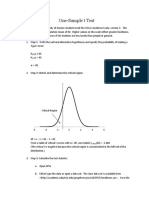
Beruflich Dokumente
Kultur Dokumente
Lachnit and Kinder
Hochgeladen von
jorge9000000Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Lachnit and Kinder
Hochgeladen von
jorge9000000Copyright:
Verfügbare Formate
THE QUARTERLY JOURNAL OF EXPERIMENTAL PSYCHOLOGY, 2000, 53B (3), 209224
Stimulus representations in human Pavlovian
conditioning: Implications of missing negative
transfer across response systems
Harald Lachnit and Annette Kinder
Philipps-Universitat Marburg, Marburg, Germany
Three Pavlovian conditioning experiments with human participants are reported, which
investigated whether common or separate stimulus representations are involved in solving
nonlinear discrimination tasks in different response systems. In our experiments we made use
of a negative transfer effect between positive and negative patterning. Experiment 1 specied
the conditions under which such a negative transfer effect occurs in human eyelid conditioning. Experiments 2 and 3 investigated whether a similar effect also occurs if two response
systemsthe eyelid and the skin conductance response systemare trained with trials of
both types being randomly interleaved. The presence or absence of a negative transfer effect
indicates whether or not the stimulus representations involved in the two conditioning
processes overlap. The ndings are discussed within the framework of a neuropsychological
model of hippocampal function. The results suggest that the representations are distinct and
thus support the idea of acquired equivalence and distinctiveness of stimulus representations.
Modern theories of Pavlovian conditioning (e.g., Pearce, 1994; Rescorla & Wagner, 1972;
Wagner & Brandon, 1989) describe the associative processes that underlie the observable
changes in an organisms behaviour. Although these processes are thought to be
functionally equivalent in different response systems, neuropsychological models typically assume that they are located in different neural pathways. Eyelid conditioning, for
example, is supposed to be mediated by localized sites in the cerebellum (Daum et al.,
1993; Swain & Thompson, 1993). In fear conditioning, by contrast, the amygdala (e.g.,
LeDoux, 1995) is thought to be important. However, in most types of classical conditioning central neural areas such as the associative cortex and the hippocampus are also
thought to be involved (e.g., Fanselow, 1999; Gluck & Myers, 1993, 1997; Hugdahl,
1998; Myers & Gluck, 1996; Rudy & Sutherland, 1989, 1995; Schmajuk & Buhusi,
Requests for reprints should be sent to Harald Lachnit, Department of Psychology, Philipps-University
Marburg, Gutenbergstr. 18, D-35032 Marburg, Germany. Email: lachnit@mailer.uni-marburg.de
This work was supported by the National German Science Foundation (Deutsche Forschungsgemeinschaft,
DFG) with grants DFG LA 564/71 and DFG LA 564/72 to the rst author. We thank Birte Barthel, Oliver
Goldmann, and Ralf Reinecke for running most of the experimental groups and David Lieberman, Catherine
Myers, Mark Gluck, and an anonymous reviewer for helpful comments on an earlier version of this manuscript.
q 2000 The Experimental Psychology Society
http://www.tandf.co.uk/journals/pp/02724995.html
210
LACHNIT AND KINDER
1997; Schmajuk & DiCarlo, 1992). Thus, different conditioning pathways do not seem to
be completely separated from each other.
Cortical and hippocampal areas are considered as especially important in nonlinear
discrimination tasks, where the discrimination cannot be solved by merely summing the
associative strengths of the elements when the compound is presented. Congural representations, which are crucial for solving such tasks, are thought to be built or enhanced in
these areas. Two well-known nonlinear discrimination tasks are positive and negative
patterning. In both types of patterning, two stimuli are presented either separately, as
elements, or together, as a compound. In positive patterning (PP), only the compound is
followed by an unconditioned stimulus (US), whereas the elements are followed by
nothing (AB+, A2 , B2 ). Organisms accordingly show conditioned responses (CRs) in
the presence of the compound and no or much fewer (or smaller) CRs in the presence of
the elements. In negative patterning (NP), the elements are followed by the US, whereas
the compound is followed by nothing (A+, B+, AB2 ). Organisms accordingly learn to
respond when the elements are presented but to refrain from responding when the
compound is presented.
The empirical observation that both patterning discriminations are solved successfully
can be explained by assuming not only that the elements gain associative strength but that
a representation of the stimulus conguration is built, which also gets associated with the
US. According to this hypothesis (e.g., Whitlow & Wagner, 1972), an NP discrimination
is explained by assuming that the elements gain positive associative strength whereas the
congural representation becomes inhibitory. PP can be explained by assuming that only
the congural representation gets positively associated to the US but not the elements.
Whereas there is no way of explaining NP without assuming a congural representation,
PP also could be explained by a mere elemental principle. One only has to assume that the
two elements gain positive associative strength, each of which is too small to elicit a
response on its own. On the compound presentation, the sum of the associative strength
of both elements could nevertheless be large enough to elicit a response. However, as
Bellingham, Gillette-Bellingham, and Kehoe (1985) pointed out, although this hypothesis
can explain responding at any given time of training, it is unable to account for the
development of responding normally observed in the course of training. Initially, responding both to the compound and to the elements increases. Thereafter, responding to the
compound still rises, whereas responding to the elements declines. Apparently, the summation principle cannot account for this nding. Therefore, congural representations
should be involved not only in NP but also in PP discriminations.
The current article deals with the question of whether or not the stimulus representations that are used in solving PP and NP problems overlap. We investigated two response
systems in humans, the eyelid response system and the skin conductance response (SCR)
system. Both systems are capable of acquiring the correct response pattern both in PP and
in NP (Kinder & Lachnit, 2000; Lachnit, 2000; Lachnit & Kimmel, 1993). In our experiments, we made use of a negative transfer effect, which was rst reported by Bellingham
et al. (1985). These authors trained rats to approach a water magazine according to either
a PP or an NP schedule. The two conditioned stimuli (CSs)a light (L) and a tone (T)
were presented either separately or together. In NP, water was given when either the tone
or the light was presented but not when both stimuli were presented (T+, L+, TL2 ). In
STIMULUS REPRESENTATIONS
211
PP, water was given only if both stimuli were presented together (TL+, T2 , L2 ).
Bellingham et al. trained two groups of rats, which experienced a different sequence of
PP and NP training phases. In the rst phase, one group was trained on PP and the other
group on NP. Both groups learned well to discriminate between compounds and elements. In the second phase, the reinforcement schedule was changed. The rats previously
trained on NP were then trained on PP, whereas the rats previously trained on NP were
then trained on PP. Surprisingly, only the rats changing from NP to PP successfully
mastered the new PP discrimination. By contrast, rats changing from PP to NP completely failed to learn the new NP discrimination. In the third phase, the reinforcement
schedule was switched again, back to the pattern used in the rst phase. Again, only the
rats trained on PP in the third phase showed signicant response differentiation. The rats
trained on NP in the third phase did not learn the discrimination although they had
already acquired the correct discrimination in the rst phase. To summarize, Bellingham
et al. observed PP training to have a negative effect on subsequent acquisition of NP. This
negative transfer effect was an asymmetric one because acquisition of NP was disturbed
by prior PP training whereas acquisition of PP remained unaffected by prior NP training.
