Beruflich Dokumente
Kultur Dokumente
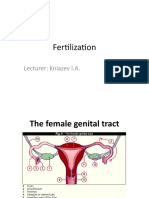
A&P CH 28a
Hochgeladen von
Sam KimOriginaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
A&P CH 28a
Hochgeladen von
Sam KimCopyright:
Verfügbare Formate
7/28/16
Chapter 28 Part A
Pregnancy
and Human
Development
Annie Leibovitz/Contact Press Images
2016 Pearson Education, Inc.
PowerPoint Lecture Slides
prepared by
Karen Dunbar Kareiva
Ivy Tech Community College
Why This Matters
Understanding of pregnancy and human
development is critical so that you can counsel
your pregnant patients about bodily changes
they will experience and educate them on fetal
development, as well as stages of the birthing
process.
2016 Pearson Education, Inc.
Video: Why This Matters
2016 Pearson Education, Inc.
7/28/16
Pregnancy and Development
Pregnancy: events that occur from fertilization
until infant is born
Conceptus: developing offspring
Gestation period: time from last menstrual
period until birth (~280 days)
Embryo: conceptus from fertilization through
week 8
Fetus: conceptus from week 9 through birth
2016 Pearson Education, Inc.
Figure 28.1 Diagrams showing the approximate size of a human conceptus from fertilization to the early fetal stage.
Embryo
Fertilization 1-week
conceptus
3-week
embryo
(3 mm)
5-week embryo
(10 mm)
8-week embryo
(22 mm)
12-week fetus
(90 mm)
2016 Pearson Education, Inc.
28.1 Fertilization
Before fertilization can occur, sperm must reach
secondary oocyte
Oocyte viable for 12 to 24 hours
Sperm viable 24 to 48 hours after ejaculation
For fertilization to occur, coitus must occur no
more than 2 days before and at least 24 hours
after ovulation
Fertilization: sperms chromosomes combine
with those of secondary oocyte to form fertilized
egg, called a zygote
2016 Pearson Education, Inc.
7/28/16
Sperm Transport and Capacitation
Most ejaculated sperm do not make the 12-cm
(5-inch) trip to join with egg
Some leak out of vagina immediately after
deposition
Some are destroyed by acidic vaginal
environment
Some fail to make it through cervix
Some are dispersed in uterine cavity or
destroyed by phagocytes
Only a few thousand out of millions reach
uterine tubes
2016 Pearson Education, Inc.
Sperm Transport and Capacitation (cont.)
Sperm must be capacitated before they can
penetrate oocyte
Motility must be enhanced, and cell membranes
must become fragile enough to release hydrolytic
enzymes
Secretions of female tract help to weaken and
thin out acrosome membrane
Sperm have olfactory receptors that can follow
chemical trail released by by egg or surrounding
cells
Sperm sniff their way to oocyte
2016 Pearson Education, Inc.
Acrosomal Reaction and Sperm Penetration
Sperm reaches oocyte by several steps:
1. Approach: aided by enzymes, sperm weaves
through corona radiata
Hyaluronidase on cell surface of sperm acts to digest
connection between granulosa cells, causing them to
separate
Sperm heads then bind to sperm-binding receptors in
zona pellucida, causing sperm membrane calcium
channels to open
Ca2+ flows into each sperm, triggering acrosomal
reaction
2016 Pearson Education, Inc.
7/28/16
Focus Figure 28.1-1 Sperm use acrosomal enzymes and receptors to approach, bind, and enter the oocyte. Blocks to polyspermy prevent further sperm entry,
ensuring that only two copies of each chromosome are present in the fertilized ovum.
Sperm, delivered to the vagina and
capacitated in the female reproductive
tract, stream toward a secondary
oocyte.
1 Approach. Aided by enzymes
on its surface, a sperm cell weaves
its way past granulosa cells of the
corona radiata.
