
in physics. Matter may have potential energy
by virtue of its physical arrangement (i.e., a
coiled spring) or its position (i.e., a weight
raised to a height). The potential energy is
actualized by an appropriate stimulus, releasing the spring or dropping the weight.
In the same way, the primes are seen as
having motivational potential by virtue of
their particular qualities. These qualities of
the primes have evolved to respond to stimuli
and events that have proved challenging over
the course of evolution. Their function is to
activate homeostatic responses, expressive behaviors, and subjective experiences that have
proved adaptive in the face of these challenges.
Motivation is the potential for the activation
of these responses that is built into the primes
and is released by challenging events. Challenging events can be anything from the
neurologist's tap on the knee (activating a
relatively simple spinal reflex), to a receptive
sexual partner or menacing enemy, to a lack
of food, to Piaget's ailments (activating the
process of assimilation and accommodation
leading to cognitive growth). The primes that
these events challenge get progressively more
complex, of course, and the effects of the
individual's prior learning experiences get
progressively greater.
Thus, the primes are seen to be the basis
of biological motives, in that they are the
sources of the biological forces controlling
activating and directingbehavior. I argue
that other sorts of motives are inherent in
the structure of another system of behavior
control, that of language.
Input from the primes is organized and
integrated at higher levels in the nervous
system. Evidence indicates that the right
and left cerebral hemispheres play markedly
different roles in motivation and emotion.
There is evidence that the input of the primes
MOTIVATION AND EMOTION
to higher brain mechanisms is lateralized.
The ascending aminergic fiber tracts important in motivation and emotion (i.e., the
media) forebrain bundle) are right lateralized,
and Denenberg and his colleagues (Denenberg, 1981; Denenberg, Garbanti, Sherman,
Yutzey, & Kaplan, 1978) have shown that
right-hemisphere damage causes decreased
emotionality even in rats. Recent studies have
demonstrated anatomical assymetries in the
brain in most right-handed humans. The
Sylvian fissure rises more steeply on the right
side of the human brain than on the left, and
this is associated with a larger area on the
top surface of the temporal lobecalled the
plenum temporaleon the left (Galaburda,
LaMay, Kemper, & Geshwind, 1978). This
region forms part of Wernicke's areaan
area long associated with languageand microscopic studies have demonstrated cellular
abnormalities in this area in dyslexics (Galaburda & Kemper, 1979). In addition, the
left hemisphere has been found to be longer
than the right and wider in the posterior
region in most right-handed humans, whereas
in contrast, the right hemisphere tends to be
wider in the frontal regions (Geshwind, 1979).
The frontal cortex, in turn, has long been
known to be important in emotion. Geshwind
(1979) suggested that this characteristic shape
of the human brain reflects the right hemisphere's specialization for emotion and the
left hemisphere's specialization for language.
It has long been known that the left hemisphere is involved with linguistic functions,
with damage being associated with crippling linguistic communication disorders
the aphasias. The functions of the right hemisphere were not as apparent, and for many
years it was relegated to the status of the
minor, silent hemisphere. Recent evidence
suggests that the right hemisphere acts as a
synthesizer, organizing input into complex
and global wholes, and that this kind of
syncretic conceptualization is involved in
emotion (Tucker, 1981). Thus Sperry, Zaidel,
and Zaidel (1979) demonstrated that the disconnected right hemisphere is capable of
producing an awarenessincluding an affective aurain the absence of the verbal cognition of the left hemisphere. I use the term
syncretic cognition to refer to this sort of
conceptualization.3
A large number of studies using a wide
395
variety of experimental measures and subject
populations have shown that the right hemisphere plays a special role in both the expression and recognition of emotion. For example,
the following findings have been reported: (a)
Facial asymmetry orfacedness is significantly
left sided during the posing of emotional
expression in normal right-handed subjects,
suggesting relative right-hemisphere activation
(or, perhaps, relative left-hemisphere inhibition). The question of whether this is true of
spontaneous expression as well, is unresolved
(cf. Borod & Caron, 1980; Borod, Caron, &
Koff, 1981; Campbell, 1978; Ekman, 1980;
Ekman, Hagar, & Friesen, 1981; Sackheim,
Gur, & Saucy, 1978). (b) In normal righthanded subjects, right-hemisphere activation
occurs when subjects answer emotion-related
questions (cf. Schwartz, Davidson, & Maer,
1975) and are under stress (Tucker, Roth,
Arneson, & Buckingham, 1977). Also, there
is considerable evidence that emotional information is processed more efficiently when
presented to the right hemisphere via the left
ear (Carmon & Nachson, 1973; Haggard &
Parkinson, 1971; Safer & Leventhal, 1977)
or the left visual field (cf. Safer, 1981, for
references), (c) In cases of hysteria, conversion
systems tend to occur more frequently on the
left side (Galin, Diamond, & Braff, 1977;
Stern, 1977). (d) Patients with certain kinds
of right-hemisphere damage and also persons
3
The controversy between Lazarus (1982, 1984) and
Zajonc (1980, 1984) is relevant here. Lazarus (1984)
argued that cognitive appraisal is a necessary condition
for emotion: "To experience an emotion, people must
comprehend . . . that their well being is implicated in a
transaction" (p. 124). Such comprehension need not
involve a "highly differentiated symbolic process," in fact
it may take the form of a "primitive evaluative perception"
(p. 124). Zajonc (1984) replied that cognition must
require some kind of mental work in the form of a
transformation of sensory input and that "Lazarus has
broadened the definition of cognitive appraisal to include
even the most primitive forms of sensory excitation" (p.
117). This controversy would be clarified by the distinction
between syncretic and analytic cognition: Both Zajonc
and Lazarus appear to agree that some form of sensory
information is necessary for emotion; what they appear
to disagree about is what would constitute cognition.
The concept of syncretic cognition might satisfy both,
for it involves more than mere sensation, yet does not
require a transformation of input, involving instead
directly perceived analog information (see Footnote 9).
Analytic cognition, in contrast, involves the linear and
sequential organization of digital information (cf. Buck,
1984a; Tucker, 1981).
396
ROSS BUCK
whose right hemispheres are temporarily inactivated by intracarotid injections of amobarbitol have manifested symptoms of emotional indifference (Gainotti, 1969, 1972;
Galin, 1974;Rossi&Rosandini, 1967;Terzian,
1964; Terzian & Ceccotto, 1959). (e) Righthemisphere damage is associated with reduced
skin conductance responding to pain (Heilman, Schwartz, & Watson, 1978) and emotionally loaded color slides (Morrow, Vrtunski,
Kim, & Boiler, 1981). (f) Right-hemispheredamaged patients have particular difficulties
with emotional speech as opposed to prepositional speech (Heilman, Scholes, & Watson,
1975; Tucker, Watson, & Heilman, 1977) and
with the recognition and discrimination of
emotional faces and pictures (Benowitz, Bear,
Rosenthal, & Mesulam, 1983; Cicone, Wapner, & Gardner, 1980; DeKosky, Heilman,
Bowers, & Valenstein, 1980).
Buck and Duffy (1980) demonstrated that
left-hemisphere-damaged patients are more
expressive on measures of spontaneous communication than are right-hemisphere-damaged patients, even though there is much
evidence that the former are much less able
to communicate via intentional gesture and
pantomime (cf. Duffy, Duffy, & Pearson,
1975). This observation suggested the basic
importance of a distinction between spontaneous and intentional symbolic communication (Buck, 1982a, 1984a). Data in the Buck
& Duffy study also suggested that the right
hemisphere is responsible for spontaneous
emotional expression, whereas the left hemisphere acts to alter, modulate, and inhibit
such spontaneous expression with facial management strategies, such as those described
by Ekman and Friesen (1975). It is possible
that the left facedness observed in posed
emotional expression may be related to the
inhibitory action of the left hemisphere on
the right side of the face (Buck, 1982b). This
view is compatible with the suggestion by
Tucker and his colleagues that the left hemisphere is associated with anxiety and the
verbal modulation of the emotionality of the
right hemisphere (Shearer & Tucker, 1981;
Tucker, 1981; Tucker & Newman, 1981).4 In
effect, the syncretic cognition associated with
the right hemisphere interacts with the sequential, analytical, and verbal sort of cognition associated with the left hemisphere. I
follow Tucker (1981) in terming the latter
analytic cognition.
Emotion as a Readout Device
Emotion is a readout mechanism associated
with motivation. Emotion is generally defined
in terms of subjective experiences or feelings,
goal-directed behaviors (attack, flight), expressive behavior (smiling, snarling), and
physiological arousal (heart rate increases,
sweating). One of the major assumptions of
the present analysis is that emotion has
evolved as a readout mechanism carrying
information about motivationthat is, about
the state of the primesin a kind of running
progress report. The source of the information
is the motivational potential as activated by
a challenging stimulus. Motivation and emotion are thus seen to be different aspects of
the primes, in that motivation is the potential
for behavior inherent in the neurochemical
structure, and emotion involves the means
by which that potential is realized or read
out, when activated by challenging stimuli.
The relation of motivation and emotion in
this view is analogous to the relation of
energy and matter in physics: Just as energy
is a potential that manifests itself in matter,
motivation, as seen here, is a potential that
manifests itself in emotion.5 Thus motivation
and emotion are seen to be two sides of the
same coin, two aspects of the same process.
In contrast to those who argue that emotion
occurs only when a motivational sequence is
disrupted, this view suggests that emotion is
a constantly occurring phenomenon, reflecting
peacefulness and contentment when the organism is satisfied, as well as alarm when
adaptation is necessary. The organism may
tend to ignore emotional information under
conditions of satisfaction, just as one tends
to ignore the feeling of comfortable shoes on
one's feet, but attention can easily be drawn
to both.
I have suggested in another context that
facial expression involves an external readout
4
See Gray (1982) for a discussion of the neurophysiological bases of anxiety.
5
1 am grateful to Gary Schwartz for suggesting this
analogy.
MOTIVATION AND EMOTION
of those motivational/emotional processes
that have had social implications during the
course of evolution (Buck, 1980). This concept is here extended to include three different
kinds of readout of motivational/emotional
states that have appeared successively during
evolution: Emotion I, involving bodily adaptation and homeostasis; Emotion II. involving
facial expression and other mechanisms of
outward expression; and Emotion III, involving an internal cognitive readout. Thus, although the source of the emotional readout
is the motivational potential inherent in the
primes, the targets of the readout are of three
sorts: systems of bodily adaptation and homeostasis, systems of outward expression,
and systems of subjective experience. I discuss
each of these in turn.
Emotion I involves systems directly responsible for bodily adaptation and the maintenance of homeostasis. The basic evolutionary
function of the primes involves the adaptation
of the organism to the environment, insuring
a proper homeostatic balance involving food,
water, oxygen, temperature, and so forth.
Thus, the most primitive and universal kind
of readout of motivational/emotional states
involves the systems directly concerned with
the maintenance of homeostasis, and this
remains true in the nervous system of more
complex creatures. In all mammals including
humans, Emotion I involves the influence of
the motivational/emotional systems on bodily
functioning via the endocrine, immune, and
autonomic nervous systems.
The Emotion I systems, like other kinds
of readout mechanisms, show species differences that are consistent with ecological requirements. Thus, species adapted to warm
versus cold climates have differences in their
systems of temperature regulation, species
adapted to dry versus wet environments have
differences in systems of water retention and
regulation, and so forth.
Emotion II involves an external readout,
with the state of the primes reflected in overt
expressive behavior. Once the survival of a
species requires the coordination of behaviors
between individuals (for sexual reproduction,
care of the young, etc.), it becomes necessary
for certain motivational/emotional states to
be expressed outwardly, allowing communication between conspecifics. Andrew (1963,
397
1965) argued that certain kinds of emotional
expression allow a coordination of behavior
between individuals that is necessary to species survival. The threatening and submissive
displays of many species allow the establishment of dominance without harmful fights,
courtship displays allow the coordination of
sexual behaviors, certain displays of infants
encourage caregiving, and so forth. In his
analysis of primate expressions, Andrew
(1963, 1965) noted that the use of facial
expression and other kinds of communicative
displays is a function of the extent of socialization of the given species and the communication demands of the environment, with
the consequent need to coordinate social
behavior. Social species show a greater range
of displays than do species that lead solitary
lives. The highly social plains-dwelling baboon, for example, has a wider range of
displays than the more solitary forest-dwelling
mandrill and drill baboon.