An analogous negative transfer effect was observed by Lachnit and Kimmel (1993) as
well as by Lachnit and Kinder (2000) in SCR conditioning in human participants.
Lachnit and Kimmel (Experiment 1) conditioned PP and NP in an interleaved fashion
by presenting trials of both types in random order. The participants could distinguish
between the two types of trial because different CSs were used in PP and NP trials. The
results showed that the participants had learned the PP discrimination but had failed to
learn the NP discrimination. By contrast, participants in Experiment 2 who did not
experience PP trials were able to learn the NP task. Thus, the result of Lachnits and
Kimmels experiments can also be interpreted as an asymmetric negative transfer effect.
That analogous effect occurred despite the fact that the procedure was quite different to
Bellingham et al.s (1985) procedure (e.g., PP and NP trials were interleaved in Lachnits
and Kimmels experiment but separated from each other in Bellingham et al.s experiment). In another study with human SCR conditioning (Lachnit & Kinder, Experiment 1)
the result that NP was disturbed by interleaved training of PP was replicated. In the same
experiment, it was shown that NP training was disturbed not only by interleaved training
of PP but also if subjects were pre-trained with PP. This procedure resembled the one
used by Bellingham et al. more closely. Thus, in several experiments and under different
experimental conditions, PP training was shown to have a negative effect on the acquisition of the NP discrimination. Apparently, this is a reliable effect.
In the experiments reported in this article, we made use of the negative transfer effect
in order to nd out whether the representations emerging during the acquisition of PP
and NP in different response systems overlap. If different response systems use identical
representations in accomplishing patterning tasks, we expected to observe negative transfer effects similar to those observed if PP and NP are trained within a single system. In
Experiment 1, we determined conditions under which transfer effects between PP and
NP can be observed in human eyelid conditioning. In Experiments 2 and 3, participants
experienced both eyelid and SCR-conditioning trials. We investigated whether or not
similar negative transfer effects as in Experiment 1 still occurred if two different response
systems were trained.
212
LACHNIT AND KINDER
EXPERIMENT 1
The purpose of Experiment 1 was to determine experimental conditions under which
there is a negative transfer between PP and NP training in human eyelid conditioning.
Two procedures have been described in which negative transfer effects occurred: sequential training (Bellingham et al., 1985, with appetitive instrumental conditioning in rats;
Lachnit & Kinder, 2000, with aversive SCR conditioning in humans) and interleaved
training of PP and NP (Lachnit & Kimmel, 1993, with aversive SCR conditioning in
humans). In the three experimental conditions of Experiment 1, sequential as well as
interleaved training was used. There were two sequential conditions in which participants
were trained with one kind of patterning in the rst phase of the experiment and with the
other type of patterning in the second phase. That way, responding in the second phase,
after pre-training with the opposite type of patterning, could be compared across groups
with responding in the rst phase, without pre-training. The third group of participants
was trained with PP and NP trials interleaved. Different CSs were used for the two types
of patterningthe letters C and N for PP and the letters M and J for NPso as to make
the two types of patterning distinguishable. The same was true for the sequential conditions in which the letters changed when the second phase of the experiment began.
Method
Participants
A total of 73 University of Marburg students took part in the experiment. They either received
course credit or were paid for participation. The data of 13 participants were excluded from the
analyses because they habituated to the airpuff during the experiment and did not show unconditioned responses (URs) on at least 10 trials or because they did not follow the instructions. Thus, the
analyses were performed on a total of 60 data sets (20 in each condition).
Stimuli and apparatus
CSs were two pairs of letters: C, N, and M, J. The two letters of each pair were presented either
alone or together on a computer display, which was positioned 1.5 m in front of the subject. Letter
compounds were presented in the centre of the display (each letter was 4 cm high and 2.5 cm broad).
Single letters appeared on the same display position as that in the compound presentations (that is, C
or M were always presented left from the centre, and N or J were always presented right from the
centre).
The US was a corneal airpuff with 100 ms duration. Air pressure was adjusted individuallybefore
the experiment started, for every subject to show a strong and immediate UR. The CSUS interval
was 700 ms for PP and 1,200 ms for NP. These intervals have been demonstrated to yield large and
similar levels of response differentiation under the two reinforcement schedules (Kinder & Lachnit,
2000). CS offset coincided with US onset. Thus, CS duration was identical to the CSUS interval.
The experiment was conducted in a sound-proof, well-illuminated room. Participants wore an
adjustable headband, supporting a small airjet for US application and an apparatus for response
measurement. The eyeblink response was measured by a modication of a device originallydescribed
by Gormezano and Gibbs (1988). A thin aluminium tab was attached to the subjects eyelid by a
cosmetic adhesive. The tab was connected with the end of a piano wire, which permitted the move-
STIMULUS REPRESENTATIONS
213
ments of the lid to be transmitted to the shaft of a photoelectric transducer. The signal from the
transducer was sampled by a computer (at a frequency of 500 Hz), which also controlledtiming of the
stimuli. The sequence but not the timing of the CSs was controlled by another computer. Thus, all
stimulus events were exactly synchronized.
Procedure
Participants were instructed that during the experiment they would occasionally feel an airpuff to
their eye. They were told neither to aid nor to inhibit their eyelid responses and to carefully watch
what was presented in front of them on the computer screen. Every participant received both PP and
NP training. The two types of training consisted of 96 trials each, on half of which a US was
presented. PP training consisted of 48 CN+, 24 C2 , and 24 N2 trials. NP training consisted of
24 M+, 24 J+, and 48 MJ2 trials. The CSs were presented in a pseudo-randomized order with a
maximum of three reinforced or nonreinforced CSs occurring consecutively.
Participants assigned to different experimental conditions received different sequences of training. Under the condition PPNPsequential, participants were presented with 96 trials of PP training in the rst phase of the experiment and with 96 trials of NP training in the second phase. Under
the condition NPPPsequential the sequence was reversed: Participants were trained rst with NP
and secondwith PP. Under the conditionPPNPinterleaved, PP and NP trials were administered in
random sequence. The intertrial interval was 8 s on average, with a minimum length of 7 s and a
maximum length of 9 s.
