Beruflich Dokumente
Kultur Dokumente
05 - 19-23 - The Changing Brain Across The Lifespan - Development, Repair & Regeneration
Hochgeladen von
Pedja LeyOriginalbeschreibung:
Originaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
05 - 19-23 - The Changing Brain Across The Lifespan - Development, Repair & Regeneration
Hochgeladen von
Pedja LeyCopyright:
Verfügbare Formate
The Changing Brain Across the Lifespan: Development, Repair & Regeneration
Medical Neuroscience | Tutorial Notes
The Changing Brain Across the Lifespan: Development, Repair &
Regeneration
MAP TO NEUROSCIENCE CORE CONCEPTS1
NCC4. Life experiences change the nervous system.
NCC8. Fundamental discoveries promote healthy living and treatment of disease.
LEARNING OBJECTIVES
After study of the assigned learning materials, the student will:
1. Discuss the neurobiological basis for changes in gray matter and white matter volume in the
developing human brain throughout childhood.
2. Discuss the mechanisms of regeneration of peripheral nerves following injury.
3. Discuss the mechanisms of plasticity in adult sensorimotor maps following peripheral injury.
4. Discuss the mechanisms of plasticity in adult neural circuits following central injury.
5. Discuss the evidence regarding neurogenesis in the adult CNS.
TUTORIAL OUTLINE
I.
Introduction
A.
B.
mechanisms of neural development
1.
genetic specification: phenotypes produced by spatial and temporal patterns of
gene expression in cells derived from common precursors
2.
self-organization: phenotypes produced by cell-cell interactions mediated by
endogenous patterns of activity in neural networks
3.
sensorimotor experience: the modulation of endogenous neural activity by the
activation of sensory receptors during environmental interactions
as development proceeds across the lifespan, these fundamental forces continue to
shape the structure of function of neural circuitsalbeit with limited capacity to learn,
repair and regenerate outside of the critical periods that characterize early life
Visit BrainFacts.org for Neuroscience Core Concepts (2012 Society for Neuroscience) that offer fundamental principles
about the brain and nervous system, the most complex living structure known in the universe.
The Changing Brain Across the Lifespan: Development, Repair & Regeneration
II.
Ongoing development of the human brain throughout childhood
A.
commensurate with the onset and duration of many known critical periods in early life,
there is an explosive increase in synaptogenesis across the cortical mantle (see Figure
24.122)
1.
in rhesus monkey (and presumably in humans):
a.
there is an explosive increase in synapse formation (synaptogenesis) in
the neonatal period that continues through early childhood
b.
although there is likely both synapse construction AND synapse pruning
occurring concurrently, the major developmental theme of early life is
the construction of neural circuits with a several-fold increase in the
total numbers of synaptic connections in most gray matter structures
2.
presumably, the capacity to build new synaptic connections is an important
component to critical period plasticity and the rapid learning that occurs in early
life (see Figure 24.11)
3.
however, the rate of synaptogenesis and/or the proper construction of
functional, adaptive neural circuits may be altered in disorders of cognition and
behavior, such as autism spectrum disorders, schizophrenia, and attention
deficit-hyperactivity disorder (ADHD)
a.
b.
autism spectrum disorder
i.
there appears to be an accelerated rate of synaptogenesis in the
frontal and temporal lobes, and in the amygdala and
cerebellum, resulting in an overgrowth of neural circuits and an
early increase in overall brain size
ii.
by adolescence, differences in gray matter trend toward normal
volumes
iii.
however, aberrant wiring patterns of cortical and subcortical
networks are likely to persist, especially in networks subserving
social cognition and verbal and non-verbal communication
attention deficit-hyperactivity disorder (ADHD)
i.
B.
beginning in later childhood (pre-adolescent), there is a net reduction in the numbers of
synapses in the cerebral cortex (see Figure 24.12)
1.
there appears to be decreased rate of synaptogenesis
resulting in a developmental delay in circuit construction
and an overall decrease in the thickness of cortical gray
matter, especially in the frontal and temporal lobes
in most cortical areas, there is 20-50% reduction in the numbers of synaptic
connections from the peak in early childhood to a stable plateau in young
adulthood
th
Figure references to Purves et al., Neuroscience, 5 Ed., Sinauer Assoc., Inc., 2012. [click here]
The Changing Brain Across the Lifespan: Development, Repair & Regeneration
2.