Extracellular
space
Sperm
Sperm
Zona pellucida
Oocyte nucleus
arrested in meiotic
metaphase II
Polar body
Granulosa
cells of corona
radiata
Zona pellucida
Extracellular space
Oocyte plasma membrane
2016 Pearson Education, Inc.
Acrosomal Reaction and Sperm Penetration
(cont.)
2. Acrosomal reaction
When triggered by calcium influx, enzymes from many
sperm are released that digest holes in zona pellucida
Enzymes include hyaluronidase, acrosin, proteases,
and others
Hundreds of acrosomes in region all release enzymes
at same time to digest zona pellucida
Many are needed to clear a path to oocyte membrane
2016 Pearson Education, Inc.
Focus Figure 28.1-2 Sperm use acrosomal enzymes and receptors to approach, bind, and enter the oocyte. Blocks to polyspermy prevent further sperm entry,
ensuring that only two copies of each chromosome are present in the fertilized ovum.
2 Acrosomal reaction. Binding of
the sperm to receptors in the zona
pellucida causes Ca2+ levels within the
sperm to rise, triggering the acrosomal
reaction. Acrosomal enzymes from many
sperm digest holes through the zona
pellucida, clearing a path to the
oocyte membrane.
3 Binding. The
sperms membrane
binds to the oocytes
sperm-binding
receptors.
Oocyte sperm-binding
membrane receptors
Zona pellucida
sperm-binding
receptors
4 Fusion. Sperm
and oocyte plasma
membranes fuse.
Sperm contents enter
the oocyte.
5 Blocks to polyspermy.
Oocyte sperm-binding membrane
receptors are shed. Ca2+ levels
in the oocytes cytoplasm rise,
triggering the cortical reaction
(exocytosis of cortical granules).
As a result, the zona pellucida
hardens and the zona pellucidas
sperm-binding receptors
are clipped off.
Microtubules from
sperm flagellum
Cortical
granules
Mitochondria
Sperm
nucleus
2016 Pearson Education, Inc.
7/28/16
Acrosomal Reaction and Sperm Penetration
(cont.)
3. Binding
After path has been cleared in zona pellucida,
a single sperm forcibly swims towards oocyte
membrane
Acrosomal collar on rear portion of acrosomal
membrane binds to oocyte plasma membrane spermbinding receptor
Binding causes:
Oocyte to form microvilli that wrap around sperm head
Trigger fusing of oocyte and sperm membranes
2016 Pearson Education, Inc.
Focus Figure 28.1-2 Sperm use acrosomal enzymes and receptors to approach, bind, and enter the oocyte. Blocks to polyspermy prevent further sperm entry,
ensuring that only two copies of each chromosome are present in the fertilized ovum.
2 Acrosomal reaction. Binding of
the sperm to receptors in the zona
pellucida causes Ca2+ levels within the
sperm to rise, triggering the acrosomal
reaction. Acrosomal enzymes from many
sperm digest holes through the zona
pellucida, clearing a path to the
oocyte membrane.
3 Binding. The
sperms membrane
binds to the oocytes
sperm-binding
receptors.
Oocyte sperm-binding
membrane receptors
Zona pellucida
sperm-binding
receptors
4 Fusion. Sperm
and oocyte plasma
membranes fuse.
Sperm contents enter
the oocyte.
5 Blocks to polyspermy.
Oocyte sperm-binding membrane
receptors are shed. Ca2+ levels
in the oocytes cytoplasm rise,
triggering the cortical reaction
(exocytosis of cortical granules).
As a result, the zona pellucida
hardens and the zona pellucidas
sperm-binding receptors
are clipped off.
Microtubules from
sperm flagellum
Cortical
granules
Mitochondria
Sperm
nucleus
2016 Pearson Education, Inc.
Acrosomal Reaction and Sperm Penetration
(cont.)
