Beruflich Dokumente
Kultur Dokumente
Upstream Waste
Hochgeladen von
Humaira MirzaOriginalbeschreibung:
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Upstream Waste
Hochgeladen von
Humaira MirzaCopyright:
Verfügbare Formate
3/24/2016
Upstream pollution
prevention and waste
management
CGE686
Learning outcome
To understand the environmental impact of produced water, drilling muds and
drilling cuttings.
To identify the possible ways to minimize the generation of upstream waste.
3/24/2016
Contents
Produced water
Drilling waste mud and cuttings
3/24/2016
Produced water
Produced Water
Also known as
Brine
Saltwater
Sources of this water from an oil reservoir may include flow from
above or below the hydrocarbon zone
within the hydrocarbon zone
injected fluids and additives resulting from production activities
Also known as connote water or formation water
3/24/2016
1. Produced Water
In the production of natural gas produced water is separated from the gas at or
near the wellhead
Additional water is also injected into the reservoir to
Sustain pressure
Achieve greater recovery levels
Produced water production volume
Source: US National Energy Technology Laboratory (NETL), 2015
3/24/2016
Produced water production volume
Water to oil ratio
US National Energy Technology Laboratory (NETL) reports
US 5:1 and 8:1
Worldwide 2:1 and 3:1
BCC research (market research company based in the US)
North America increase in the ratio over next 12 years to 12:1 up to 50:1
(worst condition)
Producers need to pay between $3 and $12 per barrel to dispose of produced
water
3/24/2016
Why produced water is important for
O&G industry?
The cost of managing produced water is a significant factor in the profitability of oil and
gas production.
The cost of constructing treatment and disposal facilities, including equipment
acquisitions
The cost of operating those facilities, including chemical additives and utilities
The cost of managing any residuals or byproducts resulting from the treatment of
produced water
Permitting, monitoring, and reporting costs
Transportation costs.
Once these cost exceeds the value of the hydrocarbon produced from the well, the well is
usually shut down.
Factors affecting production volume
1. Volume of produced water increases for mature fields
2. Method of well drilling
Horizontal well produces more than vertical well at a similar drawdown
Horizontal well produces similar production rate at lower drawdown
3. Location of well
Horizontal well used in homogenous reservoirs - reduce the water
production
4. Different types of completion
Open hole permits testing of drilling zones and avoids drilling into water
But perforated completion method offers better control
3/24/2016
Factors affecting production volume
5. Single zone and commingled
As oil rate decline because of the maturing to the well, other zones are
opened to maintain the production of oil hence, water production
increases
6. Type of water separation technologies
Different methods are used to reduced the costs of lifting and/or water
handling for wells that produced large quantities of saline water.
Such as:
Water shut-off treatment using gelled polymer
Reducing beams pump lifting costs
Power options for reduce electricity costs
Separation technologies
Factors affecting production volume
7. Water injection or water flooding for EOR
Water flooding increase the percentage of water produced
As flood progresses, the volume of required water with suitable chemical
characteristics is necessary.
Because poor quality of treated makeup water enable sealing, clay swelling
and brine incompatibilities.
8. Poor mechanical integrity
Caused by mechanical problems of the casting holes corrosion or wear
Excessive pressure allow unwanted reservoir fluids to enter the casing and
increase water production
3/24/2016
Factors affecting production volume
9. Underground communications near wellbores or reservoirs, increases the
production of water.
Near wellbores channels behind casing, barrier breakdown and
completions into and near water.
Reservoirs coning, cresting, channeling through higher permeability
zones or fractures, fracturing out of zones
Types of
hydrocarbon
produced
Lifetime of
the reservoir
Factors
affecting
characteristics
of PW
Geographic
location of
the field
Geological
formation
3/24/2016
Characteristics of produced water
The major compounds of produced water
Dissolved and dispersed oil compounds
Dissolved formation minerals
Production chemical compounds
Production of solids
Dissolved gases
Dissolved and dispersed oil compounds
Oil is a mixture of hydrocarbon which include
Benzene, toluene, ethylbenzene and xylenes (BTEX)
Naphthalene, phenantherene, dibenzothiophene (NPD)
Polyaromatic hydrocarbons (PAHs)
Phenols
The amount depends on
Oil composition
pH, salinity, total dissolve solids (TDS), temperature
Oil/water ratio
Type and quantity of oilfield chemicals
Type and quantity of various stability compounds (waxes, asphaltenes, fine solids)
3/24/2016
Dissolved and dispersed oil compounds
Dissolved oil compounds concentration depends on pH and temperature
Organic acids (formic acid, propionic acid)
Aromatic compounds BTEX and phenol
Dispersed oil (consists of small droplets of oil suspended in the produced
water)
PAHs, C6 C9 alkylated phenols
Depends on the density of oil, amount of oil precipitation and interfacial
tension
Dissolved formation minerals
1. Anions Na+, K+, Ca2+, Mg2+, Ba2+, Sr2+, Fe2+
2. Cations Cl-, SO42-, CO32-, HCO3-
Affect the water chemistry:
buffering capacity, salinity
and scale potential
3. Heavy metals concentration depends on the age of wells and formation
geology.
Cadmium, chromium, copper, lead, mercury, nickel, silver and zinc
4. Naturally occurring radioactive materials (NORM)
226radium
Barium
& 228radium
10
3/24/2016
Production chemical components
Chemicals that are added to treat or prevent operational problems.
Production treating chemicals scale and corrosion inhibitors, biocides,
emulsion breakers, antifoam and water treatment chemicals.
Linear alkylbenzene sulfonate (LAS)
Alkyldimethlybenzenylammonium compounds
2-alkyl-1-ethylamine-2-imidazolines
Production solids
Formation solids
Corrosion and scale products
Bacteria
Waxes
Asphaltenes
Inorganic crystalline substances such SiO2, Fe2O3, Fe3O4 and BaSO4 are found in
the suspended solids
11
3/24/2016
Dissolved gases
CO2
O2
H2S
Natural gas produced water
12
3/24/2016
Oilfield produced water
Potential pathways to impact the
environment
Spills and Leaks
Discharge to
Surface Water
Underground
Injection
Air Emissions
13
3/24/2016
Fate and impact of produced water
Large spill can move into surface
water bodies
Salt problem in freshwater
Toxic compounds and oil &
grease can damage aquatic
life
Spills and Leaks
Salt can contaminate soil near
wellheads, pipelines and other
facilities
Kill plants
Damage soil to impact future
growth
Leakage from tanks can enter
groundwater
May enter source of drinking
water
Fate and impact of produced water
Discharge of salty produced
water to fresh water cause
damage to aquatic animals and
plants.
Discharge of oil & grease and
toxic chemicals can cause
damage to living organism
Discharge to
Surface Water
14
3/24/2016
Fate and impact of produced water
Underground
Injection
Checking the compatibility of the injected
produced water with the chemicals in the
ground is important to avoid:
Incompatibility of the chemicals can
cause precipitates to form block
pores and require additional
injection pressure
Potential microbial problems that
can lead to the formation of
hydrogen sulphide
Fate and impact of produced water
Air Emissions
Evaporation ponds and pits or mechanical evaporators
can create plumes of vapor that deposit on the ground
downwind of the site.
Salty deposits can harm plants and soil
15
3/24/2016
Fate and impact of produced water
The main constituent of produced water that causes impact salt
Other constituents can create problems in certain settings
Effects of marine life near production platforms.
Some fish species are attracted to productions
platforms.
Effects of these fishes
Oxidative stress
Altered fatty acid composition
Genotoxicity effects
Reproductive disturbance
These effects are local within 1 -2 km from
point of discharge
However, there is no published study on
human populations or communities.
Produced water management
Employing technologies to minimize
produced water production
Reuse and recycling
If neither of these tiers is practical, disposal
is the final option
16
3/24/2016
Water minimisation
Reducing the volume of water produced allows
More oil to be produced
Decreases the cost of lifting a heavier fluid to the surface
By minimizing the water, cost saving can be done
Reduced cost of equipment maintenance
Reduced produced water handling and treatment
Environment point of view
Less usage of chemicals for water separation
Less volume of produced water and associated pollutants discharge to the
environment
Technologies to minimize and reuse
produced water
1.
2.
3.
4.
5.
Use a closed-loop drilling fluid system
Drill horizontal wells
Optimize production rate to minimize the influx of water
Mechanically block water from entering the well
Treat the producing formation with polymers that decrease the permeability of
water, while maintaining the permeability of hydrocarbons
6. Use down-hole oil/water separation (DOWS) using hydrocyclone to separate water
and oil inside the well space
7. Seafloor separation technologies
8. Conduct hydrotest of pipelines, equipment and tanks with produced water
construction
17
3/24/2016
Water minimization technologies
limitations
The effectiveness in mechanically blocking the water from entering the well depends
on the type of reservoir and well construction. Examples of mechanical blocking
Straddle packers
Bridge plugs
Tubing patches
Cement
Chemical treatment polymers are used to shut off water-bearing channel or
fractures within the formation.