C.
in ADHD, there appears to be a modest increase in the rate of gray
matter thickness reduction (see Figure 24.14)
b.
consequently, cortical gray matter volumes are (on average) reduced in
adults with ADHD
this increase likely reflects an increase in myelination of axonal pathways in the
brain; and it could also reflect an increase in the numbers of axons within
pathways
thereafter, overall brain size gradually decreases throughout adulthood, presumably
reflected the loss of synaptic connections, axons and myelin (see Figure 31.18)
1.
III.
a.
throughout childhood and into adulthood, there is an increase in white matter volume
(see Figure 24.13B)
1.
D.
this reduction in synaptic connections is the major factor responsible for a
reduction in the volume of gray matter in the cerebral cortex observed in the
same time frame (recall that synapses are localized to gray matter) (see Figure
24.13)
however, a loss of neurons is not typical in adult aging, unless
neurodegenerative disease is manifest
Plasticity in sensory and motor maps
A.
the organization of sensory and motor maps may be altered by damage to peripheral
nerves and/or changes in ongoing patterns of neural activity
B.
the degree of plasticity is much greater during early critical periods, but some plasticity
in map structure persists into adulthood
C.
in the somatic sensory cortex
1.
2.
D.
E.
when peripheral nerves are lesioned, central representations reorganize (see
Figures 9.14; consider also phantom limb sensations Box 10D)
a.
in the short-term, newly deafferented cortical zones become
unresponsive
b.
however, over time, deafferented cortical zones become responsive to
sensory stimuli that drive adjacent regions of cortex
reorganization may also be seen in an intact system when patterns of neural
activity are habitually reinforced (e.g., with extensive practice or non-use) ((see
Figures 9.15)
in the motor cortex
1.
over training can induce an expansion of the motor representation of the
practiced movements in the primary motor cortex
2.
similar plasticity has been observed in animals recovering from brain injury (e.g.,
stroke) who were rehabilitated using specific motor sequences
mechanisms of circuit plasticity in the cerebral cortex
The Changing Brain Across the Lifespan: Development, Repair & Regeneration
1.
plasticity may occur at multiple stations along a sensory or motor pathway
2.
however, plasticity is understood best at the level of the cerebral cortex where
intrinsic cortical circuits are modified by experience
3.
long-range horizontal connections are a likely mediator of cortical plasticity in
sensory and motor maps
a.
IV.
(i)
normally, effects of horizontal connections are weak (or maybe
even silent see Box8B)
(ii)
in response to deafferentation (see below), disuse or overuse,
the synapses made by horizontal connections may become
strengthened and more effective at driving neuronal responses;
they may mediate at least the early phases of cortical
reorganization
(iii)
long term consequences may involve growth of new
connections that reinforce longer lasting effects on the
structure of cortical maps
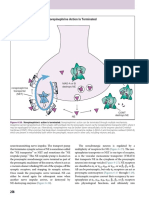
Peripheral nerve regeneration
A.
following damage (severing, evulsing or crushing a nerve), a sequence of events plays
out that results in partial restoration of sensory and motor function (see Figure 25.6)
1.
2.
3.
4.
5.
B.
V.
horizontal connections span many cortical columns and interconnect
different parts of maps that are functionally synergistic
macrophages rapidly remove myelin and axonal debris as the distal portion of
the axon degenerates
Schwann cells proliferate, express adhesion molecules and other growthpromoting signals
the neuronal cell body expresses genes that reactivate growth programs that
allow for the formation of a growth cone and the transduction machinery that is
necessary to respond to factors produced by Schwann cells
regenerating axons elongate as growth cones migrate along extracellular matrix
and the relatively orderly array of Scwhann cells that guide growth cones to
their peripheral targets
for motor axons and some sensory afferents (e.g., Merkel cell-neurite
complexes), synapses form on target tissues using similar mechanisms that
established synaptic connectivity in development
however, the fidelity of regeneration is limited and full functional recovery may not be
achieved
Plasticity of neural circuits in response to brain injury
A.
in response to brain injury (following trauma, hypoxia or the onset of
neurodegenerative disease), neuronal loss is often exacerbated by programmed cell
death and the inability to restore long axonal projections
1.
fatally injured neurons die acutely or undergo apoptosis (see Figure 25.9)
The Changing Brain Across the Lifespan: Development, Repair & Regeneration
2.