Thus overt emotional expression may be
seen as an external readout device, with the
prime state being reflected in the overt behavior. The nature and extent of the external
readout depends on the ecological requirements associated with a given species: Turtle
infants have little need of adult caretakers,
thus there is no signaling system associated
with infant needs that may be seen in birds,
mammals, and particularly in primates, with
the long periods of helplessness of the infant.
Emotion III is a direct internal cognitive
readout of the primes that evolved in ways
that are analogous to their external readout.
Another major assumption of this analysis is
that a cognitive readout of motivational/emotional states has evolved as an internal readout
in much the same way that external expressive
behavior evolved, and that this readout is
direct, involving syncretic as opposed to analytic cognition. In effect, I am suggesting
that the above analysis of the evolution of
emotional expression may be applied also to
an internal syncretic-cognitive registration of
motivational/emotional states that is a kind
of direct subjective experience of emotion.
The reasoning is as follows: Once the nervous
system of a species has evolved sufficiently
to develop even the rudiments of a generalpurpose cognitive systeman internal representation of reality1 suggest that it be-
398
ROSS BUCK
comes important that that cognitive system
have direct access to the state of the primes.
This process is presumably based particularly
on the right hemisphere, although the details
of this are still unclear.
This kind of arrangement would seem to
have at least two major advantages. First, the
readout of the prime state in syncretic cognition would presumably facilitate its handling
via analytic cognition. In effect, the syncretically experienced state becomes an object
for analytic cognition. This would encourage
cognitive participation in adaptive functions
and increase the organism's capacity for selfregulation. Thus we appear to know we are
hungry not via stomach contractions or other
signals of tissue deficit, but through a more
direct, albeit little understood, form of syncretic information. Such information may be
necessary for the kinds of adaptive anticipatory behavior and incentive motivation discussed above that precede homeostatic deficits
and that have been recognized in animals
(Mogenson & Phillips, 1976) and certainly
occur in humans. Thus it may be that the
Emotion III readout is useful and perhaps
necessary for analytic cognition to influence
bodily adaptation and engage in self-regulation.
Second, this system would allow analyticcognitive control over the outward expression
of the prime state. I noted the importance of
facial management techniques by which one
controls one's outward display to fit the requirements of the situation by intensifying,
deintensifying, substituting for, or masking
one's true feelings (Ekman & Friesen, 1975):
We look sad at funerals and happy at parties
even though we may feel quite differently.
Observation suggests that the same phenomenon occurs in animals; a frustrated and
apparently angry monkey may express its
"true feelings" only to a lower-ranking member of the group. Thus Delgado (1969) reported that a monkey's aggressive reaction to
electrical stimulation in aggression-eliciting
brain areas is affected by the dominance of
the individuals in the immediate vicinity: A
relatively dominant animal will attack others
when stimulated in such areas, whereas a
relatively submissive animal will be attacked
by others when so stimulated. Some degree
of analytic-cognitive control over the fight-or-
flight response must be necessary in social
species where fighting and fleeing must be
regulated for the good of the group. The
analysis of such emotion "sending abilities"
and their implications for personality and
social functioning in humans is just beginning
(Buck, 1979).
This kind of control is presumably part of
what is learned by the infant rhesus monkeys
studied by Harlow and his colleagues (Harlow
& Mears, 1983). These studies demonstrated
how the maturation of emotion systems
within the brain is coordinated with social
experience in young rhesus monkeys. The
infant monkey experiences an initial period
in which fear and aggression are absent, and
it is in this period that a lifelong attachment
with other monkeys is formed. Harlow argued
that this provision for social experience has
evolved within the species as a necessary
feature of the rhesus monkey's social organization: In effect the individual must learn
how to use in a social context the complex
of displays and feelings associated with certain
primes (Buck, 1983).
The readout process is summarized in
Figure 2. The source of the readout is biological motivation, which involves potentials for
the activation and direction of behavior that
are inherent in the hierarchically organized
neurochemical systems that I have termed
primes. When activated by challenging stimuli, this potential is read out to the appropriate
target systems in the body; this readout process is emotion. There are three general sorts
of targets for this readout: systems involved
in adaptation and homeostasis (Emotion I),
systems involved in external expression
(Emotion II), and systems involved in syncretic cognition (Emotion III). The functions
of these readout processes involve, respectively, (a) bodily adaptation and the consequent maintenance of homeostasis, (b)
spontaneous communication and consequent
social coordination, and (c) subjective experience and consequent self-regulation. The
readout process involves special-purpose processing systems that do not in themselves
include general-purpose systems of learning
or cognition. However, these do of course
interact with one another. Indeed, I suggest
that the Emotion III process has evolved in
part to foster this interaction.
MOTIVATION AND
399
EMOTION
midiml Soure.
RMdouI Function
II
S^nc^ion
[ S^l^.
Expressive beDavi
]~
1
Spontaneous commumcatio
Social coordination
Autonomic/endocrlmie/
ise
immune sys. respon:
f
1
Adaptation!
hornaoatasia
Figure 2. The readout process: Source, target, and functions served.
Emotion and Subjective Experience
Each type of emotional readout is associated with a distinct type of subjective emotional experience. I suggest that Emotion I
is particularly associated with interoceptive
feedback from autonomic and endocrine responses. Emotion II is particularly associated
with proprioceptive feedback from the skeletal
muscles involved in the external expression
of the prime state, and Emotion III is associated with a direct syncretic cognitive registration of emotion experience that is relatively
distinct for each prime state.
Emotion I: Visceral Feedback
The role of autonomic feedback in emotional experience involves the classic controversy between the James-Lange theory of
emotion (James, 1884) and Cannon's (1927)
critique of that theory. Essentially, the JamesLange theory argued that emotional experience is based on feedback from the bodily
response (both the visceral and skeletal muscle
response) to an emotion-arousing situation,
whereas Cannon argued that the effects of
this feedback are negligible. The considerable
research devoted to this issue has suggested
that autonomic nervous-system feedback does
indeed contribute to some kinds of emotion
experience, although it clearly is neither necessary nor sufficient for all kinds of emotion
experience (Buck, 1976, 1980). For example,
Hohmann (1966) noted that veterans with
spinal cord injuries reported a lessening of
emotional experience that was directly related
to the degree of loss of bodily sensation
caused by the spinal lesion; Delgado (1969)
cited the case of a man who underwent a
unilateral sympathectomy for cancer, who
later reported that he could no longer be
thrilled by music on the sympathectomized
side of his body; and Bertrand Russell (1927)
reported that an epinepherine injection by a
dentist (which causes sympathetic nervoussystem arousal) produced an as if emotional
experiencehe felt as if he was joyful, or
angry, or afraid, but the cognitive element
was absent and the emotional experience was
incomplete. This latter phenomenon is at the
heart of Schachter's (1964) self-attribution
theory of emotion, which suggested that a
complete emotional experience involves an
interaction between a state of autonomic
arousal plus cognitions associated with that
state of arousal. Schachter's theory seems
consistent with most of the findings concerning the contributions of autonomic feedback
to emotional experience, but it cannot handle
all forms of emotional experience.
Emotion II: Facial/Proprioceptive Feedback
The importance of proprioceptive feedback
from external expression in determining
emotion experience was suggested by Darwin
(1872/1965, p. 365), emphasized by Tomkins
(1962, 1963), and has been widely accepted
by contemporary investigators (Izard, 1977;
Kleinke & Walton, 1982; Kraut, 1982, Laird,
1974; Lanzetta, Cartwright-Smith, & Kleck,
1976). These authors argued that the subjective experience of emotion depends on feedback from the expression of that emotion,
particularly its expression on the face. The
strongest form of this facial feedback hypothesis holds that if there is no expression of
emotion, the emotion would not exist (cf.
Tomkins, 1983).
The evidence for the facial feedback hypothesis has not been conclusive, and there
is reason to doubt the stronger statements of
the hypothesis (cf. Buck, 1980; Ellsworth &
Tourangeau, 1981; Tourangeau & Ellsworth,
1979). It would seem to imply, for example,
that facially nonexpressive persons would
400
ROSS BUCK
show less evidence of emotion on other measures of emotion than would expressive persons. However, there is evidence that facially
nonexpressive persons show larger autonomic
(skin conductance and heart rate) responses
than do expressive persons (Buck, 1979,
1980). Also, the facial feedback hypothesis
would seem to imply that a person's emotional experience should mirror his or her
outward expression: If one looks happy, one
should feel happy. However, despite the sentiments of the song "Whistle a Happy Tune,"
the ubiquity of the facial management techniques of Ekman and Friesen (1975) described
above must guarantee that many facial
expressions occur in contexts unrelated to
the actual state of the primes. Several of the
investigators who have advanced the facial
feedback hypothesis in the past have recently
restated their positions in response to the
results of the Tourangeau and Ellsworth
(1979) study, and in no case has the strong
version of the hypothesis been defended (Izard, 1981; Hagar & Ekman, 1981; Tomkins,
1981).6
Emotion HI: Direct SyncreticCognitive Readout
It is difficult to substantiate the claim that
there are central sources of emotional experience that directly reflect the state of the
primes in syncretic cognition apart from any
peripheral visceral or proprioceptive feedback.
Perhaps the closest one can come to demonstrating such experience is with the direct
manipulation of motivational/emotional states
via the artificial electrical or chemical stimulation of the brain in humans.
The effects of drugs in this regard are
readily recognized. There is a substantial
literature on drug effects that makes it clear
that certain drugs affecting the central nervous
system can cause experiential effects that,
although not completely independent of cognitive expectations, can be overwhelming.
Regarding electrical stimulation, several
relevant findings stand out (cf. Buck, 1976,
pp. 77-102; Moyer, 1971; Valenstein, 1973).
First, it appears that strong emotional experience is not usually evoked by stimulation
of the hypothalamus in humans, even though
substantial autonomic changes have been observed (Sem-Jacobsen, 1968; J. White, 1940).
However, stimulation of limbic system structures has led to dramatic alterations in emotional behavior, expression, and apparent experience, which at least in some cases seem
beyond the control of the patient. Stimulation
in the circuit associated by MacLean (1968)
with fear and anger (i.e., the amygdala) has
resulted in uncontrolled rage; one female
patient stimulated in such an area via telemetery while playing a guitar suddenly
smashed her guitar against the wall (Mark &
Ervin, 1970); another woman stimulated during an interview demanded papers to rip up
and pleaded "don't let me hit you" to the
interviewer (King, 1961). In contrast, stimulation in the circuit associated with sociability
and sex (i.e., the septal area) often induces
sexual thoughts (Heath, 1964a). One depressed male patient
described his father's near-fatal illness and condemned
himself as somehow responsible, but when the septal
region was stimulated, he immediately terminated his
conversation and within 15 seconds exhibited a broad
grin as he discussed plans to date and seduce a girl
friend. When asked why he changed the conversation so
abruptly, he replied that the plans concerning the girl
suddenly came to him. [Another depressed patient] smiled
broadly and related a sexual experience of his youth
within one minute after onset of septal stimulation.
(Heath, 1964b, p. 225)
Other evidence comes from observations
of patients whose left and right cerebral
hemispheres were surgically separated to
combat epilepsy. Gazzaniga (1972) cited the
case of a female patient who was occasionally
shown a picture of a nude women in the
middle of a series of pictures of ordinary
objects. She had an amused reaction to the
nude whether it was presented to the left or
right hemisphere, although she could verbally
report the reason for her reaction only if it
was presented to the left hemisphere.
Sperry et al. (1979) cited similar instances
in which emotionally loaded photographs
of the patient; of his or her relatives, pets, or
belongs; of pretty girls or fat women in swim
suits; of public figures like Hitler, Churchill,
6
It is interesting that although the James-Lange theory
also suggested that feedback from the skeletal muscles of
the body should also contribute to emotional experience,
most of the research has focused on the face. Studies by
Riskind and his colleagues (cf. Riskind & Ootay, 1982)
are beginning to address the role of the body.
MOTIVATION AND EMOTION
or Richard Nixonproduced appropriate
emotional expressions and reports of subjective experience, even though the subject could
not verbally explain the reactions. Sperry et
al. noted that "if anything, the emotional
responses from the right hemisphere were
somewhat more intense and less restrained
and qualified than those from the left" (p.