DOWS is restricted to type of wells.
Seafloor technologies very expensive.
Produced water re-injection
Onshore, treated produced water can be used for irrigation
Offshore, the primary re-use of produced water is to enhance oil production
water reinjection
Limitations - the produced water must be treated to meet certain quality levels
to
Prevent plugging of wellbore
Prevent plugging of reservoir pores
Prevent system failure
18
3/24/2016
Produced water re-injection
Factors that limits reinjection
Dispersed oil
Suspended solids
Fatty acids
Dissolved gases
Salts
pH
Temperature
Objective of produced water treatment
De-oiling
Dissolved gas
removal
Soluble
organics
removal
Desalination
Disinfection
Suspended
solids removal
Softening
Miscellaneous:
Removing
NORM
19
3/24/2016
Can be done via
Physical
Adsorption
Sand filters
Cyclones
Evaporation
Dissolved air
precipitation
C-Tour
Freezethaw/evaporation
Electrodialysis
Chemical
Chemical
precipitation
Chemical oxidation
Electrochemical
process
Photocatalytic
treatment
Fenton process
Treatment with
ozone
Room temperature
ionic liquids
Demulsifier
Biological
Aerobic
Anaerobic
Membrane
Microfiltration (MF)
Ultrafiltration (UF)
Nanofiltraion (NF)
Reverse osmosis (RO)
(Hayes & Arthur, 2004)
20
3/24/2016
(Hayes & Arthur, 2004)
(Hayes & Arthur, 2004)
21
3/24/2016
(Hayes & Arthur, 2004)
(Hayes & Arthur, 2004)
22
3/24/2016
(Hayes & Arthur, 2004)
General PW treatment
23
3/24/2016
Combination system
24
3/24/2016
Adsorption
25
3/24/2016
Adsorption theory
Liquid
Gas/ Vapor
Adsorbed solute: Adsorbate
Solid material: Adsorbent
Definition
Adsorption is a process
whereby a gas or liquid
(adsorbate) accumulates on
the surface of a solid
(adsorbent) to form a
molecular or atomic film.
Adsorption
For produced water treatment, adsorbent used are
Activated carbon
Organoclay
Copolymers
Zeolite
Resins
These are used to remove
Organic compounds
Heavy metals
26
3/24/2016
ADSORBENT
Shape
Small pellets, beads, granules, cylindrical, powders
Size ranging from 50 m to 1.2 cm
Porous structure
Macropore ( > 500) 50 nm
Mesopore (20 - 500 )
Micropore ( < 20 )
Based on International Union of Pure and Applied Chemistry (IUPAC)
Specific surface area: 300 to 1,200 m2/g
Pores of Activated Carbon
27
3/24/2016
Schematic of carbon
contactor.
Example: Process flow diagram of TORR
technology.
By EARTH Canada
Corporation.
TORR Total Oil
Remediation and
Recovery
Remove and recover
dispersed oil in water
2m and larger
28
3/24/2016
MECHANISM:ADSORPTION
MECHANISM: ADSORPTION
Molecules/atoms/ions in a gas or liquid diffuse to the surface of an adsorbent
where:
are held there physically by weak inter-molecular forces (physical adsorption)
they bond chemically with the solid surface (chemisorption)
58
29
3/24/2016
MECHANISM: ADSORPTION
Physical adsorption
Van der Waals adsorption
Low heat of adsorption
Non specific
Monolayer or multilayer
No dissociation of adsorbed species.
Only significant at relatively low
temperatures.
Rapid, non-activated, reversible.
No electron transfer.
Chemisorption
Covalent bonds
High heat of adsorption
Highly specific
Monolayer only
May involve dissociation
Possible over a wide range of
temperature
Activated, may be slow and
irreversible
Electron transfer leading to bond
formation between sorbate and
surface.
59
MECHANISM: ADSORPTION
Movement of an organic and/or inorganic molecule to a surface site requires
four separate phenomena:
Bulk fluid transport
Film transport
Intraparticle (pore and/or surface diffusion)
Attachment
60
30
3/24/2016
61
62
31
3/24/2016
MECHANISM: DESORPTION
Adsorption-Saturated-Desorption
63
MECHANISM: DESORPTION
Regeneration process:
To recover Adsorbate
To reuse Adsorbent
Bed regeneration method (cyclic system)
Temperature-swing adsorption (TSA)
Pressure-swing adsorption (PSA)
64
32
3/24/2016
MECHANISM:TEMPERATURE SWING
ADSORPTION (TSA)
Increase in temperature
Increase in temperature leads to a decrease in the quantity adsorbed
Important note - regeneration temperature does not cause degradation of the
adsorbents
Mechanism passage of a hot purge gas or steam
Important characteristic to treat feeds with low concentrations of adsorbates
65
MECHANISM: TEMPERATURE EFFECT
For a single adsorbate:
If the partial pressure remains
constant at P1, increasing the
temperature from T1 to T2 will
decrease the equilibrium
loading from q1 to q2.
66
33
3/24/2016
Bed TSA System
Cycle Steps
1. Adsorption at T1 to
breakthrough
2. Heating of the bed to
T2 (T2 > T1)
3. Desorption at T2
4. Cooling of the bed to
T1
67
MECHANISM: PRESSURE SWING
ADSORPTION
Reducing the partial pressure of the adsorbate.
2 ways:
Reduction in the system total pressure
Introduction of an inert gas while maintaining the total system pressure
Cycle time very quick (minutes or second)
Operate PSA close to ambient temperature at a given partial pressure, the
loading is increased as temperature decreased
Popular for performing bulk separation of gases controlled by adsorption
isotherm or adsorption kinetics
68
34
3/24/2016
MECHANISM: PRESSURE EFFECT
For a single adsorbate:
Reducing the partial pressure
from P1 to P2 causes the
equilibrium loading to be
reduced from q1 to q2
69
Bed PSA System
Each bed operates alternately in 2 half-cycles of
equal duration:
Pressurisation followed by adsorption
Desorption by depressurisation (blowdown)
followed by a purge
Adsorption pressure greater than
atmospheric
Desorption pressure being atmospheric
Pressurisation feed gas
Purging effluent (non-adsorbed) product gas
70
35
3/24/2016
Operation Modes: Stirred Tank (Batch)
Powdered adsorbent e.g. activated carbon particle diameter less
than 1 mm
Main application removal of very small amounts of dissolved,
large molecules e.g. colouring agents
Spent adsorbent removed by sedimentation or filtration
71
Operation Modes: Cyclic Fixed Bed
(Batch)
Adsorbent particle size 0.05 cm to 1.2 cm
Optimal particle size bed pressure drop & solute transport rate
Main application removal of organic compounds from water
Spent adsorbent regenerated at high temperature
72
36
3/24/2016
Operation Modes: Continuous
Countercurrent
Need to circulate solid adsorbent as moving bed to achieve steady
state operation
Difficult in regenerating adsorbent when heavier HC presents
73
THEORY: EQUILIBRIUM RELATIONS
Concentration of a solute
in a fluid phase
T, P
Data is plotted as
adsorption isotherms
Concentration of a
solute in a solid phase
74
37
3/24/2016
Adsorption isotherms
q, kg adsorbate / kg adsorbent
Usage of adsorption isotherm
To select suitable adsorbent
To select separation process
c, kg adsorbate / m3 fluid
75
Adsorption Isotherms
The relationship between the concentration of solute in fluid phase and its
concentration on the solid.
76
38
3/24/2016
Types of Isotherms: Linear
Linear Isotherm
Similar to Henrys law:
q = Kc (12.1-1)
c : concentration (fluid is liquid)
p : partial pressure (fluid is a gas)
q : mass, moles or volumes of adsorbate per unit mass or per unit
surface area of adsorbent
K : an empirical, temperature-dependent constant
77
Types of Isotherms: Freundlich
q Kc n
K = Freundlich constant
n = constant
Determined
experimentally
c = equilibrium concentration
The dimensions for K depends on the value of
n.
78
39
3/24/2016
Types of Isotherms: Langmuir
Assumptions:
qc
q 0
K c
K, q0 = constant
c = equilibrium concentration
Monolayer coverage on adsorbent
No interactions between adsorbent molecules
All adsorbate molecule/adsorbent interactions are the same
Only a fixed number of active sites available
Adsorption is reversible and reached an equilibrium condition
79
Example: Adsorption Isotherms
Batch tests were performed in the laboratory using solutions of phenol in water
and particles of granular activated carbon. The equilibrium data at room
temperature are shown in the table below. Determine the isotherm that fits the
data.
