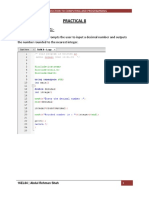
Beruflich Dokumente
Kultur Dokumente
9701 s12 Ms 31
Hochgeladen von
abdulrehman999Originaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
9701 s12 Ms 31
Hochgeladen von
abdulrehman999Copyright:
Verfügbare Formate
w
w
ap
eP
e
tr
.X
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS
for the guidance of teachers
9701 CHEMISTRY
9701/31
Paper 31 (Advanced Practical Skills 1),
maximum raw mark 40
This mark scheme is published as an aid to teachers and candidates, to indicate the requirements of
the examination. It shows the basis on which Examiners were instructed to award marks. It does not
indicate the details of the discussions that took place at an Examiners meeting before marking began,
which would have considered the acceptability of alternative answers.
Mark schemes must be read in conjunction with the question papers and the report on the
examination.
Cambridge will not enter into discussions or correspondence in connection with these mark schemes.
Cambridge is publishing the mark schemes for the May/June 2012 question papers for most IGCSE,
GCE Advanced Level and Advanced Subsidiary Level syllabuses and some Ordinary Level
syllabuses.
om
.c
MARK SCHEME for the May/June 2012 question paper
s
er
GCE Advanced Subsidiary Level and GCE Advanced Level
Page 2
Mark Scheme: Teachers version
GCE AS/A LEVEL May/June 2012
Syllabus
9701
Paper
31
Question
Sections
Indicative material
Mark
PDO layout
Constructs a table for results
PDO recording
II
Appropriate headings and units for data given.
Volume / V in cm3, / cm3 or (cm3) Time/t in
seconds, /s or (s)
PDO recording
III All times recorded to the nearest second.
MMO decision
IV 3 additional volumes chosen with intervals not less
than 2.00 cm3 and all volumes of FA 1 greater or
equal to 6.00 cm3
MMO collection
In all 3 additional experiments water is added to
make a total of 20.00 cm3
MMO quality
Round times to nearest second.
VI + VII Compare time for 20.00 cm3 of FA 1 with that
of supervisor.
VIII + IX Compare time for 10.00 cm3 of FA 1 with that
of supervisor.
The range for award of 1 or 2 depends on the
supervisor value.
(a)
Supervisor value:
< or = 15 for 2 is 2 and for 1 is 4
16 to 30 for 2 is 3 and for 1 is 6
31 to 45 for 2 is 4 and for 1 is 8
46 60 for 2 is 5 and for 1 is 10
> 60 for 2 is 6 and for 1 is 12
(b)
(c)
[9]
PDO display
(i)
Working to show ans = 5 105 mol
ACE interpretation
(ii)
0.5 x ans to (b)(i) = 2.5 105 mol
PDO display
(iii) Working to show that:
(2.5 105) / 0.050 =
(5 104 mol dm3)
ACE interpretation
Rate correctly calculated using ans (b)(iii) / time (or
4.25 104). Min 2 s.f. rounded correctly and minimum
4 results.
PDO recording
Unit for rate given as mol dm3s1.
[3]
[2]
University of Cambridge International Examinations 2012
Page 3
Question
(d)
(e)
(f)
Mark Scheme: Teachers version
GCE AS/A LEVEL May/June 2012
Paper
31
Sections
Indicative material
PDO layout
Rate on y-axis and volume on x-axis. Axes clearly
labelled (ignore units)
II
Linear scale chosen to use at least half of each
axis (need not include 0, 0)
If no point at 0, 0 cannot count for > half.
ACE conclusion
ACE interpretation
ACE improvement
ACE conclusion
Mark
III Plotting of points.
Minimum of 3 readings.
IV Draws a line of best fit. Minimum 4 readings
including 0, 0 (if plotted).
Rate is proportional to peroxodisulfate concentration
Rate increases as concentration (volume) increases
would score one
(i) correctly calculates (0.5 / time from Expt 1) 100.
Minimum of 2 s.f.
(ii)
(g)
Syllabus
9701
[4]
ans (b)(iii)
106 mol dm3 s1
Expt 1 time + 0.5
or
Rate (% from (i) rate)
[2]
(iii) Any reasonable suggestion e.g. difficult to judge
colour change / measurement of volumes /
variation in T
use of colorimeter / burettes for all volumes /
(thermostatic) waterbath.