B.
a.
cell death may be triggered by over-activation of glutamatergic synaptic
inputs (excitotoxicity see Box 6C)
b.
cell death may be triggered by inflammatory mediators (cytokines), DNA
damage, loss of neurotrophic support, and other means of cellular
stress
c.
whatever the trigger, lost neurons cannot be replaced via mitosis and
differentiation of neuroblasts (with few exceptions see below)
in compact white matter structures, damage to long pathways in the CNS have
limited capacity to regenerate
a.
glial cells respond to signals produced by damaged tissues and immune
cells that infiltrate the region of damage, and proliferate forming scars
that congest the local volume of brain tissue (see Figure 25.3B)
b.
glial reactions create an environment that is non-permissive for the
regrowth of long axonal projections (see Figure 25.11)
c.
oligodendrocytes produce signaling molecules (myelin associated
proteins) that inhibit axonal growth and elongation
however, in gray matter, surviving circuit elements may undergo functional and
structural plasticity; the key to recovery is the capacity of these elements to reorganize
and reshape the strength and distribution of their synaptic connections
1.
cortical (and subcortical) connections may be modified by central injury; e.g.,
following stroke
a.
C.
injury to brain tissue surrounding the core of infarction will undergo a
sequence of activity-dependent changes in:
i.
patterns of neural activity
ii.
patterns of gene expression
iii.
patterns of synapse formation and axonal outgrowth
b.
changes in the patterns of gene expression (and their consequences)
often involve expression of the same genes (i.e., encoding tropic
molecules, trophic molecules, and their receptors) that were initially
induced in the early construction of neural circuits
c.
thus, the plastic response of injured neural tissue likely involves the
reactivation of developmental programs
the capacity for neuronal regeneration (i.e., mitosis and production of new neurons) is
limited to special populations of neural stem cells close to the lateral wall of the lateral
ventricle (in the so-called subventricular zone) (see Figure 25.13 & 25.14)
1.
these new neurons provide interneurons for local circuits in the dentate gyrus (a
component of the hippocampus)
2.
despite some recent controversy, it now appears clear that few if any new
neurons contribute to neural circuits in the neocortex (see Box 25C)
The Changing Brain Across the Lifespan: Development, Repair & Regeneration
3.
nevertheless, the addition and integration of new neurons in the dentate gyrus
may be important for neuropsychiatric disease and treatment
STUDY QUESTIONS
Q1.
At roughly what age would you expect that highest density of synapses in the human cerebral
cortex?
A.
B.
C.
D.
E.
Q2.
third trimester of gestation
first 6 (postnatal) months of infancy
near the middle of the first decade of life
near the middle of the second decade of life
after the second decade of life
What factors contribute to the limited regenerative capacity of corticospinal tract axons
following spinal cord injury?
A.
B.
C.
D.
E.