(kg
phenol/m3
0.322
solution)
0.117
0.039
0.0061
0.0011
(kg phenol/kg carbon)
0.150
0.122
0.094
0.059
0.045
Refer to Geankoplis C. J. Transport Processes and Separation Proces Principles, 4th80Edition
40
3/24/2016
Solution
1. Rearrange the isotherm equation to become a linear equation.
2. Plot the graph
3. The suitable isotherm is when the plot is a straight line.
Graph was plotted on log graph paper.
Suitable isotherm is Freundlich isotherm
where
Slope, n = 0.229
Constant, K = 0.199
Adsoprtion isotherm equation is
q = 0.199c0.229
BATCH ADSORPTION
Used when quantities to be treated are of small amount.
Isotherms and material balance are needed.
Material balance on the adsorbate:
qF M + cF S = q M + cS
where:
qF = initial concentration of solute on the solid
q = final concentration at equilibrium
M = amount of adsorbent, kg
S = volume of feed solution, m3
82
41
3/24/2016
Example: Values of q and c at equilibrium
A wastewater solution having a volume of 1.0 m3 contains 0.21 kg
phenol/m3 of solution . A total of 1.40 kg of fresh granular activated
carbon is added to the solution , which is then mixed thoroughly to
reach equilibrium. Use the isotherm q = 0.199c0.229, find
a) the final equilibrium values (q & c)
b) the percentage of phenol extracted?
83
Solution
Information given:
This intersection gives the
equilibrium value.
q = 0.106 kg phenol/kg carbon
c = 0.062 kg phenol/m3
cF = 0.21 kg phenol/m3
qF = 0 (assumed)
% phenol extracted =
100 = 70.5
q = 0.199c0.229
S = 1.0 m3
M = 1.40 kg carbon
qF M + cF S = q M + cS
0(1.40) + 0.21(1.0) = q(1.40) + c(1.0)
42
3/24/2016
FIXED BED ADSORPTION
85
FIXED BED ADSORPTION
Concentration Profiles
Fluid to be treated passed down through the packed bed at a constant flow rate
Concentration of solute in the fluid phase and of the solid adsorbent phase
change with time and position MTZ
Mass Transfer Zone (MTZ) width and shape depends on:
the adsorption isotherm
flowrate
mass transfer rate to the particles
diffusion in the pores
86
43
3/24/2016
Mass Transfer Zone
Adsorption only occurs in a particular
region of the bed, known as Mass
Transfer Zone (MTZ) which moves
through the bed.
87
Breakthrough Concentration Curve
1.0
c/c0
At t2;
At tb;
t1
0.5
0
At t1;
mass-transfer
zone
H1
t2
tb
t3
H2
Height of adsorption bed, H
H3
HT
Typical solute conc. profiles for fluid as a function of distance through the bed at increasing times t1, t2
and tB from start of the flow through bed.
no part of bed is saturated
the bed is almost saturated for a distance H1. At H2 the bed is still clean. The region H1 and H2 is the
mass transfer zone, where adsorption takes place.
the leading point of mass transfer zone just reach the end of the bed. This is called breakthrough
point.
88
44
3/24/2016
Breakthrough Concentration Curve
1.0
mass-transfer
zone
c/c0
0.5
0
t1
t2
t3
break point,
cb
t4
tb
cd
t6
Ratio of outlet solute concentration to inlet solute concentration in the fluid as a function of time from the
start of flow. The S-shaped curve is called the breakthrough curve.
Prior to tb, the outlet solute concentration is less than the maximum allowable of c/c0 between 0.01 to
0.05.
At tb this value is reached. The adsorption step should be discontinued.
If the adsorption step is allowed to be continued for t>tB , the outlet solute concentration would be
observed to rise rapidly, eventually approaching the inlet concentration as the outlet end of the bed
became saturated.
89
Breakthrough Concentration Curve
Important points:
Break point (tB)
cout/cF is often taken as 0.01 to 0.05
Most bed capacity is used at the break point
Mass transfer zone: Region where most of
the concentration change occurs.
90
45
3/24/2016
Evaporation
Evaporation
Evaporation
Vapor from boiling liquid is removed
Remains concentrated solution
Properties which affect the processing methods :
Concentration in the liquid
Solubility
Temperature sensitivity of materials
Foaming or frothing
Pressure and temperature
Scale deposition and materials of construction
46
3/24/2016
Concentration in the liquid
Liquid feed to an evaporator is relatively dilute.
As evaporation proceeds, the solution may become very
concentrated.
Adequate circulation and/or turbulence must be present to keep
the heat transfer coefficient from becoming not too low.
93
Solubility
As solutions are heated and the concentration of solute
(salt) increases, the solubility limit of the material in the
solution may be exceeded and crystals may form.
May limit the max concentration in solution which can be
obtained by evaporation.
94
47
3/24/2016
Temperature sensitivity of materials
Temperature-sensitive materials such as food & biological
materials such as milk, vegetable extracts may degrade at
higher temperature.
Degradation is a function of temperature & time.
95
Foaming or frothing
Some solutions form a foam or froth during boiling (eg. Food
solutions such as skim milk, some fatty acid solutions)
Entrainment losses occur - suspended droplets of liquid are
carried in the escaping vapor
96
48
3/24/2016
Pressure & Temperature
The higher the operating pressure of the evaporator, the higher
the temperature at boiling.
To keep the temperature low in heat-sensitive material, its
often to operate under 1 atm pressure
97
Scale deposition
Some solutions deposit solid materials on the heating surfaces
due to the products decomposition or decrease in solubility.
May decrease the overall heat transfer coefficient and the
evaporator must be cleaned.
98
49
3/24/2016
Materials of construction
Evaporators are made of steel (carbon steel). However, many
solutions attack ferrous metal and/or contaminated by them.
Use special material (nickel, stainless steel, aluminium), however
its expensive
99
Evaporation
The basic factors that affect the rate of evaporation are the:
rate at which heat can be transferred to the liquid
quantity of heat required to evaporate each kg of water
maximum allowable temperature of the liquid
pressure at which the evaporation takes place
changes that may occur in the solution during the course of the evaporation
process
Methods
Once-through sensitive materials (food industry)
Circulation used in oil and gas industry and others
50
3/24/2016
Type of evaporators used in oil and gas
Type of evaporators used in oil and gas
Vapor compression evaporation
Vertical tube evaporators
51
3/24/2016
Benefits of evaporation for produced
water treatment
Eliminate physical and chemical treatments so no chemical sludge is produced
and costs of waste and life cycle is lowered.
Require less maintenance materials and maintenance labor.
Reduce the amount of produced water de-oiling required.
Increase Once Through Steam Generators (OSTG) feed-water quality and
improve OSTG reliability.
OSTG used for heavy oil recovery processes.
Produces high pressure steam for injection into geological formations
containing heavy oil.
The heat given by the condensing steam fluidizes the heavy and allow the
oil/water mixture to be brought to the surface.
Heat and mass balance for single effect
evaporators
Feed F, TF , xF , hF
Saturated Steam S, Ts , Hs
F, L, S = mass flow, kg/h
T = temperature,
x, y = solids content, mass fraction
H = vapor enthalpy, J/kg
h = liquid enthalpy, J/kg
Vapor V, yV T1, HV
V is pure solvent,
yV = 0
Condensate S, TS, hs
Concentrated liquid L, T1, xL, hL
Refer to Geankoplis C. J. Transport Processes and Separation Proces Principles, 4th Edition
52
3/24/2016
Heat and mass balance for single effect
evaporators
Important basis of calculation
S is assumed to leave the evaporator at TS
(saturation temperature) with enthalpy hs.
Therefore, steam gives only its latent heat,
=
(1)
V is in equilibrium with L, the
temperatures are the same (T1)
T1 at the concentrated liquid side is at
boiling point with a saturation vapor
pressure of P1
Assume that the system is at steady state
rate of mass in = rate of mass out
Heat and mass balance for single effect
evaporators
Material balance
Overall material balance: F = L+V (2)
Balance on the solute (solids): FxF = LxL (3)
Heat balance
Total heat entering = Total heat leaving
Heat in feed + Heat in steam = Heat in concentrated liquid
+ Heat in vapor + Heat in condensed steam
Assume no heat lost via radiation or convection
Fhf + SHs = LhL + VHV + ShS
simplified using equation (1)
Fhf + S = LhL + VHV (4)
53
3/24/2016
Heat and mass balance for single effect
evaporators
Heat transfer to evaporator
Heat transfer to evaporator
q U AT UA(Ts T1 ) (6)
q = S(HS hS) = S (5)
Latent heat of steam can be found in steam
tables
If the enthalpies of the feed and products are
not available, approximations can be made via
Latent heat of evaporation of 1 kg mass of
water solution can be obtained from steam
table using the temperature of the boiling
solution, T1.