Dependent variable
A CR was dened as any eyelid response occurring during a designated interval following CS
onset and exceeding 1% of the participants maximal eyelid response. The interval began 200 ms
after CS onset and terminated at CS offset, thus excluding alpha responses (Gormezano, 1966) as
well as URs from the results. Multiple responses during a CS were counted as a single CR. The
proportions of CRs in 12 successive either CS+ (omitting the randomly interspersed CS2 trials) or
CS2 trials (omitting the randomly interspersed CS+ trials) were determined. Four blocks, each
containing 12 CS+ and 12 CS2 trials, resulted for each type of patterning. Finally, a square root
transformation of the proportions was performed, in order to achieve normality of the positively
skewed distributions.
Results
All analyses of variance (ANOVAs) reported in this section include the independent
variables contingency (with the levels reinforced vs. nonreinforced blocks of trials) and
block (with the levels block 14). A signicant effect of contingency indicates that the
level of responding to reinforced CSs differs signicantly from the level of responding to
nonreinforced trials (or briey, that response differentiation is statistically signicant).
This effect is important because it indicates that participants actually discriminate
between reinforced and nonreinforced CSs.
Negative transfer is indicated by a reduced amount of response differentiation in a
transfer condition from that in a control condition. To estimate whether or not there was
any negative transfer between PP and NP training, 2 3 4 3 2 factorial ANOVAs with the
within-subjects variables contingency and block and the between-subjects variable
214
LACHNIT AND KINDER
condition were computed. In all of these ANOVAs, a transfer condition (e.g., NP, second
phase), was compared with the corresponding control condition (in that example NP, rst
phase). Thus, the independent variable condition had two levels in all ANOVAs.
In these ANOVAs, transfer effects were indicated by a signicant Condition 3
Contingency interaction. For the sake of simplicity, we refrain from reporting all effects
of the ANOVAs that we computed, but give only the F and p values for the Condition
3 Contingency interaction, because this is the effect that we are interested in. The
main effect of contingency was statistically signicant in all ANOVAs that we computed
(Fs ranging from 29.6 to 84.5). The level of signicance was .05 in all statistical
analyses.
Figure 1 shows the (root-transformed) percentages of CRs to the letter compounds
and their elements (the single letters) in blocks of 12 trials each. The upper panels show
conditioned responding during PP training, and the lower panels show conditioned
responding during NP training. From the left to the right the panels show responding
in the rst phase of the sequential conditions (Panels 1 and 4), in the second phase of the
sequential conditions (Panels 2 and 5), and in the PPNPinterleaved condition (Panels 3
and 6). Note that the results in Panel 1 and Panel 5, in Panel 4 and Panel 2, and in Panel 3
and Panel 6 belong to the same group of participants, respectively. All panels show a
higher level of responding to reinforced than to nonreinforced CSs. The amount of
differentiation, however, differs among the six sets of data.
Figure 1. Square-root-transformed proportions of eyelid responses to the single letters and letter compounds
in Experiment 1. The upper panels show responding under a positive patterning schedule and the lower panels
under a negative patterning schedule. The error bars indicate the standard error of the mean.
STIMULUS REPRESENTATIONS
215
Response differentiation during PP training when NP was trained interleaved (Panel 3)
as compared to the control condition (PP, rst phase, Panel 1) was considerably reduced.
Accordingly, the ANOVA including these two conditions revealed a signicant Condition
3 Contingency interaction, F(1, 38) 5 8.5, MSE 5 0.087, p , .01. Response differentiation during NP training when PP was trained interleaved (Panel 6) as compared to the
control condition (NP, rst phase, Panel 4), was also reduced. This negative transfer
effect again was conrmed statistically: Contingency 3 Condition interaction, F(1, 38)
5 6.7, MSE 5 0.092, p , .02.
Comparisons of PP, second phase (Panel 2) with PP, rst phase (Panel 1) and of NP,
second phase (Panel 5) with NP, rst phase (Panel 4) revealed that pre-training with the
opposite type of patterning had no impact on the amount of response differentiation. The
numerical data did not indicate any negative transfer in either case. This result was
conrmed by the statistical analyses, which in both cases revealed the Contingency 3
Condition interaction to be far from statistically signicant (F , 1).
In all three groups of participants, response differentiation with PP was numerically
larger than that with NP. Three 2 3 2 3 4 factorial ANOVAs with the independent
variables patterning, contingency, and block were computed to compare the two types of
patterning in the rst and in the second phase of the sequential condition and in the PP
NPinterleaved condition, respectively. With the data of the sequential conditions, patterning was a between-subject variable and with the PPNP interleaved condition it was a
within-subject variable. There were no signicant differences in the amount of response
differentiation either between PP and NP in the rst phase, F(1, 38) 5 2.6, MSE 5
0.100, p , .15, or between PP and NP in the second phase, F , 1. However, response
differentiation with PP was signicantly larger than that with NP when both types of
patterning were trained interleaved, F(1, 19) 5 7.5, MSE 5 0.025, p , .02.
Discussion
In Experiment 1, we observed that NP and PP interfered with each other when both types
of trials were presented interleaved. It might be argued that this result is not surprising
because contingencies in PP are opposite to those in NP. Thus, a participant will learn
that the compound is reinforced in one trial but that it is nonreinforced in the next trial.
Animal learning theories have no problem in explaining why PP and NP interfere under
such conditions. However, things get more complicated if we consider that the two letters
presented during NP trials (M, J) were different from the two letters presented during PP
trials (C, N). One can only assume that organisms not only learn to respond to a particular
pattern, but also learn about the pattern as a new relevant dimension (e.g., Sutherland &
Mackintosh, 1971). This point of view goes beyond the idea that only features of individual stimuli enter into the associative structure. Alvarado and Rudy (1992) suggested
that organisms can even learn to attend to a more abstract type of conguration. In
positive and negative patterning this may be ``Number (levels ``One for elements
and ``Two for compounds) or ``Size (elements: small; compounds: large; see also
Lachnit, 2000). Support for the involvement of such representations is given by Lachnit
and Kimmel (1993), who demonstrated that participants were capable of correctly
responding to completely new letters when they had experienced PP training with a
216
LACHNIT AND KINDER
different pair of letters. The interference observed in the interleaved conditions of
Experiment 1 then can be explained as follows. In case of PP ``Large was followed by
US and ``Small was not, whereas in NP the reverse held at the same time. Thus, size (or
number) was a poor predictor of the airpuff.
In the sequential conditions of Experiment 1 there was no negative transfer either
when PP was trained after NP or when NP was trained after PP. This result is at variance
with the data reported by Bellingham et al. (1985), showing that acquisition of NP was
weak after PP training. One reason for the results being different could be that Bellingham et al. used identical CSs (tone, light) for both types of patterning whereas we used
different letter pairs. Using different letter pairs, however, did not prevent interference in
the group of participants who were trained interleaved. But in contrast to the interleaved
conditions, size (or number) could be used as a predictor for airpuff within each phase of
the sequential groups.
Another explanation for the failure to nd negative transfer in the sequential conditions can be adopted from Bellingham et al. (1985). In order to explain their result that
negative transfer occurred only with NP but not with PP, the authors suggested that the
transfer task must be sufciently difcult for interference to occur. If that assumption is
true, acquisition of NP simply might have been too easy for human participants to be
disturbed by prior PP training, at least with the large number of training trials that we
used.