157). This seems consistent with the notion
that the left hemisphere exerts a verbally
mediated control of the emotionality of the
right (Buck & Duffy, 1980; Tucker, 1981),
and it suggests, in contrast to Schachter's
(1964) theory, that emotional experience may
occur in the absence of verbalizable (i.e.,
analytic) cognitions associated with that emotion.
Each prime state is associated with a particular pattern of expressive behaviors and a
particular quality of directly registered emotional experience. Whereas the interoceptive
feedback that constitutes the subjective element of the Emotion 1 process may be relatively similar for different motivational/emotional states, it has been well established that
patterns of expressive behavior differ for different states (Ekman & Friesen, 1975). Thus
the proprioceptive feedback that constitutes
the subjective element of the Emotion II
process must differ for different motivational/
emotional states and could serve as one means
by which different states could be subjectively
differentiated. However, due to the aforementioned difficulties with the facial feedback
hypothesis, it seems probable that the primary
means of subjective differentiation of motivational/emotional states is through the
Emotion III process, that is, through the
different qualities of directly registered emotional experience associated with the different
prime states.
This proposition is consistent with the
evidence presented above regarding the subjective effects of different kinds of psychoactive
drugs and brain stimulation. Also, it logically
follows from the present line of argument.
Thus, I am arguing that the internal cognitive
readout of emotion evolved in a way analogous to the external expressive readout; it is
useful for self-regulation just as the expressive
readout is useful for social coordination. The
pattern of expressive behaviors differs for
different prime states because of this social
401
coordination function; it would clearly be
maladaptive if the expressions reflecting happiness and sadness were similar, for example.
For analogous reasons, the syncretic-cognitive
registration of different prime states would
be expected to differ from one another, to
foster self-regulation.
However, just as the external expression of
emotion may differ for different individuals
due to temperament and social learning (witness Harlow's monkeys), the subjective experience of emotion may also come to differ
for different individuals.
Motivational/emotional experience constitutes a kind of knowledge by acquaintance,
which then interacts with knowledge by description. I suggested that the direct experiential readout of motivation and emotion
involves the holistic, syncretic conceptualization associated with right-hemisphere functioning, as opposed to the verbal and analytic
cognition associated with left-hemisphere
processing (cf. Tucker, 1981). Syncretic cognition may be associated with what has long
been termed knowledge by acquaintance, involving the self-evident presentation of immediate experience. William James described
knowledge by acquaintance as follows:
I know the color blue when I see it, and the flavor of a
pear when I taste it ...
but about the inner nature of
these facts or what makes them what they are I can say
nothing at all . . . I cannot impart acquaintance with
them to anyone who has not made it himself . . . At
most, I can say to my friends, "Go to certain places and
act in certain ways, and these objects will probably
come." (1890, p. 221).
In contrast, analytic cognition may be associated with knowledge by description, which
involves the interpretation of sense data, requires inference, and is prepositional in that
knowledge by description can be false. The
distinction between knowledge by acquaintance and knowledge by description has been
made as far back as St. Augustine in De
Magistro (Marsh, 1956), and it is reflected in
many languages, for example, the French
connaitre versus savoir (James, 1890). More
recently, it occupied a central place in Bertrand Russell's (1912, 1956) epistemological
theory.
Both James and Russell suggested that
knowledge by description depends on knowledge by acquaintance and follows it in time:
402
ROSS BUCK.
"Feelings are the germ and starting point by
cognition, thoughts the developed tree"
(James, 1890, p. 222). This is consistent with
the evidence presented by Zajonc (1980) that
affective responses precede cognitive operations in time and determine their character
(cf. Kunst-Wilson & Zajonc, 1980; Wilson,
1979). However, in addition to contrasting
affect and cognition, it is important to recognize the differing roles of syncretic and
analytic cognition (Tucker, 1981, see Footnote 3).
In applying this point of view to the analysis
of motivation and emotion, it seems reasonable to suggest that the basic, raw experience
of these states involves knowledge by acquaintance. The brain knows how to experience such states, just as it knows how to
characterize the color blue or the taste of a
pear. However, as suggested above, this experience itself can become an object of
knowledge by description; the responder may
learn much about motivation and emotion
that may (or may not) be useful and adaptive.
There are important implications of this
view that there are multiple potential sources
of emotional experience. One is that the
responder may learn to attend to some of
them and to ignore others. Also, different
aspects of emotion and motivation are differentially accessible to the responder, both because of the nature of the response systems
involved and because of social and cultural
factors. My next points deal with these issues,
on which relatively little work has been done. .
Using Emotional Information
The extent to which emotional experience
depends on Emotion I processes (feedback
from adaptive responses), Emotion II processes (feedback from expressive behavior), or
Emotion III processes (direct registration in
syncretic cognition) may differ according to
the individual, the situation, and the motivational/emotional state involved. There is little
information available on how these different
readout mechanisms might interact with each
other in determining how emotional information is managed. Studies are needed that
simultaneously analyze autonomic, expressive,
and cognitive responses to a variety of motivational/emotional states.7
One area where some study has been done
is in the relation between cognitive and expressive responding on the one hand, and
autonomic and endocrine responding on the
other. It is interesting that the results are
relevant to the analysis of some of the classic
defense mechanisms and to how they might
contribute to emotion-related physical disorders. For example, denial and intellectualization might be thought of as response patterns that emphasize the cognitive handling
of the prime state, and it is noteworthy that
studies by Lazarus and his colleagues (Lazarus
& Alfert, 1964; Lazarus, Opton, Nomikos, &
Rankin, 1965; Spiesman, Lazarus, Mordkoff,
& Davidson, 1964) have demonstrated that
such coping strategies can reduce the autonomic response to an emotional stimulus.
Conversely, repression might be conceptualized as a process in which expressive and
cognitive responses are suppressed, and it
might be expected that reliance on basic
homeostatic and adaptive response may be
increased under those circumstances. This is
supported by the findings noted above that
facially and/or verbally expressive persons
have smaller autonomic and endocrine responses to emotional stimuli than do nonexpressive persons (Buck, 1975, 1979, 1981b;
Buck, Miller, & Caul, 1974; Frankenhauser,
1978). Together, these findings suggest the
general hypothesis that the strength of the
Emotion I process may be negatively correlated with the strength of the Emotion II and
Emotion III processes. Put another way, the
degree of autonomic/endocrine responding
to a prime state may be negatively related to
the expressive and cognitive responses to that
state.8
This autonomic/endocrine response could
well have disruptive effects, perhaps involving
the immune system, which could contribute
7
1 have argued elsewhere that Emotion II and Emotion
III responses tend to vary together because both are open
to social influence, albeit in different ways, whereas
physiological responses (Emotion I) remain relatively free
of such influences (see Buck, 1971, 1984a). Much more
research is needed in this area.
8
It should be noted that these studies used betweensubjects designs. Thus they suggest negative intersubject
correlations between autonomic and other modes of
response and are not relevant to the analysis of intrasubject
relations between these variables (cf. Buck, 1980).
MOTIVATION AND EMOTION
to disease (Hurst, Jenkins, & Rose, 1976;
Selye, 1976). Impaired emotional expression
has been associated with a number of quite
different disease processes. McClelland (1982)
has reported relations between inhibited personality patterns, sympathetic activation, impaired immune system functioning, and cardiovascular disease. Sifneos (1973) coined the
term alexithymia to describe the inability to
express one's feelings verbally. This quality
has been found to be prevalent in patients
with a variety of psychosomatic illnesses
(Flannery, 1977; Pierloot & Vinck, 1977;
Wolff, 1977), and Anderson (1981) has suggested that "impairment in the expression of
affect is a necessary but not sufficient factor
in the development of psychosomatic disease"
(p. 149). There is also evidence that a limited
ability to express depression, anger, and anxiety is associated with a predisposition to the
development of cancer (Hagnell, 1966; Thomas & Greenstreet, 1973), and the loss of
significant personal relationship and feelings
of hopelessness often occur near the time of
the clinical onset of cancer (Bahnson &
Bahnson, 1966; Greene, 1966; LeShan &
Worthington, 1956; Schmale & Iker, 1966).
Emotional expression is also important in
the process of coping with cancer and may
play a significant role in its clinical course
(Mclntosh, 1974; Peterson, Popkins, & Hall,
1981; Weisman, 1976; Weisman & Sobel,
1979; Weisman & Worden, 1977).
Social learning relevant to motivation and
emotion is influenced by the accessibility of
the responses involved in the life of the individual. The apparent medical consequences
of unhealthy forms of emotional expression
lend urgency to the understanding of how
positive patterns of expression might be fostered in the developing child. The importance
of social learning by observation and imitation
are well-known (Bandura, 1977; Bandura &
Walters, 1963). One of the features of the
realm of motivation and emotion that contributes to its complexity is that many of the
relevant responses are virtually invisible or
inaccessible to observers under most conditions, and may be only vaguely experienced
by the responder. This is particularly the case
with the Emotion I types of autonomic, endocrine, and immune system responses. Other
responses (expressive responses associated
403
with Emotion II and syncretic-cognitive responses associated with Emotion III) may or
may not be accessible to others depending on
the responder's expressive behavior and selfreports. As a result, learning to deal with
hunger, pain, sexuality, anger, and so forth,
is not a simple matter from an observational
learning point of view (Buck, 1971, 1981a,
1983).
Many studies of modeling and imitation
imply that the ways in which children learn
to deal with emotional informationto express emotion to others and attend to emotional information in themselvesmust be
affected by the ways that others around them
respond. In a 1933 study of muscle relaxation,
M. A. Wenger (cited in Wenger & Bagchi,
1961) was surprised to note that the ability
of a student from India to control his physiological activity far surpassed the ability of
the American students in the study. Later
studies performed in India confirmed that
the Yogic exercises and other practices learned
in that culture allow substantial control of
autonomic activity (Wenger & Bagchi, 1961;
Wenger, Bagchi, & Anand, 1961). Perhaps in
the course of such learning, the responder
comes to attend to interoceptive and proprioceptive information regarding the motivational/emotional states to a greater extent
than an American student would be likely
to do.
Such an education of attention could potentially lead to markedly different sorts of
emotional experience in different persons.
Thus there is evidence that adult males in
our culture are less facially and verbally
expressive and more physiologically responsive
than are females to certain affective stimuli,
and that this difference may begin to appear
in childhood (Buck, 1975, 1977, 1981b;
Frankenhauser, 1978). Perhaps this is associated with the dearth of male models that
show expressive behavior or discuss their
ways of coping with certain motivational/
emotional states. Conversely, young females
in our culture may have relatively few adult
models for coping openly with achievement
strivings or anger. Thus, although, as noted
above, an affect like anger is based on a
specific neural system common to all and the
experience of anger caused by arousal in this
system is similar in all and is to some extent
404
ROSS BUCK
self-explanatory, an individual may learn to
attend to or to ignore this experience to
different extents, and may learn to label the
experience incorrectly. Thus it is quite conceivable that a child would never connect the
verbal label anger with that experience. Thus,
learning about how to handle emotional information may vary greatly from individual
to individual.
Models in the mass media may have considerable impact in this area. It seems possible
that much of the powerful interest in music,
drama, and literature shown by humans derives from the fact that they can make accessible aspects of emotion and ways of handling
emotional information that are otherwise
hidden, providing in fantasy a model of motivational and emotional experience and
expression that is informative about many
things that remain inaccessible in normal
life. One can follow the experiences and
thoughts of the characters in a novel or in a
soap opera, or witness displays of feeling on
the stage or screen, that in normal life are at
least fragmented and often unavailable, and
can compare these to one's own experiences,
thoughts, and feelings. McClelland's (1961)
finding that high levels of achievement in a
culture are preceded by high levels of
achievement imagery in literature is consistent
with the notion that the content of these tales
may provide important models for the individual; the child who learns how it feels to
achieve through such models may be more
likely to be an achiever.