Heat capacities of liquid feed, cpF and
product, cpL
q = heat transferred per unit time
U = overall coefficient of heat transfer
A = heat transfer surface
T = temperature difference between the two
streams
Example
A continuous single-effect evaporator concentrates 9072 kg/hr of a 1.0 wt% salt
solution entering at 311.0 K (37.80C) to a final concentration of 1.5 wt%. The
vapor space of the evaporator is at 101.325 kPa (1.0 atm abs) and the steam
supplied is saturated at 143.3 kPa. The overall heat transfer coefficient, U = 1704
W/m2 K.
Calculate:
a) The amount of vapor (V) & liquid product (L)
b) The heat transfer area required (A)
Assume cpF is of water = 4.14 kJ/kg.K
54
3/24/2016
Solution for (a)
Information that we have:
Material balance:
xF = 1.0 wt% = 0.01
FxF = LxL
F = L+V 9072 = L+V
F = 9072 kg/h
9072(0.01) = L(0.015)
xL = 1.5 wt% = 0.015
L = 6048 kg/h
V = 3024 kg/h
Solution for (b)
FhF + S = LhL + VHV
?
Latent heat of
condensation at
Ts
Latent heat of
evaporation at T1
55
3/24/2016
Solution for (b)
Find hF & hL
Find
T1 = choose the boiling point of water
because the salt solution is dilute.
From steam table at 143. 3 kPa (given)
hF = cpF(TF T1)
hs = 461.3 kJ/kg
TF = 311 K (given)
Since the evaporator pressure is at
101.325 kPa (1 atm), T1 = 372.2 K (100C)
= 4.14(311 373.2) = -257.5 J/kg
hL = CpL(T1 TBP)
TS = 383.2 K (100C)
Hs = 2691.5 kJ/kg
= 2230.2 kJ/kg
TBP = T1 hL = 0
Solution for (b)
Find HV
It is the latent heat at T1 = 372.2 K
(100C)
Hs = 2676.1 kJ/kg
hs = 419.04 kJ/kg
Find S
Fhf + S = LhL + VHV
9072(-257.5) + S(2230) = 6048(0) + 3024(2257)
S = 4108 kg steam/h
= 2257 kJ/kg
56
3/24/2016
Solution for (b)
Find q
q = S
q = 2 544 000 W
Finally, find A
q U AT UA(Ts T1 )
A = 149.3 m2
Steam Economy
Capacity = no. of kilograms of water vaporized per hour
Economy = no. of kilograms steam fed to the unit per hr
Steam economy = Capacity /Economy (kg vaporized water/kg steam used)
steam economy =
3024
= 0.736
4108
57
3/24/2016
Effects on processing variables
1. Effect on feed temperature
Higher feed temperature reduces the size of heat transfer area
Because if the feed temperature is above boiling point, additional vaporization is
obtained (flashing of the entering hot feed)
2. Effect of pressure
Reducing the pressure will increase the T
Resulting in decrease of heating surface area and cost of evaporator
3. Effect of steam pressure
Higher steam pressure, increases T
Resulting in decrease of heating surface area and cost of evaporator
Non dilute solutions
SOLUTIONS ARE NOT DILUTE,
HENCE..
THERMAL PROPERTIES OF THE SOLUTION IS DIFFER
CONSIDERABLY FROM THOSE OF WATER.
58
3/24/2016
BOILING POINT RISE & DUHRING
DIAGRAM
For a given pressure, the boiling point of an aqueous solution is higher than that
of pure water. The increase in boiling point over that of water is known as the
boiling point rise (BPR)
BPR = Boiling point of water at a given pressure Boiling point of solution (refer
Duhring Diagram)
NaCl-Water System
59
3/24/2016
Example
If the boiling point of water at
25.6 kPa is 65.6C,
1. What is the boiling point of
30% NaOH?
79.5C
2. What is the boiling point rise?
13.9C
Boiling point of water (0 C)
NaOH-Water System
PW Management Options for O&G
Industry
Injection
Injection of produced
water into the same
formation from which
the oil is produced or
handle to another
formation.
Discharge
Reuse in oil and gas operation
Treatment of produced
water to meet onshore
Consume in beneficial use
Treat the produced
or offshore discharge
water to meet the
regulations.
quality required to use Produced water
it for usual oil and gas treatment to meet to
fields operations
quality required for
beneficial uses such as
irrigation , rangeland
restoration, cattle and
animal consumption,
and drinking water.
60
3/24/2016
61
3/24/2016
2. DRILLING FLUID
The key to making the rotary drilling system work is the ability to circulate a fluid
continuously down through the drill pipe, out through the bit nozzles and back
to the surface.
Purpose of Drilling Fluids
1. Cooling and lubrication. As the bit drills into the rock formation, the friction
caused by the rotating bit against the rock generate heat. The heat is dissipated
by the circulating drilling fluid. The fluid also lubricates the bit.
2. Cuttings removal. An important function of the drilling fluid is to carry rock
cuttings removed by the bit to the surface. The drilling flows through treating
equipment where the cuttings are removed and the clean fluid is again pumped
down through the drill pipe string.
2. DRILLING FLUID
3. Suspend cuttings. There are times when circulation has to be stopped. The
drilling fluid must have that gelling characteristics that will prevent drill cuttings
from settling down at the bit. This may caused the drill pipe to be stuck.
4. Pressure control. The drilling mud can be the first line of defense against a
blowout or loss of well control caused by formation pressures.
The hydrostatic head produced by the mud in psi is = 0.052 x G x H
where G = density of mud in ppg
H = depth of the hole in feet.
62
3/24/2016
2. DRILLING FLUID
This hydrostatic head will counter the formation pressure in order to avoid a
blowout while drilling. For example, lets say a well is being drilled in a salt-water
basin (pressure gradient of 0.465 psi/ft), the pressure in the formation at 10,000
feet would be expected to be:
10,000 x 0.465 = 4,650 psi
The weight of mud required to counter this pressure is calculated as follows.
P = 0.052GH
4,650 = 0.052 x G x 10,000
G = 8.94 ppg
2. DRILLING FLUID
5. Data source. The cuttings that the drilling mud brings to the
surface can tell the geologist the type of formation being drilled.
6. To wall the hole with impermeable filter cake. This will give a
temporary support to the wall of the borehole from collapsing
during drilling.
63
3/24/2016
64
3/24/2016
2. DRILLING FLUID
Three basic types of DF are :
1)
Water Based Mud ( WBM) - water or brine (salt solution) as base
fluid/continuous phase. This is the most common drilling mud used in
oil drilling.
2)
Oil Based Mud (OBM) - crude oil or diesel or mineral oil as based
fluid/continuous phase.
Serve a wide range of application, some of which cannot
currently be performed by other DF
OBM is highly inhibitive, resistive to contaminants,
temperature and pressure. Its also highly lubricious, non
corrosive and flexible
3)
Pneumatic Based (PBM) - air, foam or natural gas as base fluid
65
3/24/2016
TYPES OF DRILLING FLUIDS - Examples
Non-water
based
Water
based
Pneumatic
Diesel oil
Mineral oil
Synthetic Fluid
Colloidal clay
Clay and polymer
Polymer
Dry gas
Mist
Foam
Gasified Mud
DRILLING FLUID COMPOSITION
Composition of a typical bentonite gel water based mud ,density
1300kg/m3. Components added to 1 barrel of water : (bbl=barrel,
pps=pounds per barrel); CMC :(carboxymethylcellulose)
Component
Water
Bentonite
Caustic soda
Soda ash
High viscosity cmc
Low viscosity cmc
Barite
Quantity
1 bbl
20 ppb
0,5 ppb
0,5 ppb
1,5 ppb
3,5 ppb
160 ppb
Mass(kg)
159
9,1
0,23
0,23
0,68
1,59
72,58
Volume(L)
1588,99
9,07
0,22
0,10
0,47
1,09
17,28
%mass
65,33
3,73
0,9
0,9
0,28
0,65
29,82
%volume
84,92
4,85
0,12
0,5
0,25
0,58
9,23
66
3/24/2016
DRILLING FLUID COMPOSITION
Composition of a typical oil based mud density 1318 kg/m3 , salinity 22.5%, oil to
water ratio 65:35. components combine to give a total volume of one barrel.(bbl:
barrel; ppb:pounds per barrel; gpb: gallons per barrel)
Component
Quantity
Base fluid
Viscosifier
Emulsifier 1
0,5 bbl
5 ppb
0,8 ppb
Lime
Water
CalCl2
Barite
5 ppb
0,30 ppb
30,2 ppb
167,9 ppb
Emulsifier 2
0,4 ppb
Mass(kg)
64,63
2,26
2,89
Volume(L)
1,49
2,26
47,15
13,70
76,15
%mass
83,31
1,40
3,02
30,37
1,08
1,38
1,00
47,22
3,35
18,16
1,08
22,50
6,54
36,34
1,51
0,71
%volume
52,40
0,88
1,90
0,95
0,63
29,70
2,11
11,42
DRILLING FLUID DESIGN
Synthetic Based Mud ( SBM)
The goal of SBM (also called pseudo OBM) is to provide an alternative
to OBM, without the toxicity and less environmental impact
The base fluid is a man made non aqueous liquid with different
molecular structure to the raw material it is manufactured with.