EXPERIMENT 2
The purpose of Experiment 2 was to investigate whether common or separate stimulus
representations are involved in PP and NP training of the eyelid response and the SCR. If
common representations are involved in both conditioning processes, negative transfer
effects like the ones observed in Experiment 1 should also occur across response systems.
Thus, the amount of eyelid response differentiation during NP training should decrease if
it is administered interleaved with SCRPP training. Eyelid response differentiation in
that condition should be smaller than that in a control condition in which both response
systems are trained on NP. However, on the assumption that the two processes do not
involve the same stimulus representations, the amount of eyelid conditioning discrimination should not depend on the specic type of SCR conditioning schedule.
The purpose of Experiment 2 was to test these two assumptions against each other.
Every participant was presented both with eyelid conditioning and with SCR conditioning trials in random sequence. All participants received NP eyelid conditioning trials.
Every participant received one of three different types of SCR conditioning training: PP,
NP, or ``no patterning in which 50% of each AB, A, or B presentation was reinforced.
On the assumption of separate stimulus representations, no differences in eyelid response
differentiation were expected among the three groups. On the assumption of common
stimulus representations, eyelid response differentiation in the SCRPP group would be
expected to be weaker than that in the SCRNP group.
STIMULUS REPRESENTATIONS
217
Method
Participants
A total of 63 University of Marburg students took part in the experiment. They either received
course credit or were paid for participation. The data of 15 participants were excluded from the
analyses because they habituated to the airpuff during the experiment and did not show eyelid
responses on at least ve trials or because they habituated to the shock and did not show SCRs on
at least three trials. Thus, the analyses were performed on a total of 48 data sets (16 in each
condition).
Stimuli and apparatus
Different letter pairs (C, N and M, J) were used for eyelid conditioning and SCR conditioning,
respectively. In SCR conditioningthe CSUS interval was 8 s. Letter duration also was 8 s so that the
CS offset coincided with the US onset. A DC electric shock served as US. The shock was delivered
via silversilver chloride electrodes to the volar surface of the subjects left arm from an isolated
transformercondenser shock generator (Kimmel, King, Hudy, & Gardner, 1980). The intensity of
the shock was adjusted individuallyfor each subject so that it would be ``denitely unpleasant but not
really painful. Shock duration was 10 ms. Palmar skin conductance was picked up from the thenar
and hypothenar eminences of the participantsright hand by silversilver chloride electrodes (0.8 cm
in diameter) lled with an electrolytic medium (for further details see Lachnit & Kimmel, 1993). The
apparatus for eyelid conditioning and all stimulus parameters not mentioned in this section were
identical to those in Experiment 1.
Procedure
Participants were instructed that during the experiment they would occasionally feel an electric
shock to their arm or an airpuff to their eye. They were told to watch carefully what was presented in
front of them on the computer display and neither to aid nor to inhibit their eyelid responses.
Furthermore, they were told to avoid unnecessary movements and heavy breathing. The eyelid
conditioningprocedure was identical to the one in the rst phase of the NPPPsequential condition
in Experiment 1 except for the intertrial interval, which was prolonged when an SCR conditioning
trial was administered between two eyelid conditioning trials. In this case, the (eyelid) intertrial
interval was from 14 to 16 s with an average of 15 s. Eight reinforced and eight nonreinforced
SCR trials were presented in random sequence with the 96 eyelid conditioning trials. SCR conditioning trials never followed each other immediately.
Dependent variables
In eyelid conditioning the square-root-transformed proportions of CRs in blocks of 12 trials
served as a dependent variable (see Experiment 1). In SCR conditioning the maximum conductance
change occurring during the interval from 1 to 4 s after CS onset served as a dependent variable. The
conductance changes were converted to logarithmic values (after adding 1), as recommended by
Venables and Christies (1980), and were multiplied by 1,000. These values were averaged separately
for reinforced and nonreinforced trials across two trials each. Four blocks of reinforced and four
blocks of nonreinforced trials resulted.
218
LACHNIT AND KINDER
Results
For statistical analysis of the SCR data, a 2 3 4 factorial ANOVA was computed for each
of the three groups including the independent variables contingency and block. These
ANOVAs revealed that participants showed statistically signicant response differentiation both in the SCRPP group, F(1, 15) 5 23.7, MSE 5 21037.5, p , .001, and in the
SCRNP group, F(1, 15) 5 9.3, MSE 5 12248.9, p , .001. Thus, the SCR data clearly
showed that the participant successfully learned both the PP and the NP discriminations.
As expected, there was no signicant differentiation in the no patterning condition, F , 1
(note that all three CSs were reinforced on 50% of the trials, and participants therefore
could not predict the occurrence of the US).
Figure 2 shows the eyelid responses to the single letters, which were reinforced
by an airpuff, and to their nonreinforced compound in all three groups. Differentiation in all three groups was rather low. A 3 3 2 3 4 factorial ANOVA including the
independent variables condition, contingency, and block was computed. The effect of
contingency was statistically signicant, F(1, 45) 5 10.8, MSE 5 0.075, p , .01,
thus indicating response differentiation between reinforced and nonreinforced compounds. However, the Group 3 Contingency interaction was not statistically signicant, F , 1, showing that there were no signicant differences in the amount of
differentiation among the three groups.
Discussion
In Experiment 2, eyelid response differentiation did not depend on the type of SCR
training. Thus, the results clearly do not support the hypothesis that common representations are involved in the two response systems. In that case, we would have expected
eyelid discrimination to be largest when the reinforcement schedules in SCR and in eyelid
conditioning were identical. However, the results do not seem to support the hypothesis
of separate representations either, because response differentiation in all three groups was
rather weak. By contrast, response differentiation in the rst phase of the NPPP sequential condition of Experiment 1 was much larger, thus supporting the notion that eyelid
response discrimination in Experiment 2 was disturbed in all three groups. We computed
Figure 2. Square-root-transformed proportions of eyelid responses to the single letters and letter compounds
in Experiment 2. The panels show responding in the three experimental conditions (all negative patterning
discriminations), which differed with respect to the type of interleaved SCR training. The error bars indicate the
standard error of the mean.
STIMULUS REPRESENTATIONS
219
three ANOVAs to compare response differentiation in all three conditions of Experiment
2 to response differentiation in the control condition of Experiment 1. All three ANOVAs
revealed a signicant or marginally signicant Condition 3 Contingency interaction and
thus indicated that eyelid response differentiation was reduced when SCR trials were
presented interleaved: SCRNP, F(1, 34) 5 9.5, MSE 5 0.085, p , .001; SCRPP,
F(1, 34) 5 26.2, MSE 5 0.096, p , .05; SCRno patterning, F(1, 34) 5 4.0, MSE 5
0.116, p , .06.