McClelland's methods could be employed
to analyze the social consequences of different
models of emotional expression on the emotional education within a society (Buck,
1983). It is noteworthy in this regard that in
a study of the emotional responses to music
videos, I found that the degree to which a
music video evokes the emotion it was apparently meant to evoke is related to the
liking of the video, even if the emotion
evoked is negative. Thus, just as a happy
video is liked if it elicits rated feelings of
happiness, a frightening video is liked if it
elicits feelings of fear, and a sad video is liked
if it elicits feelings of sadness. Moreover,
liking for all of the music videos was strongly
related to rated feelings of interest, regardless
of their emotional appeal (Buck, 1985). The
latter finding supports the notion that exposure to the music videos involves a kind of
exploratory behaviora process of exploring
one's own feelings. Apparently, the exploration even of feelings of fear, sadness, and
aggression can be highly rewarding, and this
may explain the high levels of interest in
some persons in horror movies, tear jerkers,
aggressive films, and the like. All of these
films could thus contribute to the emotional
education of the viewer, and this process
could in the long run have important personal
and social consequences.
The Interaction Between Primary
Motivational/Emotional Systems and
Cognition: A Model
Complex motives and emotions can be
conceptualized as an interaction between the
prime states and analytic cognition. To recap
briefly, the primary motivational/emotional
systems (primes) are innate, biologically based
special-purpose systems whose state of arousal
is read out via adaptive-homeostatic responses, expressive behavior, and direct subjective
experience. However, these responses are affected by analytic cognition. Cognition can
be regarded as a general-purpose system involving the ability to represent the nature of
the external and internal environment within
the brain, to paraphrase Galley's (1973) terms
quoted above. Analytic cognition involves
knowledge by description, which is achieved
via learning and is particularly dependent on
the sequential processing associated in humans with the left cerebral hemisphere
(Tucker, 1981). It is the interaction between
analytic cognition and the primes that makes
possible incentive motivation and anticipatory
adaptive behavior in both animals and humans. In humans, our larger analytic cognitive
capacitycombined as we shall see with the
revolutionary impact of languageunderlies
a great repertoire of motivational and emotional phenomena.
A general model of this interactive relation
between the primes and the cognitive system
is presented in Figure 3. The model assumes
that internal and external affective stimuli
initially impinge on the primes directly and
without cognitive mediation (cf. Gibson, 1966,
405
MOTIVATION AND EMOTION
Observed
stimuli
Events within the organism
Obtnvid
responses
COAL-DIRECTED
BEHAVIOR
SELF-REPORTS
FACIAL EXPRESSIONS,
BODY MOVEMENTS,
POSTURE, Etc.
PHYSIOLOGICAL
RESPONSES
Figure 3. The interactions between the primes and the cognitive system.
1979; Zajonc, 1980).9 The responses of these
systems is determined by their state of arousal
and arousability, which in turn is determined
by a variety of situational and constitutional
factors. The initial response to the stimuli is
also influenced by classically conditioned associations with the affective stimuli that are
unique to the individual. Together, the primes
and these conditioned responses determine
the impact of the affective stimuli for that
particular individual in that particular situation.
This impact is registered at both cognitive
and emotional levels. On the emotional level,
adaptive/homeostatic mechanisms are activated (Emotion I), there are tendencies toward
spontaneous expressive behaviors (Emotion
II), and subjective experiences occur, both
directly in syncretic cognition from the activation of the primes (Emotion III) and indirectly via proprioceptive and autonomic feedback (dashed lines). On the analytic-cognitive
level, the individual understands and interprets, or appraises, the stimuli on the basis
of past experience, the present situation, and
the subjective emotional experience. Once
the stimulus is understood and appraised, or
labeled, the individual has a basis for making
appropriate goal-directed coping responses
and self-reports describing the response to
the stimulus. These overt responses are affected by display rules the individual has
learned in that particular situation, which
may also interfere with spontaneous expressive
tendencies. That is, the individual may alter
his or her overt emotional/motivational behavior to fit the requirements and expectations
of a given social situation. At the same time,
the labeled emotional/motivational state may
become an internal stimulus in itself, beginning another cycle of response.
The fundamental difference between animal
and human motivation/emotion involves language. The interaction between the primes
9
Certain cognitive approaches to emotion deny that
affective stimuli per se can cause emotion without some
kind of cognitive mediation, such as Lazarus's (1984)
appraisal process. Gibson's theory, however, argued that
the organism naturally detects information that is ecologically significantthe perceptual system has evolved
to be attuned to such informationand that this process
is direct and requires no cognitive mediation (cf. Gibson,
1966, 1979; Shaw & Bransford, 1977). This argument
applies to affectively relevant information, and indeed
there is considerable evidence of an innate preparedness
to respond appropriately to affective displays in others
(Buck, 1984a, pp. 40-46). Sackett (1966) demonstrated
that isolated monkeys react with appropriate fearful
behavior when confronted with a photograph of a large
male making a threat display, and Perrett, Rolls, & Caan
(1982) found that cells in a specific area of the temporal
lobe in monkeys respond only to faces. In humans, facial
expressions of fear and anger are more readily associated
with aversive events than are happy or neutral expressions
in classical conditioning studies (Lanzetta & Orr, 1980;
Ohman & Dimberg, 1978). It appears to be critical that
the face is directed toward the subject in these studies.
Dimbetg & Ohman (1983; Dimberg, 1983) found that
the differential effects of happy versus angry faces are
reduced or lost when the face is directed away from the
subject, and Perrett et al. found reductions in response
as the facial stimulus was rotated away from a direct
orientation.
406
ROSS BUCK
and cognition portrayed in Figure 3 can be
applied to both animals and humans. However, from the time of the Greeks, Western
thought has distinguished between rational
processes unique to humans and the processes
governing animal behavior (cf. Cofer & Appley, 1964). Both Plato and Aristotle denied
rational souls to animals, but they granted
them lesser souls capable of caring for basic
bodily functions. Following them, St. Thomas
Aquinas equipped animals with a sensitive
soul and humans, in addition, with a rational soul. Descartes' dualism was similarly
grounded in the distinction between the determinants of animal and human behavior.
Part of the revolutionary impact of Darwin's theory was that it challenged the longstanding conviction that humans have a rational soul that is absent in animals. Most
have accepted this notion and regard humans
as complicated animals, and that is perhaps
accurate. However, there is one thing that all
groups of humans have that no other animals
have, and that is language. Only in humans
does behavior come so completely under the
control of factors that are mediated by language, including logic, reasoning, and social
rules. This fact is at the crux of the understanding of the uniquely human aspects of
motivation and emotion. Human motivation
and emotion are based on biological systems,
as they are in all animals. In both humans
and animals, learning and cognitive factors
build up an internal representation of reality
that influences these motives and emotions.
This kind of cognitive-emotional interaction
is clearly not unique to humans. What is
unique to humans is language, which has
created a culturally patterned system of behavior control that is functionally independent
of biology and is fundamentally different
from anything seen in animals. Linguistic
control systems enable humans to transcend
personal experience, allowing the symbolic
sharing of experience and the contemplation
of possibilities that have never been and
could never be experienced.
This is not to say that there is a fundamental biological gap between humans and
animalsthat clearly is not the casenor is
it necessarily the case that animals cannot
learn language. The point is that if an animal
did learn language it would cause a fundamental change in the animal's behavior, because that behavior would then be influenced
by a different sort of structurea different
principle of organizationin addition to the
principles of organization that had previously
determined the animal's behavior. The new
principles of organization inherent in language
are functionally independent of the old principles or organization inherent in the primes.
The development and maintenance of these
linguistic control systems also requires a kind
of motivation that is absent in animals: Needs
for understanding and for cognitive consistency, for example, underlie the uniquely
human attributes of logic and reason. Such
motivation is a potential inherent in the
structure of linguistic control systems, just as
the biologically based motivation considered
above is a potential inherent in the structure
of the primes. Linguistic control systems also
have emotionlike feelings associated with these
motives: feelings of amusement, satisfaction,
or dissonance associated with linguistic manipulation, for example. There may also be
feelings of anxiety associated with the linguistic control of the primes. The relation of
these linguistic motives and emotions to the
biologically based primes is an issue of great
interest.
I have argued that language makes humans
different in fundamental ways from animals
because it allows behavior to come under the
control of principles of logic and reasoning
that are mediated by language and are functionally independent of biology. With language
also comes the patterning of social behavior
according to the conformity and obedience
to linguistically patterned social rules (cf.
Milgram, 1974; Buck, 1976, pp. 400-414).
Perhaps this is what Plato, Aristotle, and
others meant by the human rational soul.
There is irony here, for most of these philosophers assumed that the rational soul was the
source of good and the animal passions the
source of evil. However, it is reason, logic,
and obedience to the rules that enable humans
to execute and claim justification for a holocaust, the development and use of nerve gas,
or the dropping of an atomic bomb. The
animal passions play little part in the most
monstrous of human acts.
MOTIVATION AND EMOTION
Differences With Other Approaches
The present point of view differs from both
traditional physiological approaches to motivation and recent approaches to emotion.
In contrast to Tomkins's (1962, 1963) theory
and similar analyses of emotion, the present
view (a) regards reflexes, instincts, and drives,
as well as primary affects, to be motivational/
emotional states, (b) regards homeostasis and
adaptation to be the raison d'etre for both
motivation and emotion, (c) regards major
aspects of the experience of motivational/
emotional states to be a function of a direct
experiential readout independent of external
expressive tendencies, and (d) argues that
motivation/emotion can occur without an
external readout and that indeed there may
be motivational/emotional states in which no
external readout has evolved. In contrast with
theories that define emotional states according
to whether their overt expression can be
reliably differentiated, the present analysis
defines primary emotional (and motivational)
states according to biological criteria. The
present analysis does not arrive at a single
list of primary motivational/emotional states;
such a list would differ according to the
species and the level of analysis involved. For
example, gnawing seems to be a more important phenomenon in hamsters than in
humans, some states may be present at a
physiological level (Emotion I) but not expressed in behavior (Emotion II), and so
forth.
There is considerable controversy about
what is emotion and what is not. Lazarus
(1984, pp. 124-25) questioned whether the
preferences studied by Zajonc and his colleagues should be regarded as emotions, and
Ekman (in press) discussed how the startle is
a reflex rather than emotion. The emotional
status of pain has also been questioned. The
present analysis includes a much broader
range of phenomena under the rubric of
emotion than is generally the case, and considers the distinction between reflexes, drives,
and primary affects to concern levels of emotion systems (primes) rather than emotion
versus nonemotions.
Like the approaches of Schachter (1964),
Lazarus (1966, 1984), and others, the present
407
analysis emphasizes the importance of the
interaction between cognitive factors and
physiological systems. However, the present
approach considers the biological bases of the
latter and the various aspects of response
physiological, expressive, and subjectivein
more detail. Also, different aspects of cognition, syncretic versus analytic, are considered.
Furthermore, whereas these approaches assume that cognition precedes emotionone
must know what it is before one can respond
emotionallythe present analysis agrees with
the Gibsonian view and with Zajonc's (1980,
1984) data, that initial emotional responding
precedes cognition.
This approach has more substantial differences with theories of emotion that emphasize
the analysis of emotion terminology (i.e.,
DeRivera, 1977; Plutchik, 1980; Roseman,
1979). The lists and structures of emotion
generated by such analyses usually differ substantially from one another, perhaps because
they reflect analytic-cognitive factors, the language of emotion, rather than prime systems
per se.
The hierarchical view of motivation and
emotion presented in this article is analogous
in some respects to Maslow's (1954) theory
of motivation, although there are significant
differences as well. According to Maslow,
human needs are arranged in a hierarchical
fashion, including (from the bottom) physiological needs; safety needs; needs for love,
belongingness, and esteem; aesthetic and cognitive needs; and needs for self-actualization.
Maslow suggested that these needs are inborn
and universal, or instinctoid, and that they
will always appear under favorable conditions.
However, the higher growth needs are not
strong enough to appear until the lower deficit
needs are satisfied. When a lower need is
regularly satisfied, it in effect is eliminated
from further status in the determination of
the organism's behavior (Cofer & Appley,
1964). The present analysis agrees that human
motivation is based on universal aspects of
human nature; it also agrees with the hierarchical aspects of Maslow's theory. However,
it does not agree with the implication that
when lower needs are satisfied they cease to
affect the individual's behavior, or with the
notion that higher needs are absent until the
408
ROSS BUCK
lower needs are satisfied. Instead, various
levels of behavior control are seen to be
simultaneously present and interacting with
each other.