Common base oil are Saraline and Sarapar (of Bintulu SMDS) and
Petrofree (palm oil ester based product)
Currently PCSB using Sarapar 147 as a base fluid for SBM
67
3/24/2016
ADDITIVES
Various thickeners are used to influence the viscosity of the fluid, e.g. xanthan
gum, guar gum, glycol, carboxymethylcellulose (CMC), polyanionic cellulose
(PAC), or starch.
Deflocculants are used to reduce viscosity of clay-based muds; anionic
polyelectrolytes (e.g. acrylates, polyphosphates, lignosulfonates or tannic
acid derivates such as Quebracho are frequently used.
Some other common additives include lubricants, shale inhibitors, fluid loss
additives (to control loss of drilling fluids into permeable formations).
A weighting agent such as barite (specific gravity =4.3) or ilmenite (spec. gr. 4.5)
is added to increase the overall density of the drilling fluid.
ENVIRONMENTAL IMPACT OF DRILLING FLUIDS
Drilling wastes deserve special attention. The volume of drilling wastes usually ranges
from 1,000 to 5,000 m3 for each well. Such wells can number into dozens for one
production platform and many hundreds for a large field.
As a result of many technological operations and procedures, drilling muds and cuttings
are saturated with hundreds of very different substances and compounds.
It is their discharges into the sea that pose one of the main ecological threats during
offshore oil production.
In particular, many countries express concern regarding biocides, which are used to
suppress microflora in the drilling and other circulating fluids.
The list of such compounds includes over one hundred names. The most widespread
biocides used in the oil and gas production practice include sodium salts of hypochlorite,
formalin releasers, and glutaraldehyde as well as biguanidine and quaternary
ammonium, and a number of other compounds.
68
3/24/2016
ENVIRONMENTAL IMPACT OF DRILLING FLUIDS
The composition of some compounds is not always known. Some biocides are highly toxic. Many
countries either discourage (for example, in case of carbamates and thiocarbamates) or prohibit
(for example, in case of dichlorophenols and pentachlorophenates) their use by the offshore oil
and gas industry.
Drilling discharges also contain many heavy metals (mercury, lead, cadmium, zinc, chromium,
copper, and others) and NORM (Uranium, Thorium, Radium etc) that come from components of
both drilling fluids and drilling cuttings.
Drill cutting discharges effect on benthic system around platform.
Base Oil detected in sediment up to 2km from drilling site
Less than 300 m (substantial)
500-1000 m (significant)
Up to 2 km (traces)
Similar concentrations after 8 yrs survey (low degradation).
Oil accumulated deeper in sediment (0-30cm surveyed).
Method to evaluate the environmental
impact of drilling fluid
By using BIOASSAY.
Placing chosen organism placed in the drilling fluid at
different concentration.
Use LC50 to determine the toxicity
LC50 ( lethal concentration ) : standard measure of the
toxicity of the surrounding medium that will kill half of the
sample population at specified time. It is measure in
micrograms (or milligrams) of the material per liter, or parts
per million (ppm), of air or water
138
69
3/24/2016
Drilling Waste Management
Operators must achieve a balance between
1. Minimizing environmental impact
2. Maintaining borehole stability
3. Maximizing drilling efficiency
Drilling waste management
140
70
3/24/2016
DRILLING CUTTINGS MANAGEMENT
Recently, a technology was developed to remove the drilling
wastes, especially cuttings, by reinjecting their slurry into a
geological formation. This gives some hope to achieving zero
discharge of oil-containing wastes during offshore oil and gas
production.
Some other measures (such as slim-hole drilling) to reduce
discharges, particularly in environmentally sensitive locations, are
being investigated by the industry.
Minimising drilling fluids
Some of the techniques include pipe wipers and vacuuming of spills on the rig
floors
Pneumatic drilling (air, air/water)
Developing variations of fluids that are less toxic and easily to bio treatment.
Drilling practice that reduce the generation of drilling waste.
- Directional drilling
- Drilling smaller diameter holes
- Drilling techniques that use less frilling fluids
142
71
3/24/2016
Reuse of drilling cuttings
Road spreading
One use of cuttings is to stabilize surfaces that are subject to erosion, such as
roads or drilling pads.
Reuse of Cuttings as Construction Material
Need to be treated to render the cuttings more innocuous
Used for
fill material,
daily cover material at landfills,
aggregate or filler in concrete, brick, or block manufacturing.
143
Reuse of drilling cuttings
Restoration of Wetlands Using Cuttings
as a substrate for restoring coastal wetlands
Use of Cuttings for Fuel
Cuttings were blended in at a low rate with coal, the primary fuel source
144
72
3/24/2016
Disposal
Pre treatment
Reduce the volume of the waste
Reduce the potential hazard of the
waste
Alter the state of the waste
(separate liquid and solid phases)
Disposal methods
Deep well disposal
Disposal liquid waste are injected
into a formation
-produced water, drilling
completion wastes
Evaporation pond
These pond facilitate the reduction
of waste volume through
evaporation
-drilling fluids
Thermal method
Use high temperature furnaces to
reclaim or destroy contaminated
material
-HC contaminated soil
145
Disposal
On-shore
To the sea
Reinjected into the disposal well
Off-shore
the waste fluids would be temporarily stored in earthen pits( on site or off-site
Eventually will be disposed into landfill
146
73
3/24/2016
Secured landfill diagram
147
Reducing oil waste
Solids must be removed before reusing the drilling mud.
This is to avoid the existence of ultrafine solids content in the mud that will
affect the fluids performance and general stability worn out mud that
needs to be disposed of.
Method to recover base oil from worn out mud.
M-I SWACO RECLAIM technology chemically enhanced solids-removal process
Flocculants used to remove oil-wet solids
Surfactants used to weaken muds emulsion for the flocculants to work
demulsify brine droplets from the mud
Centrifuge to remove the small solids and brine (water)
74
3/24/2016
75
3/24/2016
CRI Cuttings reinjection
1. Typically cuttings are mixed with seawater
2. Grinded
3. Or other mechanical action to form a stable viscous
slurry
4. Pumped down a dedicated disposal well or through
annulus between casing strings on an active well
5. Forced under pressure into formations
6. Which creates hydraulic fracture in the formation.
7. At the end of the injection program, the well or the
annulus are sealed with cement.
76
3/24/2016
77
3/24/2016
3. MERCURY
A concern in oil & gas industry. Found in almost all oil and gas
reservoirs
Highest concentrations normally found from reservoirs in South
America, South East Asia, Easter europe
European/African/North American gas sources also contain mercury
Mercury is classified as hazardous substance with limited use
Mercury containing waste classified as hazardous waste
Hazardous waste management requires special license
78
3/24/2016
3. MERCURY
Origin -HgS (cinnabar or mercuric sulfide) in reservoir or deposits.
Hg0(g) i.e. Elemental mercury is the major Hg containing
compound at reservoir conditions
Major mercury compoundsHg0(g) and HgS(s).
HgS(g) and Hg2(g) negligible.
Organic
mercury
components
concentrations/partial pressures
3. MERCURY
occur
in
LOCATION
ELEMENTAL MERCURY CONCENTRATION (mg/m3)
East Asia
58-193
N Europe
0.01-180
South America
North Africa
Middle East
N America
insignificant
69-119
0.3-130
1-9
0.005-0.04
79
3/24/2016
3. MERCURY
Corrosion/embrittlement
Precipitation(HgS)
Mechanisms
Plugging of compact equipment
Caused by elemental mercury
Amalgamation
Amalgam (mercury alloy) corrosion
Liquid Metal Embrittlement, LME
Fouling
Poison the precious metal catalysts
Galvanic corrosion
3. MERCURY
Cinnabar is highly toxic
Mercury poisoning via:
exposure to water-soluble forms of mercury
inhalation of mercury vapor, or
eating seafood contaminated with mercury
New health hazard from Hg released to atm undergo bio-methylation reactions
to form highly toxic DMM (dimethyl mercury), an organo-mercury compound
Refer guidelines on mercury mgmt in oil and gas industry 2011
80
3/24/2016
3. MERCURY
3. MERCURY
Traditional : packed bed with sulfur impregnated activated carbon
Drawback 1: no use for spent carbon
Drawback 2: Landfill not allowed. Only combustion followed by condensation
of mercury
Modern thinking inorganic route i.e using reactive metal sulfides
Hg + MxSy = MxSa + HgS
Advantages : spent material recycled through metal smelters
81
3/24/2016
3. MERCURY
Further reading
Mercury Removal Project: Issues and Challenges in Managing and Executing
a Technology Project 2007
Challenges in Managing Mercury in Malaysia 2009
4. NATURALLY OCCURRING
RADIOACTIVE MATERIALS
(NORM)
82
3/24/2016
4. NORM
Naturally occurring radioactive materials such as Radium -226 and Radium-228
are found in the oil sludge as the unwanted by product in the waste stream from
oil and gas exploration activities. The NORM have to be treated at suitable
facilities on-shore for long term storage to reduce volume and minimise
environmental effects.