This nding can be interpreted in two different ways. According to the rst interpretation, the two systems share general resources (e.g., concerning memory or attention)
rather than the specic resources that we are addressing in this paper. By that view, the
present result is not surprising: The eyelid conditioning process should be generally
disturbed by the concurrent SCR conditioning, independent of the type of reinforcement
schedule. According to the second interpretation, however, there are in fact differences
between the groups but these were concealed by a oor effect (note that discrimination
was rather low in all three groups). An easier task, which can be disturbed less easily,
might yield a better chance to nd differences in response differentiation being related to
the SCR conditioning schedule.
EXPERIMENT 3
Discussing the results of Experiment 2, we argued that these results alone do not allow
us to decide between the two hypotheses addressed in this paper. In order to exclude
the possibility of a oor effect concealing possible group differences, we had to choose
an eyelid conditioning task that could be solved more easily under interleaved training
conditions than in NP. In the PPNPinterleaved condition of Experiment 1, PP
discrimination was more pronounced than NP discrimination. In Experiment 3 we
therefore adopted a PP reinforcement schedule instead of the NP schedule in Experiment 2. In addition to the conditions in Experiment 2, we included one in which the
SCR training was omitted. This condition allowed for a direct comparison of eyelid
response differentiation with and without interleaved SCR conditioning within the same
experiment.
Method
Participants
A total of 91 University of Marburg students took part in the experiment. They either received
course credit or were paid for their participation. The data of 19 participants were excluded from the
analyses because they habituated to the airpuff during the experiment and did not show eyelid
responses on at least ve trials or because they habituated to the shock and did not show unconditioned SCRs on at least three trials. Thus, the analyses were performed on a total of 72 data sets (18 in
each condition).
Stimuli and apparatus
Stimuli and apparatus were the same as those in Experiment 2.
220
LACHNIT AND KINDER
Procedure
The procedure was mainly identical to that in Experiment 2. In this experiment, however, a letter
compound (MJ) was followed by an airpuff, and its elements (the single letters M and J) were
followed by nothingthat is to say, a PP schedule was employed. Furthermore, twice as many
SCR conditioning trials (with the letters C and N) as in Experiment 2 were presented, in order to
enhance a possible impact of the SCR reinforcement schedule. Participants in the fourth group
received only eyelid conditioning trials and no shocks at all. The intertrial intervals in this condition
were the same as those in Experiment 1.
Dependent variables
These were as set out in the Method section of Experiment 2.
Results and discussion
The SCR data were analysed the same way as in Experiment 2. Participants who received
PP training in the SCR showed a signicantly higher level of responding to the compound
than to the elements, F(1, 17) 5 36.4, MSE 5 24,683.7, p , .001. Furthermore, participants in the SCRNP group responded more strongly to the elements than to the
compound, F(1, 17) 5 12.7, MSE 5 3,460.3, p , .01. Thus, the SCR data revealed
that participants successfully learned both the PP and the NP discrimination. As
expected, participants in the SCRno patterning group did not show a signicant
response differentiation between reinforced and nonreinforced CSs, F(1, 17) 5 3.2,
MSE 5 10,276.7, p . .11.
Figure 3 shows the eyelid responses to the compound (MJ), which was paired with an
airpuff, and to its nonreinforced elements. In all four groups there was a considerable
difference between the levels of responding to the reinforced and to the nonreinforced
CSs. The effect of contingency was statistically signicant, F(1, 68) 5 95.2, MSE 5
0.110, p , .001, thus indicating response differentiation between reinforced elements and
nonreinforced compounds. In order to test whether there were any statistically signicant
differences in the amount of differentiation, a 4 3 2 3 4 factorial ANOVA with the
independent variables condition, contingency, and block was computed. The Condition
3 Contingency interaction was not statistically signicant, F , 1. Thus, there was no
indication of any negative transfer from the SCR conditioning process to acquisition of
PP in eyelid conditioning. The results therefore clearly support the hypothesis that
different stimulus representations are involved in solving the patterning tasks in the
two systems. Furthermore, the results suggest that eyelid differentiation being similarly
poor in all three groups of Experiment 2 was not because of a oor effect concealing the
true group differences. Rather, participants in Experiment 2 seemed to pay less attention
to the eyelid trials, because the SCR trials were more salient (the shock is much more
unpleasant than the airpuff). Of course, we do have to assume similar losses of attention in
Experiment 3. However, solving the easier PP task might have suffered to a lesser extent
from these losses than solving the more difcult NP task.
STIMULUS REPRESENTATIONS
221
Figure 3. Square-root-transformed proportions of eyelid responses to the single letters and letter compounds
in the four experimental conditions of Experiment 3 (all positive patterning discriminations). The error bars
indicate the standard error of the mean.
GENERAL DISCUSSION
The aim of our series of experiments was to investigate whether common or separate
representations are involved in solving nonlinear discrimination tasks in different
response systems. In meeting this goal, we utilized a negative transfer effect between
PP and NP, which was rst described by Bellingham et al. (1985). In Experiment 1, we
specied the conditions under which such a negative transfer effect occurs in human
eyelid conditioning. In this experiment, eyelid response discrimination in PP as well as in
NP deteriorated when the other type of patterning was trained in an interleaved fashion.
In Experiments 2 and 3 we investigated whether or not a similar transfer effect also occurs
if the interleaved training is administered in a different response system, namely the SCR
system. We argued that a negative transfer effect should occur if common representations
are used in both response systems. In that case, we would have expected a reduction of
eyelid differentiation in participants experiencing the opposite SCR reinforcement schedule from that in participants being trained on two identical reinforcement schedules.
However, neither in Experiment 2 nor in Experiment 3 did the amount of eyelid response
differentiation depend on the specic type of SCR reinforcement schedule. Although
Experiment 2 showed eyelid response differentiation to be disturbed by interleaved SCR
conditioning, this negative transfer occurred independently of the specic SCR conditioning schedule. We therefore attributed it to general attentional processes rather than to
common representations being used in the two response systems. To summarize, our
222
LACHNIT AND KINDER
results suggest that distinct rather than common representations are involved in the
conditioning of different systems.
Are our results nevertheless in accord with models that locate congural representations in central neural areas? A neuropsychological model that accounts for simultaneous
conditioning of different response systems was proposed by Schmajuk and Buhusi (1997,
see also Schmajuk, Lamoureux, & Holland, 1999). However, although we used different
USs in our experiments, Schmajuk et al.s model only assumes different CSs. This is
because the model was conceived to explain responding within the occasion-setting
paradigm, in which a single US is presented but different CSs are used (which elicit
different CRs). Therefore, the model makes no predictions for a situation in which
different USs are presented and cannot be applied to our data in its present form.