Not air conditioning.
(ii) Thiosulfate concentration / number moles / volume
is doubled (1)
Time is longer/ reaction is slower with more
thiosulfate (1)
[4]
[2]
[Total: 26]
University of Cambridge International Examinations 2012
Page 4
Question
Mark Scheme: Teachers version
GCE AS/A LEVEL May/June 2012
Sections
Syllabus
9701
Paper
31
Mark
Indicative material
FA 5 = CuCl2; FA 6 = NaOH; FA 7 = Pb(NO3)2; FA 8 = K2CrO4; FA 9 = MgSO4
2
(a)
(b)
(c)
MMO collection
ACE conclusion
Blue ppt
insol in
excess (1)
Not dark
blue
White ppt
(1)
Ignore
excess.
Yellow /
brown /
greenishbrown ppt
(1)
Not orange,
red, red /
brown
Ignore
excess.
White ppt
soluble in
excess (1)
No reaction
/ yellow
solution
and
yellow ppt
soluble in
excess
CONs ppt
(1)
[5]
Cu2+ in FA 5 AND CrO42 in FA 8
Pb2+ in FA 7 AND OH in FA 6
Cl in FA 5
MMO decision
Add Pb (NO3)2 or BaCl2 or Ba(NO3)2
MMO decision
II
Add HNO3 or HCl
PDO recording
III Presents observations in a single
table no extra reagents.
Must be > 2 boxes
MMO collection
IV White ppt
MMO collection
V No SO2 evolved or ppt insoluble
ACE conclusion
VI sulfate
[3]
[6]
[Total: 14]
University of Cambridge International Examinations 2012
Das könnte Ihnen auch gefallen
- GlossaryDokument9 SeitenGlossaryfarqaleetaliNoch keine Bewertungen
- Mit18 06scf11 Ses2.5sumDokument4 SeitenMit18 06scf11 Ses2.5sumabdulrehman999Noch keine Bewertungen
- Output Taxation, Human Capital and GrowthDokument17 SeitenOutput Taxation, Human Capital and Growthabdulrehman999Noch keine Bewertungen
- Learner Guide For As and A Level ChemistryDokument64 SeitenLearner Guide For As and A Level Chemistryabdulrehman999Noch keine Bewertungen
- Social ScienceDokument4 SeitenSocial Scienceabdulrehman999Noch keine Bewertungen
- Output Taxation, Human Capital and GrowthDokument17 SeitenOutput Taxation, Human Capital and Growthabdulrehman999Noch keine Bewertungen
- 1Dokument1 Seite1abdulrehman999Noch keine Bewertungen
- 2014 Syllabus Update A-Level ChemistryDokument1 Seite2014 Syllabus Update A-Level ChemistryseekforheavenNoch keine Bewertungen
- Common Ions and Formulae of Ionic CompoundsDokument1 SeiteCommon Ions and Formulae of Ionic Compoundsnickmirad2Noch keine Bewertungen
- Thevenin and Norton ProblemsDokument56 SeitenThevenin and Norton ProblemsSaarthak VadheraNoch keine Bewertungen
- Install CodeBlocks IDE & Write Basic C++ ProgramsDokument9 SeitenInstall CodeBlocks IDE & Write Basic C++ Programsabdulrehman999Noch keine Bewertungen
- Ee 211 - 1Dokument2 SeitenEe 211 - 1abdulrehman999Noch keine Bewertungen
- Definition: A System of Linear Equations Is Said To Be Homogeneous If It Can Be Written in The Form A X 0. Otherwise, It Is Non-HomogeneousDokument8 SeitenDefinition: A System of Linear Equations Is Said To Be Homogeneous If It Can Be Written in The Form A X 0. Otherwise, It Is Non-Homogeneousabdulrehman999Noch keine Bewertungen
- Math 123Dokument47 SeitenMath 123ShailendraPatelNoch keine Bewertungen
- 141 6.5 Lecture NotesDokument4 Seiten141 6.5 Lecture NotesAshokRockNoch keine Bewertungen
- Pureit Excella User Manual PDFDokument31 SeitenPureit Excella User Manual PDFabdulrehman999Noch keine Bewertungen
- Math 124Dokument44 SeitenMath 124ShailendraPatelNoch keine Bewertungen
- Pakistan StudiesDokument1 SeitePakistan Studiesabdulrehman999Noch keine Bewertungen
- Causes of Separation of East PakistanDokument27 SeitenCauses of Separation of East Pakistanabdulrehman99969% (16)
- 3x3 Determinants and Cramers Rule 4x4 DeterminantsDokument4 Seiten3x3 Determinants and Cramers Rule 4x4 Determinantsabdulrehman999Noch keine Bewertungen
- 141 6.5 Lecture NotesDokument4 Seiten141 6.5 Lecture NotesAshokRockNoch keine Bewertungen