83
3/24/2016
5. HAZARDOUS WASTES
5. HAZARDOUS WASTES
GUIDELINES FOR THE APPLICATION OF SPECIAL MANAGEMENT OF SCHEDULED WASTE
GUIDELINES ON STANDARD AND SPECIFICATION OF RECOVERED WASTE OIL IN
MALAYSIA
CODE OF SCHEDULED WASTE
CONTAMINATED LAND MANAGEMENT AND CONTROL GUIDELINES NO. 1: MALAYSIAN
RECOMMENDED SITE SCREENING LEVELS FOR CONTAMINATED LAND
84
3/24/2016
5. HAZARDOUS WASTES
General Definition - Hazardous Waste refers to residue of business and/or activity containing
hazardous materials which is directly and indirectly able to contaminate and/or damage the
environment, and/or endanger environment, health, performance of human and other creatures
due to its characteristics and/or concentration.
The Railroad Commission (US) has jurisdiction over oil and gas wastes, which include all wastes
generated in association with the following activities:
drilling, operation, and plugging of wells associated with the exploration, development, or production of oil and
gas, including oil and gas wells, fluid injection wells used in enhanced recovery projects, and disposal wells;
separation and treatment of produced fluids in the field or at natural gas processing plants;
storage of crude oil before it enters a refinery;
underground storage of hydrocarbons and natural gas;
transportation of crude oil or natural gas by pipeline;
solution mining of brine; and
storage, hauling, disposal, or reclamation of wastes generated by these activities.
5. HAZARDOUS WASTES
Rustamina et al., 2009
85
3/24/2016
5. HAZARDOUS WASTES
Hazardous Wastes in Malaysia are governed by
Hazardous Substances Division
Air Division
Water & Marine Division
86
3/24/2016
6. GASEOUS EMISSIONS
In Oil and gas industry, general categories of direct emissions sources are:
Production of heat, steam, or electricity, whether for use by the company or for sale to other
parties
Combustion in flares and incinerators
Production of work by engines and turbines, for example, to drive pumps or compressors
Physical or chemical process emission such as from gas processing, oil refining, and
petrochemical manufacture
Transportation in company-owned motor vehicles and vessels, such as tank trucks and oil
tankers
Fugitive losses from equipment leaks such as from gas pipeline systems
87
3/24/2016
6. GASEOUS EMISSIONS
Three main types of gas emissions in oil and gas industry, namely;
1.
2.
3.
Combustion gases consisting of carbon dioxides and minor amounts of carbon monoxide,
nitrous oxide, N2O, SO2 and uncombusted hydrocarbons (methane and VOC)
Hydrocarbons consisting of methane and mainly aliphatic VOCs vented to atmosphere or
escaping from the hydrocarbon processes through fugitive emissions
Releases of halon and other CFC gases from fire fighting and refrigeration systems.
6. GASEOUS EMISSIONS
88
3/24/2016
6. GASEOUS EMISSIONS
The assumed effect of emitted CFC gases to the depletion of the stratospheric ozone
layer has resulted in the Montreal Protocol (1985)
The Geneva Convention through its three protocols; the Helsinki protocol (1985), the
Sofia Protocol (1988), and the Geneva Protocol (1991), signed by almost all industrial
countries in Europe and North America, imposes obligations on the signature nations
to control and reduce their emissions of SOx, NOx, and VOC.
Norway government introduced a CO2 tax in 1991. This tax is now 0.80 NOK per Sm3
(3.20 USD/1000SCF) natural gas fueled or flared. A similar tax has to be paid for diesel
fuel. (Geir & Novatech, 1994)as for effectively 1 Jan 2013, the amount increased to
0.96 NOK per Sm3 (Environmental and climate considerationsin the Norwegian
petroleum Sector, 2013) to signify the importance of good management on the
gaseous emissions.
6. GASEOUS EMISSIONS
The most commonly reported greenhouse gases are those covered by the Kyoto Protocol:
Carbon Dioxide, CO2
Methane, CH4
Nitrous Oxide, N2O
Hydrofluorocarbons, HFCs
Perfluorocarbons, PFCs
Sulfur Hexafluoride, SF6
In addition to this list, some reporting programs, such as the national inventory reporting Program of the
Intergovernmental Panel on Climate Change (IPCC), include emissions of nitrogen oxides, carbon
monoxide, and non-methane volatile organic compounds when accounting for GHG emissions.
Compounds covered by the Montreal Protocol, such as chlorofluorocarbons and hydrochlorofluorocarbons,
are also sometimes included in GHG emissions inventories. While these compounds are also GHGs, they
currently receive relatively little attention as GHGs because they either have already been or are being
phased out for most applications
89
3/24/2016
6. GASEOUS EMISSIONS
It is recommended that companies within the petroleum industry account for and report
all significant emissions of each of the six GHGs listed above that fall within their
established organizational and operational boundaries. Virtually all companies within the
industry would be expected to have emissions of CO2 and to a lesser extent CH4 and
N2O since these gases are produced through combustion.
Both CH4 and CO2 are also part of the materials processed by the industry as they are
produced, in varying quantities, from gas and oil wells. Because the quantities of N2O
produced through combustion are quite small compared to the amount of CO2
produced, CO2 and CH4 are the predominant petroleum industry GHGs.
6. GASEOUS EMISSIONS
HFCs, PFCs and SF6 while not as closely associated with the petroleum industry as other
GHGs, may be emitted by various subsectors and operations. HFCs are increasingly used
in refrigeration systems, including virtually all motor vehicle air conditioners.
Both HFCs and PFCs may be used as solvents, and PFCs are used in some fire
extinguishing systems. PFCs are also emitted during the manufacture of aluminum and in
some semi-conductor manufacturing processes.
Sulfur hexafluoride is used in high-voltage electrical equipment and in the production
and casting of magnesium.
Since none of these emitting activities are core parts of the petroleum industry, total
emissions of these gases would be expected to be small. For particular facilities or
businesses in which petroleum industry companies have an interest, however, these
kinds of emissions may be significant.
90
3/24/2016
6. GASEOUS EMISSIONS
GUIDELINE FOR THE INSTALLATION & MAINTENANCE OF CONTINUOUS EMISSION
MONITORING
SYSTEMS
(CEMS)
FOR
INDUSTRIAL
PREMISES
/FACILITIESVOLUME I
GUIDELINE FOR THE INSTALLATION & MAINTENANCE OF CONTINUOUS EMISSION
MONITORING
SYSTEMS
(CEMS)
FOR
INDUSTRIAL
PREMISES
/FACILITIESVOLUME II
6. GASEOUS EMISSIONS
A large number of alternative reduction measures were identified, assessed and analysed. These measures
were grouped into 4 categories:
a)reduction of the energy demand
b)more efficient energy generation
c)extraction of CO2 from the exhaust gases
d)conversion to an alternative energy carrier
The first categories were evaluated against state-of-the-art technology used in other industries. Gas has
traditionally been a cheap and plentiful resource on the offshore oil and gas production platforms.
Consequently energy utilization has not been optimized as well as in some other industries. The following
measures within category a. and b. were found to contain the most interesting potentials for CO2 emission
reduction;
export gas pipeline optimization
gas turbine waste heat for heating purposes
combined cycle power plants
91
3/24/2016
6. GASEOUS EMISSIONS
Extraction of CO2 from the gas turbine exhaust gas, followed by disposal in subsurface formations or in
deep sea ocean layers has been proposed by research institutes as an interesting alternative to reduce the
CO2 emissions.
The technology has the potential to almost fully eliminate the energy generated CO2 emissions from
Norwegian offshore oil and gas production. It was therefore decided to evaluate the availability and
feasibility of this technology. The study concluded that technology exists, but the energy and space
requirements are very large, showing that a new and additional CO2 removal platform will be required on
each field if the technology should be applied on existing platforms, unless significant technology
improvements take place.
Hydroelectric power supply from shore, as an alternative to gas fuel, was also investigated. Except for a few
special cases, this alternative was found to be very uneconomic compared to conventional technology.
For the two latter cases, publically available documentation was established that may assist operators in
future evaluation of these reduction measures. Furthermore, the documentation can form a basis for a
constructive dialogue with authorities and other interesting parties.