A neuropsychological model that can be applied to situations involving different USs
was proposed by Gluck and Myers (1993). According to this model, the hippocampus can
be described as an autoassociative structure that is trained to reproduce its own input. In a
conditioning experiment, this input is provided by the CSs. Furthermore, the network is
trained to predict the outcomethat is, the occurrence of the US. The autoassociative
structure contains a hidden layer in which the input information is compressed and
differentiated. As a result of learning, CSs having identical outcomes are represented
by more similar activation patterns in the hidden layer, whereas CSs having different
outcomes are represented by less similar activation patterns. In our opinion (for a related
view see Honey & Watt, 1998) this autoassociative structure may serve as a neuropsychological basis for what is called acquired equivalence and distinctiveness of cues (e.g.,
Delameter, 1998; Honey & Hall, 1989) or mediated generalization. For example, Wasserman (e.g., Astley & Wasserman, 1998; Wasserman & Astley, 1994; Wasserman, DeVolder,
& Coppage, 1992) reported that perceptual dissimilar stimuli will come to be classed
together merely because they are associated with the same response or outcome. Vice
versa stimuli should become more dissimilar because they are associated with different
responses or outcomes. The existence of such a structure and its psychological implications may help to explain the pattern of results reported in this article.
In order to explain the interference between NP and PP interleaved conditions in
Experiment 1, we suggested that the letter pairs used in the two tasks must have activated
partially identical representations (e.g., small, large). If this were the case, should not the
same have happened in Experiments 2 and 3, both of which consisted of interleaved
training? The answer is no, because of the fact that Experiment 1 on the one hand and
Experiments 2 and 3 on the other hand clearly differed with respect to the number of
outcomes. In Experiment 1 there was only one outcome (occurrence or non-occurrence of
an airpuff), whereas in Experiments 2 and 3 shock was introduced as an additional outcome. Thus, in Experiment 1 the stimuli became more similar than in Experiments 2 and
3. Therefore CSs in Experiment 1 were harder to discriminate and interfered with one
another. In Experiments 2 and 3 only C and N, either as a compound or as elements, were
ever followed by shock, whereas only M and J were ever followed by an airpuff. According
to Gluck and Myers models, the internal representations of C and N therefore should
have differentiated clearly from the internal representations of M and J. Thus, distinct
stimulus representations, involving different hidden layer representations, might have
emerged as a result of different USs being presented when the different response systems
STIMULUS REPRESENTATIONS
223
were trained. This qualitative analysis suggests that Gluck and Myers (1993) model can
account for the results of Experiments 2 and 3.
Another way to explain our results would be to assume that distinct associative structures were involved in the conditioning of the two response systems. This hypothesis,
however, would be at variance with neuropsychological models, assuming that the crucial
structure for solving nonlinear discrimination tasks are located in central neural structures such as the neocortex or the hippocampus (e.g., Gluck & Myers, 1993, 1997; Rudy
& Sutherland, 1989, 1995). On the basis of the data presented in this article, we cannot
decide between the two possible explanations. Further research, for example in the
domain of neuropsychology, is needed to understand better how the conditioning processes in different response systems interact.
REFERENCES
Alvarado, M.C., & Rudy, J.W. (1992). Some properties of congural learning: An investigation of the
transverse-patterning problem. Journal of Experimental Psychology: Animal Behavior Processes, 18,
145153.
Astley, S.L., & Wasserman, E.A. (1998). Novelty and functional equivalence in superordinate categorization by pigeons. Animal Learning & Behavior, 26, 125138.
Bellingham, W.P., Gillette-Bellingham, K., & Kehoe, E.J. (1985). Summation and conguration in
patterning schedules with the rat and rabbit. Animal Learning & Behavior, 13, 152164.
Daum, I., Schugens, M.M., Ackermann, H., Lutzenberger, W., Dichgans, J., & Birbaumer, N. (1993).
Classical conditioning after cerebellar lesion in humans. Behavioral Neuroscience, 107, 748756.
Delamater, A.R. (1998). Associative mediational processes in the acquired equivalence and distinctiveness
of cues. Journal of Experimental Psychology: Animal Behavior Processes, 24, 467482.
Fanselow, M.S. (1999). Learning theory and neuropsychology: Conguring their disparate elements in
the hippocampus. Journal of Experimental Psychology: Animal Behavior Processes, 25, 275283.
Gluck, M.A., & Myers, C.E. (1993). Hippocampal mediation of stimulus representation: A computational
theory. Hippocampus, 3, 491516.
Gluck, M.A., & Myers, C.E. (1997). Psychobiological models of hippocampal function in learning and
memory. Annual Review of Psychology, 48, 481514.
Gormezano, I. (1966). Classical conditioning. In J.B. Sidowsky (Ed.), Experimental methods and instrumentation in psychology (pp. 385420). New York: McGraw Hill.
Gormezano, I., & Gibbs, C.M. (1988). Transduction of the rabbits nictitating membrane response.
Behavior Research Methods, Instruments & Computers, 20, 1821.
Honey, R.C., & Hall, G. (1989). The acquired equivalence and distinctiveness of cues. Journal of
Experimental Psychology: Animal Behavior Processes, 15, 338346.
Honey, R.C., & Watt, A. (1998). Acquired relational equivalence: Implications for the nature of associative
structures.Journal of Experimental Psychology: Animal Behavior Processes, 24, 325334.
Hugdahl, K. (1998). Cortical control of human classical conditioning: Autonomic and positron emission
tomography data. Psychophysiology, 35, 170178.
Kimmel, H.D., King, J., Hudy, J.J., & Gardner, K.A. (1980). A mutual inductance shocker. Behavior
Research Methods & Instrumentation, 12, 605606.
Kinder, A., & Lachnit, H. (2000). Classication processes in different paradigms and response systems.
Unpublished manuscript.
Lachnit, H. (2000). Rule learning and Pavlovian conditioning: An attempt to bridge the gap. Unpublished
manuscript.
Lachnit, H., & Kimmel, H.D. (1993). Positive and negative patterning in human classical skin conductance response conditioning. Animal Learning & Behavior, 21, 314326.
Lachnit, H., & Kinder, A. (2000). Evidence for secondary or response mediated generalization in human
Pavlovian SCR and eyelid conditioning. Unpublished manuscript.
224
LACHNIT AND KINDER
LeDoux, J.E. (1995). Emotion: Clues from the brain. Annual Review of Psychology, 46, 209235.
Myers, C.E., & Gluck, M.A. (1996). Cortico-hippocampal representations in simultaneous odor discrimination: A computational interpretation of Eichenbaum, Mathews, and Cohen (1998). Behavioral
Neuroscience, 110, 685706.
Pearce, J.M. (1994). Similarity and discrimination: A selective review and a connectionist model.
Psychological Review, 101, 587607.
Rescorla, R.A., & Wagner, A.R. (1972). A theory of Pavlovian conditioning: Variations in the effectiveness
of reinforcement and nonreinforcement. In A.H. Black & W.F. Prokasy (Eds.), Classical conditioning II
(pp. 6469). New York: Appleton-Century-Crofts.