4. Fusion of membranes
Oocyte and sperm membranes fuse
Cytoplasmic contents of sperm enter oocyte
Tail and other parts, such as sperm cell membrane and
mitochondria, are left behind on oocyte cell membrane
surface
2016 Pearson Education, Inc.
7/28/16
Focus Figure 28.1-2 Sperm use acrosomal enzymes and receptors to approach, bind, and enter the oocyte. Blocks to polyspermy prevent further sperm entry,
ensuring that only two copies of each chromosome are present in the fertilized ovum.
2 Acrosomal reaction. Binding of
the sperm to receptors in the zona
pellucida causes Ca2+ levels within the
sperm to rise, triggering the acrosomal
reaction. Acrosomal enzymes from many
sperm digest holes through the zona
pellucida, clearing a path to the
oocyte membrane.
3 Binding. The
sperms membrane
binds to the oocytes
sperm-binding
receptors.
Oocyte sperm-binding
membrane receptors
4 Fusion. Sperm
and oocyte plasma
membranes fuse.
Sperm contents enter
the oocyte.
5 Blocks to polyspermy.
Oocyte sperm-binding membrane
receptors are shed. Ca2+ levels
in the oocytes cytoplasm rise,
triggering the cortical reaction
(exocytosis of cortical granules).
As a result, the zona pellucida
hardens and the zona pellucidas
sperm-binding receptors
are clipped off.
Microtubules from
sperm flagellum
Cortical
granules
Zona pellucida
sperm-binding
receptors
Mitochondria
Sperm
nucleus
2016 Pearson Education, Inc.
Blocks to Polyspermy
Polyspermy does occur in some animals, but in
humans, only monospermy, in which one
sperm penetrates oocyte, is allowed
One-sperm-per-oocyte condition
Two mechanisms ensure monospermy
1. Oocyte membrane block: when a sperm binds
to sperm-binding receptor on oocyte, it causes
oocyte to shed all other sperm-binding receptors
Other sperm can no longer bind to oocyte plasma
membrane
2016 Pearson Education, Inc.
Blocks to Polyspermy (cont.)
2. Zona reaction
Also called slow block to polyspermy
Entry of sperm into oocyte triggers Ca2+ surge from
oocyte ER that causes:
Activation of oocyte to prepare for second meiotic
division
Cortical reaction: granules located just inside oocyte
plasma membrane release zonal inhibiting proteins, or
ZIP enzymes, into extracellular space below zona
pellucida
ZIPs destroy zona pellucida sperm-binding
receptors; fragments bind water and swell,
detaching any other sperm still around
2016 Pearson Education, Inc.
7/28/16
Focus Figure 28.1-2 Sperm use acrosomal enzymes and receptors to approach, bind, and enter the oocyte. Blocks to polyspermy prevent further sperm entry,
ensuring that only two copies of each chromosome are present in the fertilized ovum.
2 Acrosomal reaction. Binding of
the sperm to receptors in the zona
pellucida causes Ca2+ levels within the
sperm to rise, triggering the acrosomal
reaction. Acrosomal enzymes from many
sperm digest holes through the zona
pellucida, clearing a path to the
oocyte membrane.
3 Binding. The
sperms membrane
binds to the oocytes
sperm-binding
receptors.
4 Fusion. Sperm
and oocyte plasma
membranes fuse.
Sperm contents enter
the oocyte.
Oocyte sperm-binding
membrane receptors
Zona pellucida
sperm-binding
receptors
5 Blocks to polyspermy.
Oocyte sperm-binding membrane
receptors are shed. Ca2+ levels
in the oocytes cytoplasm rise,
triggering the cortical reaction
(exocytosis of cortical granules).
As a result, the zona pellucida
hardens and the zona pellucidas
sperm-binding receptors
are clipped off.
Microtubules from
sperm flagellum
Cortical
granules
Mitochondria
Sperm
nucleus
2016 Pearson Education, Inc.
Completion of Meiosis II and Fertilization
Events involved include:
1. Ca2+ surge triggers completion of meiosis II in
oocyte, resulting in ovum + second polar body
Ovum nucleus swells to become female pronucleus
2. As sperm nucleus moves toward oocyte
nucleus, it also swells
Forms male pronucleus
2016 Pearson Education, Inc.
Figure 28.2a-1 Events of fertilization.