glial scar formation in damaged white matter (e.g., in the lateral column of the spinal
cord)
the suppression of growth promoting genes in the cell bodies of corticospinal tract
neurons
the expression by astrocytes of semaphorins, ephrins and slits that promote growth
cone collapse
the expression by oligodendrocytes of integral membrane proteins, such as NogoA,
myelin associated protein, and oligodendrocyte myelin glycoprotein, that inhibit axon
growth
all of the above factors contribute to the limited regenerative capacity of corticospinal
tract axons following spinal cord injury
Das könnte Ihnen auch gefallen
- The Wiley Handbook of Evolutionary NeuroscienceVon EverandThe Wiley Handbook of Evolutionary NeuroscienceStephen V. ShepherdNoch keine Bewertungen
- Regulation of Axonal Regeneration After Mammalian Spinal Cord InjuryDokument18 SeitenRegulation of Axonal Regeneration After Mammalian Spinal Cord Injuryelif.onsozNoch keine Bewertungen
- 01 STR 31 1 223Dokument9 Seiten01 STR 31 1 223cah bagusNoch keine Bewertungen
- Mechanisms of B-WPS OfficeDokument22 SeitenMechanisms of B-WPS OfficeDaylan Lindo MontefalcoNoch keine Bewertungen
- Unit 2 NeuroplasticityDokument6 SeitenUnit 2 NeuroplasticityKhushi AroraNoch keine Bewertungen
- Dwnload Full Adult Development and Aging 8th Edition Cavanaugh Solutions Manual PDFDokument35 SeitenDwnload Full Adult Development and Aging 8th Edition Cavanaugh Solutions Manual PDFwilliambrowntdoypjmnrc100% (14)
- Adult Development and Aging 8th Edition Cavanaugh Solutions ManualDokument35 SeitenAdult Development and Aging 8th Edition Cavanaugh Solutions Manualkussiertheiform.a0d2z100% (19)
- Full Download Adult Development and Aging 8th Edition Cavanaugh Solutions ManualDokument35 SeitenFull Download Adult Development and Aging 8th Edition Cavanaugh Solutions ManualluliandivljabNoch keine Bewertungen
- Nihms 1753629Dokument35 SeitenNihms 1753629Afiah RahmanNoch keine Bewertungen
- Nerve Repait Tissue EngineeringDokument11 SeitenNerve Repait Tissue EngineeringMiroslav VetrikNoch keine Bewertungen
- Vanders Human Physiology The Mechanisms of Body Function 14Th Edition Widmaier Solutions Manual Full Chapter PDFDokument44 SeitenVanders Human Physiology The Mechanisms of Body Function 14Th Edition Widmaier Solutions Manual Full Chapter PDFgadsmanoutfitqcs100% (5)
- Dudai - The Consolidation and Transformation of Memory PDFDokument13 SeitenDudai - The Consolidation and Transformation of Memory PDFCami Sánchez SalinasNoch keine Bewertungen
- Neuroplasticity - or Brain PlasticityDokument2 SeitenNeuroplasticity - or Brain Plasticityjack stewartNoch keine Bewertungen
- Stress, Depression and Neuroplasticity: Shatrunjai P. Singh and Swagata KarkareDokument6 SeitenStress, Depression and Neuroplasticity: Shatrunjai P. Singh and Swagata KarkareJoeNoch keine Bewertungen
- Daniel O. Kellett Et Al - Memory Consolidation in The Cerebellar CortexDokument13 SeitenDaniel O. Kellett Et Al - Memory Consolidation in The Cerebellar CortexCortate15gNoch keine Bewertungen
- Physiological Psychology Lecture Notes: Nervous System DamageDokument3 SeitenPhysiological Psychology Lecture Notes: Nervous System DamageGeneric_PersonaNoch keine Bewertungen
- Intrinsic Control of Axon RegenerationDokument15 SeitenIntrinsic Control of Axon RegenerationYoNoch keine Bewertungen
- Bonetto 2021 Myelin - AgatekeeperofactivityDokument16 SeitenBonetto 2021 Myelin - AgatekeeperofactivityMary KarabkNoch keine Bewertungen