Rudy, J.W., & Sutherland, R.J. (1989). The hippocampal formation is necessary for rats to learn and
remember congural discriminations. Behavioral Brain Research, 34, 97109.
Rudy, J.W., & Sutherland, R.J. (1995). Congural association theory and the hippocampal formation: An
appraisal and reconguration. Hippocampus, 5, 375389.
Schmajuk, N.A., & Buhusi, C.V. (1997). Stimulus conguration, occasion setting, and the hippocampus.
Behavioral Neuroscience, 111, 124.
Schmajuk, N.A., & DiCarlo, J.J. (1992). Stimulus conguration, classical conditioning and hippocampal
function. Psychological Review, 99, 268305.
Schmajuk, N.A., Lamoureux, J.A., & Holland, P.C. (1999). Occasion setting: A neural network approach.
Psychological Review, 105, 332.
Sutherland, N.S., & Mackintosh, N.J. (1971). Mechanisms of animal discrimination learning. New York:
Academic Press.
Swain, R.A., & Thompson, R.F. (1993). In search of engrams. In F.M. Crinella et al. (Eds.), Brain
mechanisms: Papers in memory of Robert Thompson. Annals of the New York Academy of Sciences
(Vol. 702, pp. 2739). New York: New York Academy of Sciences.
Venables, P.H., & Christies, M.J. (1980). Electrodermal activity. In I. Martin & P.H. Venables (Eds.),
Techniques in psychophysiology (pp. 467). Chichester: Wiley.
Wagner, A.R., & Brandon, S.E. (1989). Evolution of a structured connectionist model of Pavlovian
conditioning (AESOP). In S.B. Klein & R.R. Mowrer (Eds.), Contemporary learning theories: Pavlovian conditioning and the status of learning theory (pp. 149189). Hillsdale, NJ: Lawrence Erlbaum
Associates, Inc.
Wasserman, E.A., & Astley, S.L. (1994). A behavioral analysis of concepts: Its application to pigeons and
children. In D.L. Medin (Ed.), The psychology of learning and motivation (Vol. 31, pp. 73132). San
Diego, CA: Academic Press.
Wasserman, E.A., DeVolder, C.L., & Coppage, D.J. (1992). Non-similarity-based conceptualization in
pigeons via secondary or mediated generalization. Psychological Science, 3, 374379.
Whitlow, J.W., & Wagner, A.R. (1972). Negative patterning in classical conditioning: Summation of
response tendencies to isolable and congural components. Psychonomic Science, 27, 299301.
Manuscript received 21 June 1999
Accepted revision received 29 February 2000
Das könnte Ihnen auch gefallen
- Cabo et al (2018)Dokument16 SeitenCabo et al (2018)jorge9000000Noch keine Bewertungen
- Alcalá et al (2022)Dokument12 SeitenAlcalá et al (2022)jorge9000000Noch keine Bewertungen
- Wilson & Groves (1973)Dokument7 SeitenWilson & Groves (1973)jorge9000000Noch keine Bewertungen
- 2020 MacDonald & BarryDokument15 Seiten2020 MacDonald & Barryjorge9000000Noch keine Bewertungen
- Nelson 2015Dokument31 SeitenNelson 2015jorge9000000Noch keine Bewertungen
- Priming URDokument19 SeitenPriming URjorge9000000Noch keine Bewertungen
- One-Sample T TestDokument4 SeitenOne-Sample T Testjorge9000000Noch keine Bewertungen
- Modifiability of Behavior in Tubicolous AnnelidsDokument9 SeitenModifiability of Behavior in Tubicolous Annelidsjorge9000000Noch keine Bewertungen
- 1999 Lim Et AlDokument9 Seiten1999 Lim Et Aljorge9000000Noch keine Bewertungen
- 2019 Barry Et AlDokument15 Seiten2019 Barry Et Aljorge9000000Noch keine Bewertungen
- Davis1992 PDFDokument23 SeitenDavis1992 PDFjorge9000000Noch keine Bewertungen
- Mertens 2018Dokument16 SeitenMertens 2018jorge9000000Noch keine Bewertungen
- Guidelines For Using Human Event-Related Potentials To Study Cognition: Recording Standards and Publication CriteriaDokument26 SeitenGuidelines For Using Human Event-Related Potentials To Study Cognition: Recording Standards and Publication Criteriajorge9000000Noch keine Bewertungen
- Guidelines For Using Human Event-Related Potentials To Study Cognition: Recording Standards and Publication CriteriaDokument26 SeitenGuidelines For Using Human Event-Related Potentials To Study Cognition: Recording Standards and Publication Criteriajorge9000000Noch keine Bewertungen
- One-Sample T TestDokument4 SeitenOne-Sample T Testjorge9000000Noch keine Bewertungen
- Zaksaite & Jones (2017, Conference)Dokument6 SeitenZaksaite & Jones (2017, Conference)jorge9000000Noch keine Bewertungen
- REM Simulator1 GuideDokument20 SeitenREM Simulator1 Guidejorge9000000Noch keine Bewertungen
- Loftus (2005)Dokument7 SeitenLoftus (2005)jorge9000000Noch keine Bewertungen
- REM Simulator1 GuideDokument20 SeitenREM Simulator1 Guidejorge9000000Noch keine Bewertungen
- Vogel 2015Dokument25 SeitenVogel 2015jorge9000000Noch keine Bewertungen
- Wills Et Al 2011Dokument9 SeitenWills Et Al 2011jorge9000000Noch keine Bewertungen
- Livesey, Lee, & Shone (2013) - Paper0523Dokument7 SeitenLivesey, Lee, & Shone (2013) - Paper0523jorge9000000Noch keine Bewertungen
- Aitken Et Al (2001)Dokument27 SeitenAitken Et Al (2001)jorge9000000Noch keine Bewertungen
- Aitken Et Al (2001)Dokument27 SeitenAitken Et Al (2001)jorge9000000Noch keine Bewertungen
- Le Pelley 2004 PDFDokument52 SeitenLe Pelley 2004 PDFjorge9000000Noch keine Bewertungen
- LePelley Et Al 2012Dokument13 SeitenLePelley Et Al 2012jorge9000000Noch keine Bewertungen
- Logie Et Al 1994Dokument16 SeitenLogie Et Al 1994jorge9000000Noch keine Bewertungen
- De Rammelaere Et Al 1999Dokument6 SeitenDe Rammelaere Et Al 1999jorge9000000Noch keine Bewertungen
- BiasDokument29 SeitenBiasAndreea ErimiaNoch keine Bewertungen
- Shoe Dog: A Memoir by the Creator of NikeVon EverandShoe Dog: A Memoir by the Creator of NikeBewertung: 4.5 von 5 Sternen4.5/5 (537)
- Never Split the Difference: Negotiating As If Your Life Depended On ItVon EverandNever Split the Difference: Negotiating As If Your Life Depended On ItBewertung: 4.5 von 5 Sternen4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureVon EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureBewertung: 4.5 von 5 Sternen4.5/5 (474)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeVon EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeBewertung: 4 von 5 Sternen4/5 (5783)