92
Das könnte Ihnen auch gefallen
- Structural Analysis 1: Statically Determinate StructuresVon EverandStructural Analysis 1: Statically Determinate StructuresNoch keine Bewertungen
- Euncl PCC 002 PDFDokument19 SeitenEuncl PCC 002 PDFAnvesh DonthulaNoch keine Bewertungen
- PDFDokument23 SeitenPDFDharmaraaj RajalinggamNoch keine Bewertungen
- 2010 Effect of PH and Scale Inhibitor Concentration On Phosphonate-Carbonate InteractionDokument18 Seiten2010 Effect of PH and Scale Inhibitor Concentration On Phosphonate-Carbonate Interactionandrea cunhaNoch keine Bewertungen
- Adsorption Isotherms in Liquid PhaseDokument33 SeitenAdsorption Isotherms in Liquid PhasetarisaiNoch keine Bewertungen
- Corrosion in Oil and Gas Industry A Perspective On Corrosion Inhibitors 2169 0022.1000e110Dokument1 SeiteCorrosion in Oil and Gas Industry A Perspective On Corrosion Inhibitors 2169 0022.1000e110Gustavo Jaime100% (1)
- Rheology and Hydraulics: Rheology Is The Science of Deformation and Flow of MatterDokument36 SeitenRheology and Hydraulics: Rheology Is The Science of Deformation and Flow of Matterhassan haddadiNoch keine Bewertungen
- Scale PresentationDokument59 SeitenScale PresentationMohamed SadekNoch keine Bewertungen
- Water TechnologyDokument58 SeitenWater TechnologyAdi Mantha اديتية منتة100% (3)
- What Are Corrosion Inhibitors.? Corrosion InhibitorsDokument3 SeitenWhat Are Corrosion Inhibitors.? Corrosion InhibitorsdiwakarngmNoch keine Bewertungen
- Bentonite Clay-Acid Activation Studies: K. Suresh, R. Samant, S.K. Jatty and H. BambhaniaDokument5 SeitenBentonite Clay-Acid Activation Studies: K. Suresh, R. Samant, S.K. Jatty and H. BambhaniaminingnovaNoch keine Bewertungen
- Inhibition of Calcium Carbonate Precipitation in NaClDokument9 SeitenInhibition of Calcium Carbonate Precipitation in NaCldalton2004Noch keine Bewertungen
- Sand Effects On Production AssetsDokument32 SeitenSand Effects On Production AssetsMohamed HashemNoch keine Bewertungen
- Water For Injection-By AnamDokument20 SeitenWater For Injection-By AnamMuhammad Bilal TahirNoch keine Bewertungen
- Hazardous Waste Identification Guidance DocumentDokument105 SeitenHazardous Waste Identification Guidance DocumentMiguel TorresNoch keine Bewertungen
- BRENT ALSPACH - Produced Water and Salinity Management The Desalination FrontierDokument7 SeitenBRENT ALSPACH - Produced Water and Salinity Management The Desalination FrontierPAOLANoch keine Bewertungen
- Aluminum Chemistry Increases Shale StabilityDokument14 SeitenAluminum Chemistry Increases Shale StabilityYracema Ochoa GutierrezNoch keine Bewertungen
- Ipc2012 90184Dokument10 SeitenIpc2012 90184Marcelo Varejão CasarinNoch keine Bewertungen
- Drilling and Complwtion FluidsDokument14 SeitenDrilling and Complwtion FluidsvipmittalNoch keine Bewertungen
- Ransom Bridge 1997Dokument14 SeitenRansom Bridge 1997Javier BosigasNoch keine Bewertungen
- Newtonian and Non-Newtonian Fluids: Velocity Profiles, Viscosity Data, and Laminar Flow Friction Factor Equations For Flow in A Circular DuctDokument9 SeitenNewtonian and Non-Newtonian Fluids: Velocity Profiles, Viscosity Data, and Laminar Flow Friction Factor Equations For Flow in A Circular DuctAmedeo Franco BonattiNoch keine Bewertungen
- Environmental Chemistry of Phosphonates: Article in PressDokument14 SeitenEnvironmental Chemistry of Phosphonates: Article in PressNathan BlecharcykNoch keine Bewertungen
- Mainlog Manual: Each Index Item in Has A Link, Just Pan and Click On The Menu or Feature You Want To Go ToDokument133 SeitenMainlog Manual: Each Index Item in Has A Link, Just Pan and Click On The Menu or Feature You Want To Go ToHafizMuhammadAzeemNoorNoch keine Bewertungen
- Polymer Support FluidDokument2 SeitenPolymer Support FluidhamsarajshettyNoch keine Bewertungen
- Formation DamageDokument26 SeitenFormation DamagerajneeshgogoiNoch keine Bewertungen
- SPE-183743-MS Maintaining Injectivity of Disposal Wells: From Water Quality To Formation PermeabilityDokument19 SeitenSPE-183743-MS Maintaining Injectivity of Disposal Wells: From Water Quality To Formation PermeabilityAminNoch keine Bewertungen
- Waste Management RegulationsDokument65 SeitenWaste Management RegulationsMIRAL JALUNoch keine Bewertungen
- Chapter 6no AnsDokument19 SeitenChapter 6no AnsGD AminNoch keine Bewertungen
- Mud Weighting Drilling LabDokument8 SeitenMud Weighting Drilling LabMuhammad S. RaniYahNoch keine Bewertungen
- Corrosion Application Library ManualDokument230 SeitenCorrosion Application Library ManualMiguel Angel Holguin MontañoNoch keine Bewertungen
- A High Performance, Damage Tolerant Fusion Bonded Epoxy CoatingDokument15 SeitenA High Performance, Damage Tolerant Fusion Bonded Epoxy CoatingpaimpillyNoch keine Bewertungen
- Well PalnningDokument30 SeitenWell PalnningPankaj Singh ChauhanNoch keine Bewertungen
- Deep Well Injection For Concentrate Disposal PDFDokument15 SeitenDeep Well Injection For Concentrate Disposal PDFmurbietaNoch keine Bewertungen
- DD1M HighwayLiveLoadsonConcretePipeDokument10 SeitenDD1M HighwayLiveLoadsonConcretePipeMauricioNoch keine Bewertungen
- PDF BrochureDokument50 SeitenPDF BrochureAnees RanaNoch keine Bewertungen
- The Effect of Norust 720 and CH1377A Inhibitors On N80 Steel Corroded by Bacterial CorrosionDokument5 SeitenThe Effect of Norust 720 and CH1377A Inhibitors On N80 Steel Corroded by Bacterial CorrosionInternational Journal of Innovative Science and Research TechnologyNoch keine Bewertungen
- Otc 10979 MS PDFDokument8 SeitenOtc 10979 MS PDFChinmaya Ranjan JenaNoch keine Bewertungen
- Polymer Systems For Water Shutoff and Profile Modification: A Review Over The Last DecadeDokument15 SeitenPolymer Systems For Water Shutoff and Profile Modification: A Review Over The Last DecadeLeopold Roj DomNoch keine Bewertungen
- Mol ReportDokument86 SeitenMol ReportAyesha KhanNoch keine Bewertungen
- Corrosion Prevention by Use of InhibitorsDokument19 SeitenCorrosion Prevention by Use of InhibitorsSai PradeepNoch keine Bewertungen
- Alcomer 74 TDSDokument1 SeiteAlcomer 74 TDSAnonymous JMuM0E5YONoch keine Bewertungen
- Fundamentals of Water Treatment Unit Processes (Feb 2013)Dokument9 SeitenFundamentals of Water Treatment Unit Processes (Feb 2013)lalo2385100% (1)
- Petroleum Geology - HalliburtonDokument34 SeitenPetroleum Geology - HalliburtonStephen FortisNoch keine Bewertungen
- Florida Guard Rail SystemDokument26 SeitenFlorida Guard Rail SystemZaher Mhd SharafNoch keine Bewertungen
- Journal of Petroleum Science and EngineeringDokument10 SeitenJournal of Petroleum Science and EngineeringMohammed Al-shargabiNoch keine Bewertungen
- Membrane Technology: By: Prof. Dr. Tien R. MuchtadiDokument47 SeitenMembrane Technology: By: Prof. Dr. Tien R. MuchtadiDwiMariaUlfahNoch keine Bewertungen
- Preface ...........................................................................Dokument6 SeitenPreface ...........................................................................Pak RioNoch keine Bewertungen
- Anti FoamDokument20 SeitenAnti FoamNduong NguyenNoch keine Bewertungen
- Spe 184815 MSDokument13 SeitenSpe 184815 MSSSNoch keine Bewertungen
- On Corrosion InhibitorsDokument41 SeitenOn Corrosion InhibitorsdiwakarngmNoch keine Bewertungen