- Introductory Discussion On Glial Function: Department of Psychology, Yale University, New Haven, Conn. U.S.A.)Dokument11 SeitenIntroductory Discussion On Glial Function: Department of Psychology, Yale University, New Haven, Conn. U.S.A.)Eusebio ChaconNoch keine Bewertungen
- T E Neuro DiseasesDokument12 SeitenT E Neuro DiseasesSanjna VinodNoch keine Bewertungen
- Neural PlasticityDokument5 SeitenNeural PlasticityJyoti SarojNoch keine Bewertungen
- Intro J O N 2 2015Dokument1 SeiteIntro J O N 2 2015YesicaNoch keine Bewertungen
- Brainsci 10 00300Dokument17 SeitenBrainsci 10 00300AhsanurNoch keine Bewertungen
- Nerve RegenDokument31 SeitenNerve RegenKAPIL SINGHNoch keine Bewertungen
- Papel de La Microglía en La SinapsisDokument13 SeitenPapel de La Microglía en La SinapsisLorena de la FlorNoch keine Bewertungen
- TUCI Data Book V 2010.02 Babys Brain Begins Now.v2Dokument12 SeitenTUCI Data Book V 2010.02 Babys Brain Begins Now.v2George ManuNoch keine Bewertungen
- Human Brain Biochemistry: American Journal of BioscienceDokument13 SeitenHuman Brain Biochemistry: American Journal of BioscienceEugen MNoch keine Bewertungen
- Acknowledgments: Gray's Anatomy, The Remarkable Work From Which Gray's Clinical Neuroanatomy IsDokument7 SeitenAcknowledgments: Gray's Anatomy, The Remarkable Work From Which Gray's Clinical Neuroanatomy IsAndreea LăzăroiuNoch keine Bewertungen
- AP Psychology Chapter 2 Study Guide (W/ Answers)Dokument24 SeitenAP Psychology Chapter 2 Study Guide (W/ Answers)JeremiahUmaleNoch keine Bewertungen
- Mechanisms of Disease: What Factors Limit The Success of Peripheral Nerve Regeneration in Humans?Dokument7 SeitenMechanisms of Disease: What Factors Limit The Success of Peripheral Nerve Regeneration in Humans?balab2311Noch keine Bewertungen
- Mapping Brain MaturationDokument12 SeitenMapping Brain MaturationandresNoch keine Bewertungen
- Neuronal Regeneration and Functional Recovery Following Peripheral Nervous System LesionsDokument12 SeitenNeuronal Regeneration and Functional Recovery Following Peripheral Nervous System LesionsMajo CambindoNoch keine Bewertungen
- JAK Neural PlasticityDokument15 SeitenJAK Neural PlasticityOwais KhanNoch keine Bewertungen
- Nhsjs SubmissionDokument8 SeitenNhsjs Submissionapi-410186016Noch keine Bewertungen
- Histologia Del SNCDokument15 SeitenHistologia Del SNCJuan Ignacio Cardenas RodriguezNoch keine Bewertungen
- Lecture 10 PlasticityDokument36 SeitenLecture 10 PlasticityDrGasnasNoch keine Bewertungen
- Review Article: Noninvasive Strategies To Promote Functional Recovery After StrokeDokument17 SeitenReview Article: Noninvasive Strategies To Promote Functional Recovery After Strokecah bagusNoch keine Bewertungen
- Functional NeuroanatomyDokument23 SeitenFunctional NeuroanatomyDaniela GrigoraşNoch keine Bewertungen
- Clinical Neuroanatomy Lange 29Th Edition Stephen Waxman Full ChapterDokument51 SeitenClinical Neuroanatomy Lange 29Th Edition Stephen Waxman Full Chaptertim.morrison630100% (14)
- (Studies in Brain and Mind 2) John Bickle (Auth.) - Philosophy and Neuroscience - A Ruthlessly Reductive Account-Springer Netherlands (2003)Dokument245 Seiten(Studies in Brain and Mind 2) John Bickle (Auth.) - Philosophy and Neuroscience - A Ruthlessly Reductive Account-Springer Netherlands (2003)Mikha ElleNoch keine Bewertungen
- The Neural Basis of Cognitive Development: A Constructivist ManifestoDokument58 SeitenThe Neural Basis of Cognitive Development: A Constructivist ManifestoA_WretchNoch keine Bewertungen
- Cerebellum: Movement Regulation and Cognitive Functions Course Title DateDokument16 SeitenCerebellum: Movement Regulation and Cognitive Functions Course Title DateDanyNoch keine Bewertungen