- 70 Vector & 3d Part 3 of 6Dokument6 Seiten70 Vector & 3d Part 3 of 6keerthyNoch keine Bewertungen
- 141 6.5 Lecture NotesDokument4 Seiten141 6.5 Lecture NotesAshokRockNoch keine Bewertungen
- Engineering MechanicDokument24 SeitenEngineering MechanicManoj BallaNoch keine Bewertungen
- Bub GB N-GdaaaambajDokument140 SeitenBub GB N-GdaaaambajJoseph VijuNoch keine Bewertungen
- Practical 13Dokument6 SeitenPractical 13abdulrehman999Noch keine Bewertungen
- Practical 11Dokument10 SeitenPractical 11abdulrehman999Noch keine Bewertungen
- C++ programs for rounding, time conversion & unit conversionDokument18 SeitenC++ programs for rounding, time conversion & unit conversionabdulrehman999Noch keine Bewertungen
- Practical 12 Object: Tasks: Working With Arrays in C++Dokument6 SeitenPractical 12 Object: Tasks: Working With Arrays in C++abdulrehman999Noch keine Bewertungen
- Shoe Dog: A Memoir by the Creator of NikeVon EverandShoe Dog: A Memoir by the Creator of NikeBewertung: 4.5 von 5 Sternen4.5/5 (537)
- Grit: The Power of Passion and PerseveranceVon EverandGrit: The Power of Passion and PerseveranceBewertung: 4 von 5 Sternen4/5 (587)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceVon EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceBewertung: 4 von 5 Sternen4/5 (890)
- The Yellow House: A Memoir (2019 National Book Award Winner)Von EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Bewertung: 4 von 5 Sternen4/5 (98)
- The Little Book of Hygge: Danish Secrets to Happy LivingVon EverandThe Little Book of Hygge: Danish Secrets to Happy LivingBewertung: 3.5 von 5 Sternen3.5/5 (399)
- On Fire: The (Burning) Case for a Green New DealVon EverandOn Fire: The (Burning) Case for a Green New DealBewertung: 4 von 5 Sternen4/5 (73)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeVon EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeBewertung: 4 von 5 Sternen4/5 (5794)
- Never Split the Difference: Negotiating As If Your Life Depended On ItVon EverandNever Split the Difference: Negotiating As If Your Life Depended On ItBewertung: 4.5 von 5 Sternen4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureVon EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureBewertung: 4.5 von 5 Sternen4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryVon EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryBewertung: 3.5 von 5 Sternen3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerVon EverandThe Emperor of All Maladies: A Biography of CancerBewertung: 4.5 von 5 Sternen4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreVon EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreBewertung: 4 von 5 Sternen4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyVon EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyBewertung: 3.5 von 5 Sternen3.5/5 (2219)
- Team of Rivals: The Political Genius of Abraham LincolnVon EverandTeam of Rivals: The Political Genius of Abraham LincolnBewertung: 4.5 von 5 Sternen4.5/5 (234)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersVon EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersBewertung: 4.5 von 5 Sternen4.5/5 (344)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaVon EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaBewertung: 4.5 von 5 Sternen4.5/5 (265)
- The Unwinding: An Inner History of the New AmericaVon EverandThe Unwinding: An Inner History of the New AmericaBewertung: 4 von 5 Sternen4/5 (45)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Von EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Bewertung: 4.5 von 5 Sternen4.5/5 (119)
- Her Body and Other Parties: StoriesVon EverandHer Body and Other Parties: StoriesBewertung: 4 von 5 Sternen4/5 (821)