- 25 One Year Experience With The Injection of Nitrate To Control Souring in Bonga Deepwater Development Offshore NigeriaDokument9 Seiten25 One Year Experience With The Injection of Nitrate To Control Souring in Bonga Deepwater Development Offshore NigeriaCatalinaManjarresNoch keine Bewertungen
- Mechanism of An Asphaltene Inhibitor PDFDokument50 SeitenMechanism of An Asphaltene Inhibitor PDFTEXOPED Parsian KishNoch keine Bewertungen
- Soil Exploration or Site Investigation: For Testing Soil Properties For Design & AnalysisDokument126 SeitenSoil Exploration or Site Investigation: For Testing Soil Properties For Design & Analysismandeso wanawNoch keine Bewertungen
- Bioreactor DesignDokument4 SeitenBioreactor DesignMahendra Varma BoopathyNoch keine Bewertungen
- 2019 Recent Development and Remaining Challenges of Iron Sulfide Scale Mitigation in Sour-Gas WellsDokument8 Seiten2019 Recent Development and Remaining Challenges of Iron Sulfide Scale Mitigation in Sour-Gas WellsBeatriz Joo LeeNoch keine Bewertungen
- On Oxygen-Induced Corrosion of An Oil Refinery Condensate Fraction at Ion UnitDokument17 SeitenOn Oxygen-Induced Corrosion of An Oil Refinery Condensate Fraction at Ion UnitAzmi Mohammed NorNoch keine Bewertungen
- BITUMENDokument35 SeitenBITUMENIZIMBA0% (1)
- Corrosive SoilDokument25 SeitenCorrosive Soilsherasiya sherasiyaNoch keine Bewertungen
- Produced Water Issues and Treatment: Group Members Roll Number Submitted To Mr. Imran AliDokument45 SeitenProduced Water Issues and Treatment: Group Members Roll Number Submitted To Mr. Imran AliU jKNoch keine Bewertungen
- Expected Level of ProficiencyDokument1 SeiteExpected Level of ProficiencyHumaira MirzaNoch keine Bewertungen
- Side View: Uitm - RX 2.80: Attachment ADokument2 SeitenSide View: Uitm - RX 2.80: Attachment AHumaira MirzaNoch keine Bewertungen
- Improving Cost EstimatesDokument4 SeitenImproving Cost EstimatesHumaira MirzaNoch keine Bewertungen
- PROSPER Assg 2 ReportDokument1 SeitePROSPER Assg 2 ReportHumaira MirzaNoch keine Bewertungen
- CHAPTER 2 OutlineDokument2 SeitenCHAPTER 2 OutlineHumaira MirzaNoch keine Bewertungen
- Leadership and Ethics 4Dokument8 SeitenLeadership and Ethics 4Humaira MirzaNoch keine Bewertungen
- Leadership and Ethics 2Dokument9 SeitenLeadership and Ethics 2Humaira MirzaNoch keine Bewertungen
- CGE416 - Chap6 - Drilling Engineering and Well CompletionDokument109 SeitenCGE416 - Chap6 - Drilling Engineering and Well CompletionHumaira Mirza50% (2)
- Carbonate Sedimentology, Reef History and MVT Deposits, Canning Basin, WADokument2 SeitenCarbonate Sedimentology, Reef History and MVT Deposits, Canning Basin, WAHumaira MirzaNoch keine Bewertungen
- CGE416 Introduction To Petroleum Technology Chapter 5: Reservoir Engineering Blended Learning 1Dokument1 SeiteCGE416 Introduction To Petroleum Technology Chapter 5: Reservoir Engineering Blended Learning 1Humaira MirzaNoch keine Bewertungen
- Saftey of Ammonia Handling PDF-libreDokument51 SeitenSaftey of Ammonia Handling PDF-libreHumaira Mirza100% (1)
- Luke Sisti Resume 2014Dokument1 SeiteLuke Sisti Resume 2014Humaira MirzaNoch keine Bewertungen
- 100 Basic Terminologies For Petroleum IndustryDokument7 Seiten100 Basic Terminologies For Petroleum IndustryHumaira Mirza100% (1)
- Chapter 1 - Directional Drilling - ADokument37 SeitenChapter 1 - Directional Drilling - AHumaira MirzaNoch keine Bewertungen
- The Most Important Part of Casing Hanger Is : Slip and Seal RingDokument130 SeitenThe Most Important Part of Casing Hanger Is : Slip and Seal RingHumaira Mirza100% (5)
- Tuto 7Dokument1 SeiteTuto 7Humaira MirzaNoch keine Bewertungen
- Boiler Heat Balance Sample Calculation Power Plant DesignDokument3 SeitenBoiler Heat Balance Sample Calculation Power Plant DesignJk Pascii100% (1)
- Evaporation PowerpointDokument16 SeitenEvaporation PowerpointZahraa HerekNoch keine Bewertungen
- A Model To Assess The Risk of Ice Accretion Due ToDokument1.666 SeitenA Model To Assess The Risk of Ice Accretion Due ToTudorache BogdanNoch keine Bewertungen
- Oxygen Plant ManualDokument54 SeitenOxygen Plant Manualmahaveen89% (18)
- Reflex Pressure Vessels Design and CalculationDokument52 SeitenReflex Pressure Vessels Design and CalculationcsharpplusNoch keine Bewertungen
- C Evapo TranspirationDokument49 SeitenC Evapo Transpirationjuliyet strucNoch keine Bewertungen
- EM2U60HLP - Data Sheet PDFDokument3 SeitenEM2U60HLP - Data Sheet PDFCayqueCasaleNoch keine Bewertungen
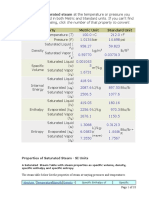
- Property Metric Unit Standard Unit: Properties of Saturated Steam - SI UnitsDokument18 SeitenProperty Metric Unit Standard Unit: Properties of Saturated Steam - SI UnitsGaapchuNoch keine Bewertungen
- A02 115Dokument29 SeitenA02 115jaime0% (1)
- CH 1Dokument7 SeitenCH 1Asad IslamNoch keine Bewertungen
- Material Balance in Unit OperationsDokument75 SeitenMaterial Balance in Unit OperationsAcademicBMNoch keine Bewertungen
- Evaporative Systems DiagnosisDokument40 SeitenEvaporative Systems DiagnosisVASEKNoch keine Bewertungen
- Evaporator 20160915091922109Dokument3 SeitenEvaporator 20160915091922109OnofreNoch keine Bewertungen
- Single Effect EvaporaterDokument16 SeitenSingle Effect EvaporaterdebdeepNoch keine Bewertungen
- A Semiquantitative Method To Assess Occupational Exposure To Harmful ChemicalsDokument0 SeitenA Semiquantitative Method To Assess Occupational Exposure To Harmful ChemicalsKyaw Kyaw Aung100% (1)
- Processing Instructions V 5.0 Refraspecial®Dokument3 SeitenProcessing Instructions V 5.0 Refraspecial®Mohammed AbdalrhmanNoch keine Bewertungen
- Boiling Liquid Expanding Vapour Explosions (BLEVEs)Dokument14 SeitenBoiling Liquid Expanding Vapour Explosions (BLEVEs)Ayoub BenkaouhaNoch keine Bewertungen
- Evaporation - Inorganic Chemistry For IndustriesDokument2 SeitenEvaporation - Inorganic Chemistry For IndustriesTimothy JonesNoch keine Bewertungen
- Final Molasses Purity ControlDokument8 SeitenFinal Molasses Purity Controlzafar Bukhari100% (1)
- TranscriptDokument29 SeitenTranscriptNurAfifah OsmanNoch keine Bewertungen
- Mechanical Engineering ThermofluidsDokument175 SeitenMechanical Engineering Thermofluidsemad11518100% (1)
- Fisherbrand Vacuum Pumps PDFDokument15 SeitenFisherbrand Vacuum Pumps PDFchiragpatel7294Noch keine Bewertungen
- Unusual Properties of WaterDokument44 SeitenUnusual Properties of WaterHanan ShehaNoch keine Bewertungen
- And D. Choose The Best Option.: Diagram 1 Diagram 3Dokument12 SeitenAnd D. Choose The Best Option.: Diagram 1 Diagram 3shng84Noch keine Bewertungen
- Ecolec Guide QuestionsDokument26 SeitenEcolec Guide QuestionsArt Julius D. Hallazgo100% (1)
- Electrohydrodynamic Enforcement of Evaporation and Gas FlowDokument7 SeitenElectrohydrodynamic Enforcement of Evaporation and Gas FlowMichael ReznikovNoch keine Bewertungen
- Lesson - 68 - 4th - Quarter - Science - 4. Role of Sun in The Water CycleDokument3 SeitenLesson - 68 - 4th - Quarter - Science - 4. Role of Sun in The Water CycleVenus Cureg100% (2)
- Volatility of Liquefied Petroleum (LP) Gases: Standard Test Method ForDokument4 SeitenVolatility of Liquefied Petroleum (LP) Gases: Standard Test Method ForDennise ChicaizaNoch keine Bewertungen
- Environmental Science and Engineering Unit - IDokument47 SeitenEnvironmental Science and Engineering Unit - IarumugamNoch keine Bewertungen
- Chemistry Class Ix For 2017 181 PDFDokument139 SeitenChemistry Class Ix For 2017 181 PDFMukul GoyalNoch keine Bewertungen