- Science Module For Grade 10: Guagua National Colleges, Inc. Guagua, PampangaDokument62 SeitenScience Module For Grade 10: Guagua National Colleges, Inc. Guagua, PampangaNorman Punla SantosNoch keine Bewertungen
- Modification of Neural Circuits in Early Neonatal LifeDokument6 SeitenModification of Neural Circuits in Early Neonatal Lifesykesg1 onsitephysicianNoch keine Bewertungen
- Pascal 2019 Ooperation of The Vestibular and Cerebellar Networks in Anxiety Disorders and DepressionDokument56 SeitenPascal 2019 Ooperation of The Vestibular and Cerebellar Networks in Anxiety Disorders and DepressionJuan Hernández GarcíaNoch keine Bewertungen
- Neurofarm Syaraf PusatDokument19 SeitenNeurofarm Syaraf PusatDHEA NURAFIDA HERMAWATINoch keine Bewertungen
- Plasticidad Neuronal Funcional With Cover Page v2Dokument12 SeitenPlasticidad Neuronal Funcional With Cover Page v2MarianaNoch keine Bewertungen
- tmpDDAE TMPDokument14 SeitentmpDDAE TMPFrontiersNoch keine Bewertungen
- Mecanismos Celulares de La Neuroplasticidad Sin ProteccionDokument22 SeitenMecanismos Celulares de La Neuroplasticidad Sin ProteccionMati Sanabria100% (1)
- 2010 - Neural Mechanisms of Ageing and Cognitive DeclineDokument19 Seiten2010 - Neural Mechanisms of Ageing and Cognitive DeclineВладимир ДружининNoch keine Bewertungen
- The Potential of Endogenous Neurogenesis For BrainDokument5 SeitenThe Potential of Endogenous Neurogenesis For BrainConstantinos ChristodoulidesNoch keine Bewertungen
- Neuronal Network Models of ADHDDokument9 SeitenNeuronal Network Models of ADHDbhasiNoch keine Bewertungen
- Option A - Neurobiology and BehaviourDokument29 SeitenOption A - Neurobiology and Behaviour玛丽娜Noch keine Bewertungen
- Cloft Et Al - 2019 - Organotypic Brain Slice Cultures To Model Neurodegenerative ProteinopathiesDokument11 SeitenCloft Et Al - 2019 - Organotypic Brain Slice Cultures To Model Neurodegenerative Proteinopathiesmarej312Noch keine Bewertungen
- ID NeurodevtDokument19 SeitenID NeurodevtManaliNoch keine Bewertungen
- NeuroplasticityDokument57 SeitenNeuroplasticityKent JuanNoch keine Bewertungen
- Review Neural Stem Cells and Strategies For The Regeneration of The Central Nervous SystemDokument13 SeitenReview Neural Stem Cells and Strategies For The Regeneration of The Central Nervous SystemgerasepNoch keine Bewertungen
- Science 10 Module 3 FinalDokument60 SeitenScience 10 Module 3 FinalNorman Punla SantosNoch keine Bewertungen
- W S Y Y S: HEN Cience Meets Oga and Oga Meets CienceDokument20 SeitenW S Y Y S: HEN Cience Meets Oga and Oga Meets CiencePedja LeyNoch keine Bewertungen
- Conscious Thinking and Cognitive PhenomeDokument493 SeitenConscious Thinking and Cognitive PhenomeAleh Valença100% (2)
- License Terms - Slideforest FreebiesDokument1 SeiteLicense Terms - Slideforest FreebiesPedja LeyNoch keine Bewertungen
- Dance of Death (2003)Dokument14 SeitenDance of Death (2003)Pedja LeyNoch keine Bewertungen
- A Matter of Life and Death (2006)Dokument15 SeitenA Matter of Life and Death (2006)Pedja LeyNoch keine Bewertungen
- Myofascial Meridians PDFDokument9 SeitenMyofascial Meridians PDFPedja Ley100% (3)
- House Rules: Things You Should and Shouldn't DoDokument9 SeitenHouse Rules: Things You Should and Shouldn't DoPedja LeyNoch keine Bewertungen
- Achilles TendinitisDokument4 SeitenAchilles TendinitisPedja LeyNoch keine Bewertungen
- Medical Neuroscience Tutorial Notes: Pain SystemsDokument6 SeitenMedical Neuroscience Tutorial Notes: Pain SystemsPedja LeyNoch keine Bewertungen