In contrast to classical physiological approaches to motivation, the present view emphasizes the importance of phenomena usually linked with emotion, even in the case of
such basic motives as hunger and thirst. Also,
it emphasizes the rich potential of using the
rigorous and detailed analysis of emotional/
motivation expression in the study of processes usually approached at a physiological
level. Many animal studies of brain functioning, to say nothing of human studies, are
based on analyses of emotional expression,
from Hess's (1928) observations of hissing
and spitting cats, to Kluver and Bucy's (1939)
observations of docility in amygdalectomized
monkeys, to J. Olds's (1956) descriptions of
frantic bar pressing. Recent authors have
emphasized the importance of describing such
behaviors more completely than they have
been described in the past (e.g., Vanderwolf
& Robinson, 1981). An example of such an
analysis is Teitelbaum's use of a movement
notation system originally developed for ballet
choreography to demonstrate specific similarities in the akinesic gait of rats lesioned in
dopaminergic systems and in human patients
with Parkinson's disease (cf. Schallert, Whishaw, Ramirez, & Teitelbaum, 1978). It is
important to note that such observations can
involve intact subjects, behaving freely and
spontaneously in their natural environments.
The rigorous analysis of the behaviors expressive of emotion/motivation may allow
significant increases in the understanding of
physiological systems in both animals and
humans.
Summary
This article has presented the view that
each species has evolved a set of primary
motivational/emotional systems, or primes,
which are special-purpose systems serving the
basic functions of bodily adaptation and homeostasis. It is assumed that the primes are
based on neurochemical substrates, that motivation consists of the potential for behavior
inherent in the prime, and that emotion
involves the readout of this motivational po-
tential when activated by challenging stimuli.
Three kinds of such readout have evolved,
the most basic (Emotion I) being the readout
of motivational/emotional states to adaptive
and homeostatic mechanisms. The other kinds
of readout have evolved as needed by particular species, an external expressive readout
(Emotion II) occurring in social species, and
an internal syncretic-cognitive readout (Emotion III) occurring to the extent of the species'
cognitive abilities. It is suggested that each of
these kinds of readout is associated with a
distinct type of subjective emotional experience and that each prime is associated with
a particular pattern of expressive behavior
and a particular quality of Emotion III experience. Thus, the experience of a particular
state (i.e., anger, surprise, happiness, hunger,
sex) represents an amalgam of feelings from
interoceptive stimuli (Emotion I), proprioceptive stimuli (Emotion II), and experience
directly registered in syncretic cognition
(Emotion III). This amalgam differs for different states, with Emotion III generally being
more important for the differentiation of
state than Emotion I stimuli or Emotion II
stimuli, although this can be influenced by
learning to attend to one aspect of emotion
or another.
It is recognized that analytic-cognitive factors interact with the prime systems, and
indeed it is suggested that Emotion III has
evolved to foster such interaction. A general
model of the interaction between the cognitive
system and emotion suggests that it is in the
context of such an interaction that complex
human motivation and emotion can be most
profitably understood. In particular, the ability
to use language in interaction with the primes
is suggested to underlie uniquely human aspects of motivation and emotion. Time will
tell whether this ability will lead to the further
advancement of the human species or to its
destruction.
References
Anderson, C. D. (1981). Expression of affect and physiological response in psychosomatic patients. Journal
of Psychosomatic Research, 25, 143-149.
Andrew, R. J. (1963). The origin and evolution of the
calls and facial expressions of the primates. Behaviour,
20, 1-109.
Andrew, R. J. (1965). The origins of facial expressions.
Scientific American, 213, 88-94.
MOTIVATION AND EMOTION
Averill, J. R. (1980). On the paucity of positive emotions.
In K. R. Blankenstein, P. Pliner, & J. Polivy (Eds.),
Assessment and modification of emotional behavior
(pp. 7-45). New York: Plenum Press.
Bahnson, C. B., & Bahnson, M. D. (1966). Role of the
ego defenses: Denial and repression in the etiology of
malignant neoplasm. Annals of New York Academy of
Sciences, 125, 827-845.
Bandura, A. (1977). Social learning theory. Englewood
Cliffs. NJ: Prentice-Hall.
Bandura. A.. & Walters, R. H. (1963). Social learning
and personality development. New York: Holt, Rinehart
& Winston.
Benowitz, L. I., Bear, D. M., Rosenthal, R., & Mesulam,
M. (1983). Sensitivity to nonverbal communication
after unilateral brain damage. Cortex. 19, 5-12.
Bindra, D. (1968). Neuropsychological interpretation of
the effects of drive and incentive motivation on general
activity and instrumental behavior. Psychological Review, 75, 1-22.
Bolles, R. D. (1972). Reinforcement, expectancy, and
learning. Psychological Review, 79, 394-409.
Borod, J., & Caron, H. (1980). Facedness and emotion
related to lateral dominances, sex and expression type.
Neuropsychologia, IS, 237-241.
Borod, J. C, Caron, H. S., & Koff, E. (1981). Asymmetries
in positive and negative facial expression: Sex differences. Neuropsychologia, 19, 819-824.
Buck, R. (1971). Differences in social learning underlying
overt behavioral, self-report, and physiological responses
to emotion [Abstract]. Research in Education, 6, 17.
Buck, R. (1975). Nonverbal communication of affect in
children. Journal of Personality and Social Psychology,
31. 644-653.
Buck, R. (1976). Human motivation and emotion. New
York: Wiley.
Buck, R. W. (1977). Nonverbal communication of affect
in preschool children: Relationships with personality
and skin conductance. Journal of Personality and
Social Psychology, 35, 225-236.
Buck, R. (1979). Individual differences in nonverbal
sending accuracy and electrodermal responding. In R.
Rosenthal (Ed.), Skill in nonverbal communication
(pp. 140-170). Cambridge, MA.: Oelgeschlager, Gunn
& Hain.
Buck, R. (1980). Nonverbal behavior and the theory of
emotion: The facial feedback hypothesis. Journal of
Personality and Social Psychology, 38, 811-824.
Buck, R. (1981 a). The evolution and development of
emotion expression and communication. In S. Brehm,
S. Kassin, & F. Gibbons (Eds.), Developmental social
psychology (pp. 127-151). New York: Oxford.
Buck, R. (I981b). Sex differences in psychophysiological
responding and subjective experience: A comment.
Psychophysio/ogy, 18, 349-350.
Buck, R. (1982a). Spontaneous and symbolic nonverbal
behavior and the ontogeny of communication. In R.
Feldman (Ed.), Development of nonverbal behavior in
children (pp. 29-62). New York: Springer-Verlag.
Buck, R. (1982b, February). A theory of spontaneous and
symbolic expression: Implications for facial lateralization. Paper presented at the International Neuropsychological Society Convention, Pittsburgh.
Buck, R. (1983). Emotion development and emotional
education. In R. Plutchik & H. Kellerman (Eds.),
409
Emotions in early development (pp. 259-292). New
York: Academic Press.
Buck, R. (I984a). The communication of emotion. New
York: Guilford Press.
Buck, R. (1984b). The physiological bases of nonverbal
communication. In W. Waid (Ed.), Sociophysiology
(pp. 139-162). New York: Springer-Verlag.
Buck, R. (1985). Emotional appeals in music: The psychology of MTV. Unpublished paper. University of
Connecticut.
Buck, R., & Duffy, R. (1980). Nonverbal communication
of affect in brain-damaged patients. Cortex, 16, 351362.
Buck, R. W., Miller, R. E., & Caul, W. F. (1974). Sex,
personality and physiological variables in the communication of emotion via facial expression. Journal of
Personality and Social Psychology, 30, 587-596.
Campbell, R. (1978). Asymmetries in interpreting and
expressing a posed facial expression. Cortex, 14, 327342.
Cannon, W. B. (1927). The James-Lange theory of
emotion: A critical examination and an alternative
theory. American Journal of Psychology, 39, 106-124.
Carmon, A., & Nachson, 1. (1973). Ear asymmetry in
perception of emotional and nonverbal stimuli. Ada
Physiologica, 37, 351-357.
Cicone, M., Wapner, W, & Gardner, H. (1980). Sensitivity
to emotional expressions and situations in organic
patients. Cortex, 16. 146-147.
Cofer, C. N., & Appley, M. H. (1964). Motivation: Theory
and research. New York: Wiley.
Darwin, C. (1965). Expression of the emotions in man
and animals. Chicago: University of Chicago Press.
(Originally published 1872)
Delgado, J. M. R. (1969). Physical control of the mind.
New York: Harper & Row.
DeKosky, S. T., Heilman, K. M., Bowers, D., & Valenstein,
E. (1980). Recognition and discrimination of emotional
faces and pictures. Brain and Language, 9, 206-214.
Denenberg, V. H. (1981). Hemispheric laterally in animals
and the effects of early experience. The Behavioral and
Brain Sciences, 4, 1-49.
Denenberg, V. H., Garbanti, J., Sherman, G., Yutzey,
D. A., & Kaplan, R. (1978). Infantile stimulation
induces brain lateralization in rats. Science, 201, 1150-
1152.
DeRivera, J. (1977). A structural theory of the emotions.
New York: International Universities Press.
Dimberg, V. (1983). Emotional conditioning to facial
stimuli: A psychobiological analysis. Uppsala, Sweden:
Uppsala University.
Dimberg, U., & Ohman, A. (1983). The effects of directional facial cues on electrodermal conditioning to
facial stimuli. Psychophysiology, 20, 160-167.
Duffy, R. J., Duffy, J. R., & Pearson, K. (1975). Pantomime recognition in aphasics. Journal of Speech and
Hearing Research, 18, 115-132.
Eibl-Eibesfeldt, I. (1970). Ethology: The biology of behavior. New York: Holt, Rinehart & Winston.
Eibl-Eibesfeldt, I. (1972). Similarities and differences
between cultures in expressive movements. In R. A.
Hinde (Ed), Nonverbal communication (pp. 297-312).
Cambridge, England: Cambridge University Press.
410
ROSS BUCK
Ekman, P. (1980). Asymmetry in facial expression. Science, 209. 833-834.
Ekman, P. (in press). Expression and the nature of
emotion. In K. Scherer & P. Ekman (Eds.), Approaches
to emotion. Hillsdale, NJ: Erlbaum.
Ekman, P., & Friesen, W. V. (1969). The repertoire of
nonverbal behavior: Categories, origins, usage and coding. Semiotica. 1, 49-98.
Ekman, P., & Friesen, M. V. (1975). Unmasking the face.
Englewood Cliffs, NJ: Prentice Hall.
Ekman, P., Hagar, J., & Friesen, W. (1981). The symmetry
of emotional and deliberate facial action. Psychophysiology, 18, 101-106.
Ekman, P., Sorenson, E. R., & Friesen, W. V. (1969).
Pan-cultural elements in the facial displays of emotion.
Science, 164, 86-88.
Ellsworth, P. C, & Tourangeau, R. (1981). On our failure
to disconfirm what nobody ever said. Journal of Personality and Social Psychology, 40, 363-369.
Flannery, J. G. (1977). Alexithymia I: The communication
of physical symptoms. Psychotherapy and Psychosomatics, 28, 133.
Frankenhauser, M. (1978). Psychoneuroendocrine sex
differences in adaptation to the psychosocial environment. In L. Zechella (Ed.), Clinical psychoneuro-endocrinology in reproduction: Serono Symposium, 1977.
New York: Academic Press.
Gainotti, G. (1969). Reactions "catastrophiques" et manifestations d'indifference au cours des atteintes cerebrales [Catastrophic reactions and manifestations of
indifference associated with cerebral damage]. Neuropsychologica, 7, 195-204.
Gainotti, G. (1972). Emotional behavior and hemispheric
side of the lesion. Cortex, 8, 41-55.
Galaburda, A. M., & Kemper, T. M. (1979). Annals of
Neurology, 6, 94-100.
Galaburda, A. M., LeMay, M., Kemper, T. M., & Geshwind, N. (1978). Right/left asymmetries in the brain.
Science. 199. 852-856.
Galin, D. (1974). Implications for psychiatry of left and
right cerebral specializations: A neurophysiological
context for unconscious processes. Archives of Genera/
Psychiatry, 31. 572.
Galin, D., Diamond, R., & Braff, D. (1977). Lateralization
of conversion symptoms: More frequent on the left.
American Journal of Psychiatry, 134, 578-580.
Gazzaniga, M. S. (1972). The split brain in man. In
T. J. Taylor (Ed.), Altered states of awareness (pp.
119-124). San Francisco: Freeman.