- Excerpt From Otherbound by Corinne DuyvisDokument9 SeitenExcerpt From Otherbound by Corinne DuyvisAbrams BooksNoch keine Bewertungen
- De Notified Nomadic and Semi Nomadic Tribes 1Dokument68 SeitenDe Notified Nomadic and Semi Nomadic Tribes 1hk81100% (1)
- The Dyslexic Reader 2006 - Issue 41Dokument32 SeitenThe Dyslexic Reader 2006 - Issue 41Davis Dyslexia Association International100% (17)
- CST Students With Disabilities Practice Exam JBB Test PrepDokument4 SeitenCST Students With Disabilities Practice Exam JBB Test Prepinquiries432156% (9)
- Resume-Kenneth Gammad 2016-01-07Dokument2 SeitenResume-Kenneth Gammad 2016-01-07api-305260671Noch keine Bewertungen
- Gonzales Cannon November 21 IssueDokument34 SeitenGonzales Cannon November 21 IssueGonzales CannonNoch keine Bewertungen
- Multitasking Practices and Task Based Management Style Classroom LeadersDokument10 SeitenMultitasking Practices and Task Based Management Style Classroom LeadersIOER International Multidisciplinary Research Journal ( IIMRJ)Noch keine Bewertungen
- English Composition Assessment For Primary SchoolDokument1 SeiteEnglish Composition Assessment For Primary SchoolPeriachi Periadevi D/OP VijayanNoch keine Bewertungen
- Smart Lungu's CV To Madam MwambaDokument4 SeitenSmart Lungu's CV To Madam MwambaSmart lunguNoch keine Bewertungen
- Educational Qualifications Attained So Far?: - How Is It Related To Your Previous Studies?Dokument8 SeitenEducational Qualifications Attained So Far?: - How Is It Related To Your Previous Studies?agam reddyNoch keine Bewertungen
- Test Question Educ 207Dokument7 SeitenTest Question Educ 207Marivic LastimozaNoch keine Bewertungen
- Field Study FormsDokument6 SeitenField Study FormsGeraldine L. GaddiNoch keine Bewertungen
- ARF Nursing School 05Dokument7 SeitenARF Nursing School 05Fakhar Batool0% (1)
- Blue Hat Green Hat by Sandra BoyntonDokument10 SeitenBlue Hat Green Hat by Sandra Boyntontanvi100% (11)
- Position Description FormDokument3 SeitenPosition Description FormGlen Mark MacarioNoch keine Bewertungen
- English Week 8 LPDokument5 SeitenEnglish Week 8 LPVina Angelie80% (5)
- BE Level 3 Interactive PDF Student Book - Complete PDFDokument237 SeitenBE Level 3 Interactive PDF Student Book - Complete PDFPinGuinteRC100% (1)
- MIT Department InfoDokument20 SeitenMIT Department InfonaitikkapadiaNoch keine Bewertungen
- Action Research ProposalDokument13 SeitenAction Research ProposalEvonne L YK100% (1)
- Summative ReportDokument12 SeitenSummative Reportapi-383497107Noch keine Bewertungen
- Mathematics Learning DisabilityDokument4 SeitenMathematics Learning Disabilityapi-502438448Noch keine Bewertungen
- February 19 LGDokument4 SeitenFebruary 19 LGapi-252811687Noch keine Bewertungen
- Ap Intervention.Dokument18 SeitenAp Intervention.bernalyn santosNoch keine Bewertungen
- Teaching ResumeDokument1 SeiteTeaching Resumeapi-514518399Noch keine Bewertungen
- AES Citizens CharterDokument29 SeitenAES Citizens CharterIan Khay CastroNoch keine Bewertungen
- Master of Education (M.Ed) ProgrammeDokument36 SeitenMaster of Education (M.Ed) ProgrammeNurulFatinNabila100% (1)
- The Effect of Social Medie On Academic Performance of Senior High School StudentsDokument4 SeitenThe Effect of Social Medie On Academic Performance of Senior High School StudentsMiguel Mangabat BeltranNoch keine Bewertungen
- Fs 41Dokument38 SeitenFs 41analynNoch keine Bewertungen
- Final Reading & WritingDokument6 SeitenFinal Reading & WritingCharisse Alvarez100% (2)
- SOGI included in Anti-Bullying Act IRR protects LGBT studentsDokument2 SeitenSOGI included in Anti-Bullying Act IRR protects LGBT studentsNaomi CartagenaNoch keine Bewertungen