- Invertebrate Nervous Systems: Thomas MathesonDokument6 SeitenInvertebrate Nervous Systems: Thomas MathesonMax MoralesNoch keine Bewertungen
- Introduction To Alcohol Withdrawal: Richard Saitz, M.D., M.P.HDokument8 SeitenIntroduction To Alcohol Withdrawal: Richard Saitz, M.D., M.P.HXavierNoch keine Bewertungen
- Child Development A Cultural Approach 2nd Edition Arnett Test Bank 1Dokument117 SeitenChild Development A Cultural Approach 2nd Edition Arnett Test Bank 1joshua100% (36)
- 7 Anand ANN PDFDokument78 Seiten7 Anand ANN PDFVaddi Dushyanth KumarNoch keine Bewertungen
- Three: Biological Foundations of BehaviorDokument13 SeitenThree: Biological Foundations of Behaviorivana maeNoch keine Bewertungen
- Neurosculpting Institute Neuroplasticity Brain TrainingDokument14 SeitenNeurosculpting Institute Neuroplasticity Brain Traininges3hadoNoch keine Bewertungen
- Study Guide (Psy 101)Dokument215 SeitenStudy Guide (Psy 101)Allison Tapia100% (1)
- The Child and Adolescent Learner ReviewerDokument11 SeitenThe Child and Adolescent Learner ReviewerArielle Grace YalungNoch keine Bewertungen
- Spinal ReflexesDokument4 SeitenSpinal Reflexesjp100% (1)
- Brain AnatomyDokument37 SeitenBrain AnatomyDr. Jayesh PatidarNoch keine Bewertungen
- Special Focus Report 55Dokument38 SeitenSpecial Focus Report 55danielwwcheong8981100% (2)
- Nerve Energy and QiDokument6 SeitenNerve Energy and Qikemet215100% (3)
- Modul Sains SPM ObjektifDokument60 SeitenModul Sains SPM ObjektifTheMovingFingerNoch keine Bewertungen
- The Learning BrainDokument222 SeitenThe Learning BrainMaria SanduNoch keine Bewertungen
- PP Class 12 p4Dokument20 SeitenPP Class 12 p4dR SHAMMIR AHMEDNoch keine Bewertungen
- Stahl' Essential Psychopharmacology 276 PDFDokument1 SeiteStahl' Essential Psychopharmacology 276 PDFMuhammad AzkaNoch keine Bewertungen
- Biology Notes For IGCSEDokument54 SeitenBiology Notes For IGCSErajeshn186% (37)
- Eeg Signal Processing PDFDokument47 SeitenEeg Signal Processing PDFAZA14Noch keine Bewertungen
- Intro Spychology Exam 1Dokument24 SeitenIntro Spychology Exam 1talk2ajalatundeNoch keine Bewertungen
- The Science of Monatomic GoldDokument6 SeitenThe Science of Monatomic GoldLaron Clark75% (4)
- ClassificationDokument32 SeitenClassificationGauravSinghNoch keine Bewertungen
- Rotc Brain FunctionDokument10 SeitenRotc Brain FunctionAdya FradiptaNoch keine Bewertungen
- s11144 023 02360 9Dokument17 Seitens11144 023 02360 9elmustafa IboustatenNoch keine Bewertungen
- The Oxford Handbook of Event-Related Potential ComponentsDokument760 SeitenThe Oxford Handbook of Event-Related Potential ComponentsGustavo Belleza75% (8)
- Light and Electron Microscopic Assessment of Progressive Atrophy Following Moderate Traumatic Brain Injury in The RatDokument15 SeitenLight and Electron Microscopic Assessment of Progressive Atrophy Following Moderate Traumatic Brain Injury in The RatKKarindha LadoNoch keine Bewertungen
- Hap-Ii Lab Practical III: Recording of Basal Mass Index. Permanent Slides of Vital Organs and GonadsDokument9 SeitenHap-Ii Lab Practical III: Recording of Basal Mass Index. Permanent Slides of Vital Organs and Gonads99 LHH Sakshi VishwakarmaNoch keine Bewertungen
- Anatomy and Physiology ReviewerDokument8 SeitenAnatomy and Physiology ReviewerBeverly DatuNoch keine Bewertungen
- (최상위) 8.It's Up to You! (01) - 능률 (김성곤) 고1 영어 (15문제) (Q) 2Dokument7 Seiten(최상위) 8.It's Up to You! (01) - 능률 (김성곤) 고1 영어 (15문제) (Q) 2nsfbz45ftbNoch keine Bewertungen
- Meeting 1 - NounsDokument5 SeitenMeeting 1 - NounsMalasari Lala0% (1)
- Ganong Physiology Mcqs & SeqsDokument193 SeitenGanong Physiology Mcqs & SeqsRichardNoch keine Bewertungen