Geshwind, N. (1979). Specializations of the human brain.
Scientific American, 241, 202-218.
Gibson, J. J. (1966). The senses considered as perceptual
systems. Boston: Houghton-Miiflin.
Gibson, J. J. (1979). The ecological approach to visual
perception Boston: Houghton-Mifflin.
Glickman, S. E., & Schiff, B. B. (1967). A biological
theory of reinforcement. Psychological Review, 74, 81109.
Gray, J. A. (1982). Precis of The neuropsychology of
anxiety. The Behavioral and Brain Sciences, 5, 469534. (Includes commentary)
Greene, W. A. (1966). The psychosocial setting of the
development of leukemia and lymphoma. Annals of
New York Academy of Science, 125, 794-801.
Grossman, S. B. (1967). A textbook of physiological
psychology. New \ork: Harper & Row, Hoeber Medical
Division.
Hagar, J. C., & Ekman, P. (1981). Methodological problems in Tourangeau and Ellsworth's study of facial
expression and experience of emotion. Journal of
Personality and Social Psychology, 40, 358-362.
Haggard, M. P., & Parkinson, A. M. (1971). Stimulus
and task factors as determinants of ear advantages.
Quarterly Journal of Experimental Psychology, 23,
168-177.
Hagnell, O. (1966). The premorbid personality of persons
who develop cancer in a total population investigated
in 1947 and 1957. Annals of New York Academy of
Science, 125, 846-855.
Harlow, H. F., & Mears, C. E. (1983). Emotional sequences
and consequences. In R. Plutchik & H. Kellerman
(Eds.), Emotion: Theory, research and experience: Vol.
2. Emotions in early development (pp. 171-198). New
York: Academic Press.
Heath, R. G. (Ed.). (1964a). The role of pleasure in
behavior. New York: Harper & Row, Hoeber Medical
Division.
Heath, R. G. (1964b). Pleasure responses of human
subjects to direct stimulation of the brain: Physiologic
and psychodynamic considerations. In R. G. Heath
(Ed.), The role of pleasure in behavior (pp. 219-243).
New York: Harper & Row, Hoeber Medical Division.
Hebb, D. O. (1955). Drives and the C.N.S. (Conceptual
nervous system). Psychological Review, 62, 243-254.
Heilman, K. M., Scholes, R., & Watson, R. T. (1975).
Auditory affective agnosia. Journal of Neurology, Neurosurgery, and Psychiatry, 38, 69-72.
Heilman, K. M., Schwartz, H. D., & Watson, R. T.
(1978). Hypoarousal in patients with the neglect syndrome and emotional indifference. Neurology, 28. 229232.
Hess, W. R. (1928). Stammganglien-reizversuche [Brainstem stimulation attempts]. Berliner Gesellschaft fur
Physiologic, 42, 554.
Hohmann, G. (1966). Some effects of spinal cord lesions
on experimental emotional feelings. Psychophysiology,
3, 143-156.
Hurst, M. W., Jenkins, C. D., & Rose, R. M. (1976).
The relation of psychological stress to onset of medical
illness. Annual Review Medicine, 27, 301-312.
Izard, C. E. (1971). The face of emotion. New York:
Appleton-Century-Crofts.
Izard, C. E. (1977). Human emotions. New York: Plenum
Press.
Izard, C. E. (1981). Differential emotions theory and the
facial feedback hypothesis of emotion activation. Journal of Personality and Social Psychology, 40, 350-354.
James, W. (1884). What is an emotion? Mind, 9, 188205.
James, W. (1890). The principles of psychology (Vol. 1).
New York: Henry Holt.
King, H. E. (1961). Psychological effects of excitation in
the limbic system. In D. E. Sheer (Ed.), Electrical
stimulation of the brain (pp. 477-486). Austin: University of Texas Press.
Kleinke, C., & Walton, J. (1982). Influence of reinforced
smiling on affective responses in an interview. Journal
of Personality and Social Psychology, 42, 557-565.
Kluver, H., & Bucy, P. C. (1939). Preliminary analysis
MOTIVATION AND EMOTION
411
Mark, V. H., & Ervin, F. R. (1970). Violence and the
brain. New York: Harper & Row.
Marsh, R. C. (Ed.). (1956). Bertrand Russell: Logic and
knowledge. London: George Allen and Unwin.
Maslow, A. H. (1954). Motivation and personality. New
York: Harper.
McClelland, D. C. (1961). The achieving society. Princeton, NJ: Van Nostrand.
McClelland, D. C. (1982). The need for power, sympathetic activation, and illness. Motivation and emotion,
6, 31-41.
Mclntosh, J. (1974). Processes of communication, information seeking and control associated with cancer: A
selective review of the literature. Social Science Medicine, S, 167-187.
Milgram, S. (1974). Obedience to authority: An experimental view. New York: Harper & Row.
Mogenson, G. J., & Phillips, A. G. (1976). Motivation:
A psychological construct in search of a physiological
substrate. In J. M. Sprague & A. N. Epstein (Eds.),
Progress in psychobiology and physiological psychology
(pp. 189-243). New York: Academic Press.
Morrow, L., Vrtunski, P. M., Kim, Y, & Boiler, F. (1981).
Arousal responses to emotional stimuli and laterality
of lesion. Neuropsychologica, 19, 65-71.
Mover, K. E. (1971). The physiology of hostility. Chicago:
Markham.
Oatley, K. (1973). Stimulation and theory of thirst. In
A. N. Epstein, H. R. Killileff & E. Stellar (Eds.), The
neuropsychology of thirst (pp. 199-224). New York:
Winston-Wiley.
Ohman, A., & Dimberg, V. (1978). Facial expressions as
conditioned stimuli for electrodermal responses: A case
of "preparedness"? Journal of Personality and Social
Psychology, 36, 1251-1258.
Olds, J. (1956, October). Pleasure centers in the brain.
Scientific American, 195, 105-117.
Olds, J., & Milner, P. (1954). Positive reinforcement
produced by electrical stimulation of septal area and
other regions of rat brain. Journal of Comparative and
Physiological Psychology, 47, 419-427.
Olds, M. E., & Fobes, J. L. (1981). The central basis of
motivation: Intracranial self-stimulation studies. Annual
Review of Psychology, 32, 523-576.
MacLean, P. D. (1968). Contrasting functions of limbic Olds, J., & Olds, M. E. (1965). Drives, rewards and the
and neocortica! systems of the brain and their relevance
brain. In T. M. Newcome (Ed.), New directions in
to psychophysiological aspects of medicine. In E. Cellpsychology (Vol. 2). New York: Holt.
horn (Ed.), Biological foundations of emotion. Glenview,
Panksepp, J. (1981). Hypothalamic integration of behavior.
IL: Scott, Foresman.
In P. Morgane & J. Panksepp (Eds.), Handbook of the
MacLean, P. D. (1969). The hypothalamus and emotional
hypothalamus: Vol. 3, Part B Behavioral studies of the
behavior. In W. Haymaker, E. Anderson, & W. J. H.
hypothalamus (pp. 289-431). New York: Marcel Dekker.
Nanta (Eds.), The hypothalamus, Springfield, IL:
Panksepp, J. (1982). Toward a general psychobiological
Charles C Thomas.
theory of emotions. The Behavioral and Brain Sciences,
MacLean, P. D. (1970). The limbic brain in relation to
5, 407-467. (Including commentary)
the psychoses. In P. H. Black (Ed.), Physiological
Papez, J. W. (1937). A proposed mechanism of emotion.
correlates of emotion. New York: Academic Press.
Archives of Neurology and Psychiatry, 38, 725-743.
MacLean, P. D. (1973). A triune concept of the brain and
Perrett, D. L, Rolls, E. T, & Caan, W. (1982). Visual
behaviour. Toronto, Canada: University of Toronto
neurones responsive to faces in the monkey temporal
Press.
cortex. Experimental Brain Research, 47, 329-342.
MacLean, P. D. (1981). Role of transhypothalamic pathPeterson, L. G., Popkins, M. K., & Hall, R. G. W.
ways in social communication. In P. Morgane & J.
(1981). Psychiatric aspects of cancer. Psychosomatics,
Panksepp (Eds.), Handbook of the hypothalamus: Vol.
22, 778-793.
3, Part B. Behavioral studies of the hypothalamus (pp.
Piaget, J. (1971). Piaget's theory. In P. Mussen (Ed.),
259-287). New York: Marcel Dekker.
Handbook of child development (Vol. 1). New York:
Mandler, G. (1975). Mind and emotion. New York: Wiley.
Wiley.
of functions of the temporal lobe in monkeys. Archives
of Neurology and Psychiatry, 42, 979-1000.
Kraut, R. E. (1982). Social presence, facial feedback,
and emotion. Journal of Personality and Social Psychology, 42, 853-863.
Kunst-Wilson, W. R., & Zajonc, R. B. (1980). Affective
discrimination of stimuli that cannot be recognized.
Science, 207, 557-558.
Laird, J. D. (1974). Self-attribution of emotion: The
effects of expressive behavior on the quality of emotional
experience. Journal of Personality and Social Psychology, 29, 475-486.
Lanzetta, 3. T, Cartwright-Smith, J., & Kleck, R. E.
(1976). Effects of nonverbal dissimulation on emotional
experience and autonomic arousal. Journal of Personality and Social Psychology, 33, 354-370.
Lanzetta, J. T., & Orr, S. P. (1980). Influence of facial
expressions on the classical conditioning of fear. Journal
of Personality and Social Psychology, 39, 1081-1087.
Lazarus, R. S. (1966). Psychological stress and the coping
process. New York: McGraw-Hill.
Lazarus, R. S. (1982). Thoughts on the relations between
emotion and cognition. American Psychologist, 37,
1019-1024.
Lazarus, R. S. (1984). On the primacy of cognition.
American Psychologist, 39, 124-129.
Lazarus, R. S., & Alfert, E. (1964). Short-circuiting of
threat by experimentally altering cognitive appraisal.
Journal of Abnormal and Social Psychology, 69, 195205.
Lazarus, R. S., Opton, E. M., Jr., Nomikos, M. S., &
Rankin, N. O. (1965). The principle of short-circuiting
of threat: Further evidence. Journal of Personality, 33,
622-635.
LeShan, L., & Worthington, R. E. (1956). Loss of
cathexes as a common psychodynamic characteristic
of cancer patients. Psychology Reports, 2, 183-193.
Lindsley, D. B. (1951). Emotion. In S. S. Stevens (Ed.),
Handbook of experimental psychology (pp. 473-516).
New York: Wiley.
Lindsley, D. B. (1957). Psychophysiology and motivation.
In M. R. Jones (Ed.), Nebraska Symposium on Motivation (pp. 44-105). Lincoln: University of Nebraska
412
ROSS BUCK
Pierloot, R., & Vinck, J. (1977). A pragmatic approach
to the concept of alexithymia. Psychotherapy and
Psychosomatics, 28, 156.
Plutchik, R. (1980). Emotion: A psychoevolulionary synthesis. New York: Harper & Row.
Riskind, J. H., & Gotay, C. C. (1982). Physical posture:
Could it have regulatory or feedback effects on motivation and emotion? Motivation and Emotion, 6, 273298.
Roseman, I. (1979, September). Cognitive aspects of
emotion and emotional behavior. Paper presented at
the meeting of the American Psychological Association,
New York.
Rossi, G. E, & Rosandini, G. (1967). Experimental
analysis of cerebral dominance in man. In C. H.
Millikan & F. L. Darley (Eds.), Brain mechanisms
underlying speech and language (pp. 167-175). New
York: Grune & Stratton.
Routtenberg, A. (1978). The reward system of the brain.
Scientific American, 239, 154-164.
Russell, B. (1912). Problems of philosophy. New York:
Oxford University Press.
Russell, B. (1927). Philosophy. New York: W. W. Norton.
Russell, B. (1956). Our knowledge of the external world.
New York: New American Library.
Russell, J. A. (1979). Affective space is bipolar. Journal
of Personality and Social Psychology, 37, 345-356.
Russell, J. A. (1983). Pancullural aspects of the human
conceptual organization of emotions. Journal of Personality and Social Psychology, 45, 1281-1288.
Russell, J. A., & Mehrabian, A. (1977). Evidence for a
three-factor theory of emotions. Journal of Research
in Personality, 11, 273-294.