- Grit: The Power of Passion and PerseveranceVon EverandGrit: The Power of Passion and PerseveranceBewertung: 4 von 5 Sternen4/5 (587)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceVon EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceBewertung: 4 von 5 Sternen4/5 (890)
- The Yellow House: A Memoir (2019 National Book Award Winner)Von EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Bewertung: 4 von 5 Sternen4/5 (98)
- On Fire: The (Burning) Case for a Green New DealVon EverandOn Fire: The (Burning) Case for a Green New DealBewertung: 4 von 5 Sternen4/5 (72)
- The Little Book of Hygge: Danish Secrets to Happy LivingVon EverandThe Little Book of Hygge: Danish Secrets to Happy LivingBewertung: 3.5 von 5 Sternen3.5/5 (399)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryVon EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryBewertung: 3.5 von 5 Sternen3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnVon EverandTeam of Rivals: The Political Genius of Abraham LincolnBewertung: 4.5 von 5 Sternen4.5/5 (234)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaVon EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaBewertung: 4.5 von 5 Sternen4.5/5 (265)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersVon EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersBewertung: 4.5 von 5 Sternen4.5/5 (344)
- The Emperor of All Maladies: A Biography of CancerVon EverandThe Emperor of All Maladies: A Biography of CancerBewertung: 4.5 von 5 Sternen4.5/5 (271)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyVon EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyBewertung: 3.5 von 5 Sternen3.5/5 (2219)
- The Unwinding: An Inner History of the New AmericaVon EverandThe Unwinding: An Inner History of the New AmericaBewertung: 4 von 5 Sternen4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreVon EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreBewertung: 4 von 5 Sternen4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Von EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Bewertung: 4.5 von 5 Sternen4.5/5 (119)
- Her Body and Other Parties: StoriesVon EverandHer Body and Other Parties: StoriesBewertung: 4 von 5 Sternen4/5 (821)
- Two Examples Can Be Found and For A More Detailed Description, VisitDokument7 SeitenTwo Examples Can Be Found and For A More Detailed Description, Visitasoisson4339Noch keine Bewertungen
- Cognitive Linguistics History and Key ConceptsDokument7 SeitenCognitive Linguistics History and Key ConceptsDoris TănaseNoch keine Bewertungen
- Experimental Teaching Technique AssignmentDokument3 SeitenExperimental Teaching Technique Assignmenteddydasilva100% (1)
- College of Education: PROFED2 - The Teaching ProfessionDokument2 SeitenCollege of Education: PROFED2 - The Teaching ProfessionMichaela Tercero SalaverNoch keine Bewertungen
- 2018 Ugwoke2018Dokument8 Seiten2018 Ugwoke2018FernandoCedroNoch keine Bewertungen
- Psychomotor DomainDokument24 SeitenPsychomotor DomainJonna Bajande100% (1)
- PDF Comparison of Freud Erikson Piaget Kohlberg Theories Developmental PhenomenaDokument3 SeitenPDF Comparison of Freud Erikson Piaget Kohlberg Theories Developmental PhenomenaJohanine VillasantiagoNoch keine Bewertungen
- Rhetorical Analysis Zombie FinalDokument12 SeitenRhetorical Analysis Zombie Finalapi-610120761Noch keine Bewertungen
- LC4 Social Lit.Dokument12 SeitenLC4 Social Lit.Shaira LiabanNoch keine Bewertungen
- Myers Psychology For AP 1st Edition by David G Myers TEST BANKDokument3 SeitenMyers Psychology For AP 1st Edition by David G Myers TEST BANKCarmen8100% (1)
- Validity and reliability in research measurementsDokument8 SeitenValidity and reliability in research measurementsVíctor Hugo En Cuarentena Fernández-BedoyaNoch keine Bewertungen
- Anish Madgula - Topic Proposal - MinorDokument1 SeiteAnish Madgula - Topic Proposal - Minorapi-477533338Noch keine Bewertungen
- Research in HR1Dokument431 SeitenResearch in HR1teamhrmclub2421Noch keine Bewertungen
- Online Verbal Coaching for MBA Entrance ExamsDokument7 SeitenOnline Verbal Coaching for MBA Entrance ExamsGaurav ShahNoch keine Bewertungen
- Ethics Zoo EssayDokument2 SeitenEthics Zoo EssayChanel SalinasNoch keine Bewertungen
- Linking Theories to Practice in Seminar PresentationsDokument8 SeitenLinking Theories to Practice in Seminar PresentationsVinay WadhwaNoch keine Bewertungen
- RQ TEST - Supplemental For Session 1Dokument2 SeitenRQ TEST - Supplemental For Session 1Bello NelsonNoch keine Bewertungen
- Discourse Analysis of English General Extenders in Nigerian Newspapers EditorialsDokument32 SeitenDiscourse Analysis of English General Extenders in Nigerian Newspapers EditorialsEwata ThompsonNoch keine Bewertungen
- Job Characteristics Impact Work Attitudes Mediated by JusticeDokument24 SeitenJob Characteristics Impact Work Attitudes Mediated by Justiceinvestigacioneducativauman2Noch keine Bewertungen
- The 7 Feedback SkillsDokument4 SeitenThe 7 Feedback SkillsArpit RathiNoch keine Bewertungen
- Understanding Literary Works Through ContextDokument11 SeitenUnderstanding Literary Works Through ContextAustineGayondato100% (1)
- JRR 13-2 RabanDokument11 SeitenJRR 13-2 RabanDL GirolettiNoch keine Bewertungen
- Connect With Your Superconscious MindDokument24 SeitenConnect With Your Superconscious MindOVVCMOULINoch keine Bewertungen
- SelfhelpgroupsDokument16 SeitenSelfhelpgroupsFiroz NpNoch keine Bewertungen
- Great Man Theory of LeadershipDokument1 SeiteGreat Man Theory of LeadershipRovelynNoch keine Bewertungen
- AdjustmentDokument33 SeitenAdjustmentAnonymous nrqk2LNoch keine Bewertungen
- Fs Practice TeachingDokument8 SeitenFs Practice TeachingVanessa Garfin TambanNoch keine Bewertungen
- Teacher Leader Qualities Self AssessmentDokument7 SeitenTeacher Leader Qualities Self Assessmentapi-499253971Noch keine Bewertungen
- Teacher Interview QuetionsDokument16 SeitenTeacher Interview QuetionsEdwin TuckerNoch keine Bewertungen
- Communication ProcessDokument15 SeitenCommunication ProcessKate ParanaNoch keine Bewertungen