Sackeim, H. A., Our, R. C, & Saucy, M. C. (1978).
Emotions are expressed more intensely on the left side
of the face. Science, 202, 434-435.
Sackett, G. P. (1966). Monkeys reared in isolation with
pictures as visual input: Evidence for an innate releasing
mechanism. Science, 154, 1468-1473.
Safer, M. (1981). Sex and hemisphere differences in
access to codes for processing emotional expressions
and faces. Journal of Experimental Psychology: General,
110, 86-100.
Safer, M. A., & Leventhal, H. (1977). Ear differences in
evaluating emotional tones of voice and verbal content.
Journal of Experimental Psychology: Human Perception
and Performance, 3, 75-82.
Schachter, S. (1964). The interaction of cognitive and
physiological determinants of emotion state. In L.
Berkowitz (Ed.), Advances in experimental social psychology (Vol. 1, pp. 49-80). New York: Academic
Press.
Schallert, T., Whishaw, I., Ramirez, V., & Teitelbaum, P.
(1978). Compulsive, abnormal walking caused by antichotinergics in akinetic, 6-hydroxydopamine treated
rats. Science, 199, 1461-1463.
Schlosberg, H. (1952). The description of facial expressions
in terms of two dimensions. Journal of Experimental
Psychology, 44. 229-237.
Schmale, A., & Iker, H. (1966). The psychological setting
of uterine cervical cancer. Annals of New York Academy
of Sciences, 125, 807-313.
Schwartz, G. E., Davidson, R. J., & Maer, F. (1975).
Right hemisphere lateralization for emotion in the
human brain: Interactions with cognition. Science,
190. 286-288.
Selye, H. (1976). The stress of life. New York: McGrawHill.
Sem-Jacobson, C. W. (1968). Deplh-eleclroencephalographic stimulation of the human brain and behavior.
Springfield, IL: Charles C Thomas.
Shaw, R., & Bransford, J. (1977). Psychological approaches
to the problem of knowledge. In R. Shaw & J.
Bransford (Eds.), Perceiving, acting, and knowing.
Hillsdale, NJ: Erlbaum.
Shearer, S. L., & Tucker, D. M. (1981). Differential
cognitive contributions of the cerebral hemispheres in
the modulation of emotional arousal. Cognitive Theory
and Research, 5, 85-93.
Sifneos, P. E. (1973). The prevalence of "alexithymic"
characteristics in psychosomatic patients. Psychotherapy
and Psychosomatics, 22, 255.
Sperry, R. W., Zaidel, E., & Zaidel, D. (1979). Self
recognition and social awareness in the disconnected
minor hemisphere. Neuropsychologia, 17, 153-166.
Spiesman, J. C, Lazarus, R. S., Mordkoff, A. M., &
Davidson, L. A. (1964). Experimental reduction of
stress based on ego defense theory. Journal of Abnormal
and Social Psychology, 68, 367-380.
Stellar, E. (1954). The physiology of motivation. Psychological Review, 61, 5-22.
Stern, D. B. (1977). Handedness and the lateral distribution of conversion reactions. Journal of Nervous and
Mental Disease, 164, 122-128.
Terzian, H. (1964). Behavioural and EEG effects of
intracarotid sodium amytal injections. Ada Neurochirurgica (Wien), 12. 230-239.
Terzian, H., & Ceccotto, C. (1959). Su un nuova metodo
per la determinazione e lo studio della dominanga
emisferica [On a new method for the determination
and study of hemispheric dominance]. Giornale de
Psichitrie et Neuropatholgie. 87, 889-924.
Terzian, H., & Dalle Ore, G. (1955). Symptoms of Kluver
and Bucy reproduced in man by bilateral removal of
the temporal lobes. Neurology, 5, 373-380.
Thomas, C. B., & Greenstreet, R. L. (1973). Psychological
characteristics in youth as predictors of five disease
states: Suicide, mental illness, hypertension, coronary
heart disease, and tumor. Johns Hopkins Medical
Journal, 132, 16-43.
Tomkins, S. (1962). Affect, imagery and consciousness:
The positive affects (Vol. 1). New York: Springer.
Tomkins, S. (1963). Affect, imagery and consciousness:
The negative affects (Vol. 2). New York: Springer.
Tomkins, S. (1981). The role of facial response in the
experience of emotion: A reply to Tourangeau and
Ellsworth. Journal of Personality and Social Psychology,
40. 355-357.
Tomkins, S. (1983). Affect theory. In P. Ekman (Ed.),
Emotion in the human face (2nd ed., pp. 353-395).
New York: Cambridge University Press.
Tourangeau, R., & Ellsworth, P. C. (1979). The role of
facial response in the experience of emotion. Journal
of Personality and Social Psychology, 37, 1519-1531.
Tucker, D. M. (1981). Lateral brain function, emotion,
and conceptualization. Psychological Bulletin. 89. 1946.
Tucker, D. M., & Newman, J. P. (1981). Lateral brain
MOTIVATION AND
function and the cognitive inhibition of emotional
arousal. Cognitive Theory and Research. 5, 197-202.
Tucker, D. M., Roth, R. S., Arneson, B. A., & Buckingham, V. (1977). Right hemisphere activation during
stress. Neuropsychologica, }5, 697-700.
Tucker, D. M., Watson, R. T., & Heilman, K. M. (1977).
Discrimination and evocation of affectively intoned
speech in patients with right parietal disease. Neurology,
27. 947-950.
Valenstein, E. S. (1973). Brain control. New York: Wiley.
Valenstein, E. S., Cox, V. C, & Kakolewski, J. K. (1970).
Re-examination of the role of the hypothalamus in
motivation. Psychological Review, 77, 16-31.
Vanderwolf, C. H., & Robinson, T. E. (1981). Reticulocortical activity and behavior: A critique of the arousal
theory and a new synthesis. The Behavioral and Brain
Sciences, 4, 459-514. (Includes commentary)
von Hoist, E., & von Saint Paul, U. (1962, March).
Electrically controlled behavior. Scientific American,
206. 50-59.
Weisman, A. D. (1976). Early diagnosis of vulnerability
in cancer patients. American Journal of Medical Science,
271, 187-196.
Weisman, A. D., & Sobel, H. J. (1979). Coping with
cancer through self-instruction: A hypothesis. Journal
of Human Stress, 5. 3-8.
Weisman, A. D., & Worden, J. W. (1977). The existential
plight of cancer. Significance of the first 100 days.
International Journal of Psychiatry Medicine, 7, 1-15.
Wenger, M. A., & Bagchi, B. K. (1961). Studies of
autonomic functions in practitioners of yoga in India.
Behavioral Science. 6. 313-323.
EMOTION
413
Wenger, M. A., Bagchi, B. K., & Anand, B. K. (1961).
Experiments in India on "voluntary" control of the
heart and pulse. Circulation, 24. 1319-1325.
Whalen, R. E. (1966). Sexual motivation. Psychological
Review, 72, 151-163.
White, J. C. (1940). Autonomic discharge from stimulation
of the hypothalamus in man. Research Publications of
the Association for Research in Nervous and Mental
Disease, 20, 854-863.
White, R. W. (1959). Motivation reconsidered: The concept of competence. Psychological Review, 66, 297333.
Wilson, W. R. (1979). Feeling more than we can know:
Exposure effects without learning. Journal of Personality
and Social Psychology, 37, 811-821.
Wise, R. A. (1968). Hypothalamic motivational systems:
Fixed or plastic neural circuits? Science, 162, 377379.
Wolff, H. H. (1977). The concept of alexithymia in the
future of psychosomatic research. Psychotherapy and
Psychosomatics, 28, 376.
Zajonc, R. B. (1980). Feeling and thinking: Preferences
need no inferences. American Psychologist, 35, 151175.
Zajonc, R. B. (1984). On the primacy of affect. American
Psychologist, 39, 117-123.
Received April 20, 1984
Revision received October 22, 1984
Psychological Documents To Be Discontinued
At its February 2-3, 1985, meeting, the Council of Representatives voted to cease
publication of Psychological Documents (formerly the Journal Supplement Abstract
Service) as of December 31, 1985, with the publication of the December 1985 issue
of the catalog. Continued low submissions, decreasing usage, and rising costs for
fulfillment of paper and microfiche copies of documents were reasons given for
discontinuing publication of the alternative format publication, which was begun in
1971 as an "experimental" publication.
Authors who wish to submit documents for publication consideration must do so
by July 1. Authors who are currently revising documents at the request of the editor
should complete all revisions and submit them for final review as soon as possible,
but no later than July 1. Orders for paper and microfiche copies of documents
presently in the system and of those documents entered during 1985 will continue
through December 31, 1986.
Das könnte Ihnen auch gefallen
- Memory and Emotion - Oxford (2003)Dokument428 SeitenMemory and Emotion - Oxford (2003)Gabriel BudianNoch keine Bewertungen
- Carroll e Izard The Structure and Person PDFDokument37 SeitenCarroll e Izard The Structure and Person PDFErick SuasteNoch keine Bewertungen
- Thiemo Breyer (Eds.) - Epistemological Dimensions of Evolutionary Psychology-Springer-Verlag New York (2015)Dokument248 SeitenThiemo Breyer (Eds.) - Epistemological Dimensions of Evolutionary Psychology-Springer-Verlag New York (2015)LandoGuillénChávezNoch keine Bewertungen
- Medicinski Engleski - SkriptaDokument12 SeitenMedicinski Engleski - SkriptaNikola Kudoić100% (1)
- 10 Basic Drives and Motives PDFDokument36 Seiten10 Basic Drives and Motives PDFHammna AshrafNoch keine Bewertungen
- Cerebrovascular DiseaseDokument54 SeitenCerebrovascular Diseaselisaagustina100% (1)
- University of Phoenix Material: Biological Psychology Worksheet PSY/340Dokument3 SeitenUniversity of Phoenix Material: Biological Psychology Worksheet PSY/340Jarra37100% (2)
- How To Drink Much and Stay SoberDokument14 SeitenHow To Drink Much and Stay SoberrichlanderNoch keine Bewertungen
- Emotion, Motivation, and Anxiety: Brain Mechanisms and PsychophysiologyDokument16 SeitenEmotion, Motivation, and Anxiety: Brain Mechanisms and PsychophysiologyJulián SaugyNoch keine Bewertungen
- Answer Key Simulated Pre Board Set 4 NagaDokument11 SeitenAnswer Key Simulated Pre Board Set 4 NagaRaymark MoralesNoch keine Bewertungen
- Spiritual Development and The BrainDokument53 SeitenSpiritual Development and The BrainNidhal Ben Khalfa100% (6)
- What Emotions Really Are: The Problem of Psychological CategoriesVon EverandWhat Emotions Really Are: The Problem of Psychological CategoriesBewertung: 4.5 von 5 Sternen4.5/5 (2)
- Eric Marcus - Modern Ego PsychologyDokument29 SeitenEric Marcus - Modern Ego Psychologylc49Noch keine Bewertungen
- Psychiatry - Evolutionary Developmental Psychopathology - Ian PitchfordDokument280 SeitenPsychiatry - Evolutionary Developmental Psychopathology - Ian PitchfordAlberto Chicayban100% (1)
- Gross Barrett 2011Dokument15 SeitenGross Barrett 2011Tomás SuárezNoch keine Bewertungen
- Ciompi, I. (1997) - The Concept of Affect Logic.Dokument13 SeitenCiompi, I. (1997) - The Concept of Affect Logic.Juan Camilo SotoNoch keine Bewertungen
- PSQIDokument4 SeitenPSQIPd Ibi Propinsi DiyNoch keine Bewertungen
- Textbook Catalogue 2009Dokument52 SeitenTextbook Catalogue 2009Lippincott Williams and Wilkins- Europe100% (2)
- Writing Practice Nursing-2Dokument4 SeitenWriting Practice Nursing-2Twila Abuedo100% (5)
- General Systems TheoryDokument2 SeitenGeneral Systems TheoryArt Christian Ramos100% (2)
- Newborn Case StudyDokument9 SeitenNewborn Case Studyitsmeaya75% (4)
- An Evolutionary Theory of Human Motivation - Bernard Et Al (2005)Dokument57 SeitenAn Evolutionary Theory of Human Motivation - Bernard Et Al (2005)Eduardo Aguirre DávilaNoch keine Bewertungen
- Izard, 2007 Basic Emotions, Natural Kinds, Emotion Schemas, and A New ParadigmDokument21 SeitenIzard, 2007 Basic Emotions, Natural Kinds, Emotion Schemas, and A New ParadigmaldogiovanniNoch keine Bewertungen
- Toward A Unified Model of Human MotivatiDokument14 SeitenToward A Unified Model of Human MotivatiTruddy HarkerNoch keine Bewertungen
- Neuroscience of EmotionDokument68 SeitenNeuroscience of Emotionstephan89100% (1)
- Burns NeliganDokument45 SeitenBurns NeliganroyvillafrancaNoch keine Bewertungen
- Human Emotions: A Conceptual Overview: Andreas Keil and Vladimir MiskovicDokument22 SeitenHuman Emotions: A Conceptual Overview: Andreas Keil and Vladimir MiskovicMariaNoch keine Bewertungen
- Arthur Staats - The Paradigmatic Behaviorism Theory of Emotion. Basis For UnificationDokument27 SeitenArthur Staats - The Paradigmatic Behaviorism Theory of Emotion. Basis For UnificationYan CorpseNoch keine Bewertungen
- Keltner - Haidt.social Functions at Four Levels PDFDokument17 SeitenKeltner - Haidt.social Functions at Four Levels PDFGabriel MedinaNoch keine Bewertungen
- Evolutionary - Psychology,.a.new - Paradigm JurnalDokument30 SeitenEvolutionary - Psychology,.a.new - Paradigm JurnalkarinatyasNoch keine Bewertungen
- Toward A Psychological Theory of Multidimensional Activation (Arousal) PDFDokument34 SeitenToward A Psychological Theory of Multidimensional Activation (Arousal) PDFCC TANoch keine Bewertungen
- Eysenck 1991Dokument17 SeitenEysenck 1991Magda MoldovanNoch keine Bewertungen
- An Evolutionary Perspective On PsychiatryDokument6 SeitenAn Evolutionary Perspective On PsychiatryLanaNoch keine Bewertungen
- How To ReadDokument39 SeitenHow To Readdrm1238475Noch keine Bewertungen
- A Critical Role For Affective Neuroscience in Resolving What Is Basic About Basic EmotionsDokument7 SeitenA Critical Role For Affective Neuroscience in Resolving What Is Basic About Basic EmotionsJ. Félix Angulo RascoNoch keine Bewertungen
- WrightPanksepp NPSA 2012Dokument73 SeitenWrightPanksepp NPSA 2012Tim PetersonNoch keine Bewertungen
- Foundations of Psychology PaperDokument6 SeitenFoundations of Psychology PaperCathyrn BullerNoch keine Bewertungen
- The Science of Psychology615765Dokument34 SeitenThe Science of Psychology615765Robert SloaneNoch keine Bewertungen
- Work PsychologyDokument19 SeitenWork PsychologyAsadulla KhanNoch keine Bewertungen
- Psychological Topics 2011Dokument23 SeitenPsychological Topics 2011Lama ElghoulNoch keine Bewertungen
- PSYCHODYNAMICSDokument2 SeitenPSYCHODYNAMICSNiño PerialdeNoch keine Bewertungen
- The Emerging Field of Emotion Regulation: An Integrative ReviewDokument29 SeitenThe Emerging Field of Emotion Regulation: An Integrative ReviewAnonymous Csk4UVPONoch keine Bewertungen
- Autonomic Nervous System Activity in Emotion: A Review: Sylvia D. KreibigDokument52 SeitenAutonomic Nervous System Activity in Emotion: A Review: Sylvia D. KreibigeddyputriNoch keine Bewertungen
- KoriatMaayan&Nussinson2006 PDFDokument34 SeitenKoriatMaayan&Nussinson2006 PDFpvsptaNoch keine Bewertungen
- Psych Construction DarwinianDokument11 SeitenPsych Construction DarwinianJervin Abuda CuaysonNoch keine Bewertungen
- Personality and Individual Differences: Philip J. CorrDokument9 SeitenPersonality and Individual Differences: Philip J. Corrsomya mathurNoch keine Bewertungen
- Pages-from-Francois Clarac Auth. C. Giovanni GaliziaDokument28 SeitenPages-from-Francois Clarac Auth. C. Giovanni GaliziaMaria Cristina ConduracheNoch keine Bewertungen
- Well-Being and Affective Style: Neural Substrates and Biobehavioural CorrelatesDokument17 SeitenWell-Being and Affective Style: Neural Substrates and Biobehavioural CorrelatesGustavo Adolfo DomínguezNoch keine Bewertungen
- Psychology and The Study of Inter-Group ConflictDokument34 SeitenPsychology and The Study of Inter-Group ConflictsomeNoch keine Bewertungen
- The Dark Side of Emotion The Addiction PerspectiveDokument35 SeitenThe Dark Side of Emotion The Addiction PerspectiveDiana SgNoch keine Bewertungen
- Work Psychology1 Sabah 3 2007Dokument23 SeitenWork Psychology1 Sabah 3 2007Buy SellNoch keine Bewertungen
- Toward An Ecological Theory of Autism: January 2001Dokument31 SeitenToward An Ecological Theory of Autism: January 2001carola1344Noch keine Bewertungen
- 02chapters1 6Dokument137 Seiten02chapters1 6paulmazziotta20Noch keine Bewertungen
- Work Psychology1 Sabah 3 2007Dokument23 SeitenWork Psychology1 Sabah 3 2007Asadulla KhanNoch keine Bewertungen
- Expressions of Emotions Across Species - 2021 - Current Opinion in NeurobiologyDokument10 SeitenExpressions of Emotions Across Species - 2021 - Current Opinion in NeurobiologyOfelia KerrNoch keine Bewertungen
- Thomas Sharp CPS Final SubmissionDokument51 SeitenThomas Sharp CPS Final SubmissionJane DoeNoch keine Bewertungen
- Emotion and Motivation I: Defensive and Appetitive Reactions in Picture ProcessingDokument25 SeitenEmotion and Motivation I: Defensive and Appetitive Reactions in Picture ProcessingfranciscoNoch keine Bewertungen
- Social Science Information-2007-Parrott-419-23Dokument5 SeitenSocial Science Information-2007-Parrott-419-23Lília Bittencourt SilvaNoch keine Bewertungen
- Unit-1 Module1 BiopsychDokument28 SeitenUnit-1 Module1 BiopsychPat MaravillaNoch keine Bewertungen
- Rice Shelby 1967Dokument49 SeitenRice Shelby 1967Todva MhlangaNoch keine Bewertungen
- Embodied Cognition - Monica CowartDokument15 SeitenEmbodied Cognition - Monica CowarttomavinkovicNoch keine Bewertungen
- KognisiDokument5 SeitenKognisisgjrstujtiNoch keine Bewertungen
- Jung Was Thinking SystemsDokument23 SeitenJung Was Thinking SystemsRawan AlashramNoch keine Bewertungen
- Definisi Stress 1Dokument30 SeitenDefinisi Stress 1chatarinaNoch keine Bewertungen
- Toward A Scientific Analysis of Motivation) - R.: Indiana UniversityDokument51 SeitenToward A Scientific Analysis of Motivation) - R.: Indiana UniversityJuliánAndrésZanguñaNoch keine Bewertungen
- Exploring The Philosophy of MindDokument6 SeitenExploring The Philosophy of MindMark Russel Sean LealNoch keine Bewertungen
- 3 Lazarus 1988Dokument12 Seiten3 Lazarus 1988claudia osorioNoch keine Bewertungen
- Bladder Management Intrapartum and Postpartum 160517Dokument5 SeitenBladder Management Intrapartum and Postpartum 160517PspduntanDuaribusebelasNoch keine Bewertungen
- Case Report: Unilateral Primary Glaucoma With Immature Senile CataractDokument2 SeitenCase Report: Unilateral Primary Glaucoma With Immature Senile CataractPspduntanDuaribusebelasNoch keine Bewertungen
- Chapter IIIDokument6 SeitenChapter IIIPspduntanDuaribusebelasNoch keine Bewertungen
- Chapter VDokument1 SeiteChapter VPspduntanDuaribusebelasNoch keine Bewertungen
- Isolasi Dna: Dna and Rna Extractions Dna and Rna ExtractionsDokument5 SeitenIsolasi Dna: Dna and Rna Extractions Dna and Rna ExtractionsPspduntanDuaribusebelasNoch keine Bewertungen
- Senile Cataract in Diabetic: Case ReportDokument1 SeiteSenile Cataract in Diabetic: Case ReportPspduntanDuaribusebelasNoch keine Bewertungen
- Anesthesia and Acupuncture: Gerhard Litscher, Holger Simonis, Wolfgang KroöllDokument13 SeitenAnesthesia and Acupuncture: Gerhard Litscher, Holger Simonis, Wolfgang KroöllPspduntanDuaribusebelasNoch keine Bewertungen
- Student Registration Fact Sheet and FAQ For StudentsDokument5 SeitenStudent Registration Fact Sheet and FAQ For StudentsPspduntanDuaribusebelasNoch keine Bewertungen
- K01299 - 20211018160945 - SBF3043 Chapter 3 - Digestive SystemDokument52 SeitenK01299 - 20211018160945 - SBF3043 Chapter 3 - Digestive SystemChimChim UrkNoch keine Bewertungen
- BISC 303 Unknown ReportDokument10 SeitenBISC 303 Unknown ReportAnonymous UQF3TC0Noch keine Bewertungen
- Injuries Around The ElbowDokument82 SeitenInjuries Around The ElbowNitisha SethiNoch keine Bewertungen
- Lesson 1 "Modern Health Problems": A. Before-Reading ActivitiesDokument3 SeitenLesson 1 "Modern Health Problems": A. Before-Reading ActivitiesRomantische BohemienNoch keine Bewertungen
- Anatomy - Kidney'sDokument18 SeitenAnatomy - Kidney'sOlivia MorrisonNoch keine Bewertungen
- Parturient Paresis in CowsDokument5 SeitenParturient Paresis in Cowsابراهيم القويعىNoch keine Bewertungen
- Case PresentationDokument11 SeitenCase PresentationHira Rafique100% (1)
- DR Ara Khan Marwat: Join My Watssap Group For Part-1 Books, Papers and UpdatesDokument25 SeitenDR Ara Khan Marwat: Join My Watssap Group For Part-1 Books, Papers and UpdatesmisdduaaNoch keine Bewertungen
- Measurement of Energy Expenditure in Man: A Review of Available MethodsDokument20 SeitenMeasurement of Energy Expenditure in Man: A Review of Available Methodsशशी कुमारNoch keine Bewertungen
- The Importance of Biopharmaceutics and PharmacokineticDokument9 SeitenThe Importance of Biopharmaceutics and PharmacokineticWella AfrianiNoch keine Bewertungen
- Physiology of Motor TractsDokument29 SeitenPhysiology of Motor Tractsrj100% (1)
- Acsm Cnews 20-1Dokument16 SeitenAcsm Cnews 20-1CinthiaDReisNoch keine Bewertungen
- Carbon Dioxide MSDSDokument12 SeitenCarbon Dioxide MSDSRaja DhanasekaranNoch keine Bewertungen
- Septic Peritonitis in DogsDokument7 SeitenSeptic Peritonitis in DogsTactvisNoch keine Bewertungen
- Mechanisms of Action of AntibioticsDokument4 SeitenMechanisms of Action of AntibioticsClarissa Marie LimmangNoch keine Bewertungen
- Sphaerotilus NDokument2 SeitenSphaerotilus NLuis M. Telleria CondeNoch keine Bewertungen
- Gen Bio 2 - Chapter 1Dokument79 SeitenGen Bio 2 - Chapter 1Samantha AcostaNoch keine Bewertungen
- Group 8 Oxygenation AtionDokument46 SeitenGroup 8 Oxygenation AtionPrince Masroor Ali AbroNoch keine Bewertungen
- Growth Factors and Wound Healing 1997Dokument374 SeitenGrowth Factors and Wound Healing 1997Vo Ha Phuong NguyenNoch keine Bewertungen
- Introduction To Chiral or Optical IsomersDokument3 SeitenIntroduction To Chiral or Optical IsomersSeepana Dayakar100% (1)
- Bradycardia GuidelineDokument2 SeitenBradycardia Guidelinekannan73dr100% (1)