Sperm nucleus
Extracellular
space
Corona
radiata
Zona
pellucida
Second meiotic
division of oocyte
1 After the sperm
penetrates the
secondary oocyte,
the oocyte completes
meiosis II, forming
the ovum and second
polar body.
Second meiotic
division of first
polar body
2016 Pearson Education, Inc.
7/28/16
Figure 28.2a-2 Events of fertilization.
Male pronucleus
Female pronucleus (swollen
ovum nucleus)
2 Sperm and ovum
nuclei swell, forming
pronuclei.
Polar bodies
2016 Pearson Education, Inc.
Completion of Meiosis II and Fertilization
(cont.)
3. DNA in each pronucleus replicates, and as
pronuclei get closer, a mitotic spindle forms
between them
Nuclear envelopes dissolve, releasing chromosomes
together in vicinity of mitotic spindle
4. Maternal and paternal chromosomes combine,
forming diploid zygote
Fertilization: moment chromosomes combine
2016 Pearson Education, Inc.
Figure 28.2a-3 Events of fertilization.
Male
pronucleus
Mitotic spindle
Centriole
Female
pronucleus
3 The DNA in each
pronucleus replicates.
The pronuclei
approach each other
and a mitotic spindle
forms between them.
2016 Pearson Education, Inc.
7/28/16
Figure 28.2a-4 Events of fertilization.
4 Chromosomes of
the pronuclei intermix.
Fertilization is
accomplished and the
cell, now called a
zygote, is ready for the
first cleavage division.
Zygote
2016 Pearson Education, Inc.
Figure 28.2 Events of fertilization.
Sperm nucleus
Extracellular
space
Corona
radiata
Zona
pellucida
Second meiotic
division of oocyte
1 After the sperm
penetrates the
secondary oocyte,
the oocyte completes
meiosis II, forming
the ovum and second
polar body.
Second meiotic
division of first
polar body
Male pronucleus
Female pronucleus (swollen
ovum nucleus)
2 Sperm and ovum
nuclei swell, forming
pronuclei.
Polar bodies
Male
pronucleus
Mitotic spindle
Centriole
Female
pronucleus
Zygote
3 The DNA in each
pronucleus replicates.
The pronuclei
approach each other
and a mitotic spindle
forms between them.
Male and female
pronuclei
4 Chromosomes of
the pronuclei intermix.
Fertilization is
accomplished and the
cell, now called a
zygote, is ready for the
first cleavage division.
Polar bodies
2016 Pearson Education, Inc.
Figure 28.2b Events of fertilization.
Male and female
pronuclei
Polar bodies
2016 Pearson Education, Inc.
7/28/16
28.2 Zygote to Blastocyst Implantation
Embryonic development continues as embryo
travels through uterine tube to uterus, where it
floats freely until it implants
Significant events in this process:
Cleavage
Blastocyst Formation
Implantation
2016 Pearson Education, Inc.
Cleavage
Occurs while zygote moves toward uterus
Rapid mitotic divisions of zygote occur
Produces cells with high surface-to-volume ratio
that enhances uptake of nutrients and oxygen
and disposal of wastes
First cleavage occurs after ~36 hours and
produces two daughter cells called blastomeres,
which continue to divide
After 72 hours, cluster of cells contains16 or
more cells and is referred to as a morula
2016 Pearson Education, Inc.
Blastocyst Formation
Around day 4 or 5, embryo, which consists of
~100 cells and is now referred to as a
blastocyst, reaches uterus
Blastocyst is fluid-filled hollow sphere composed
of:
Trophoblast cells
Display immunosuppressive factors
Participate in placenta formation
2016 Pearson Education, Inc.
10
7/28/16
Blastocyst Formation (cont.)
Inner cell mass: cluster of 2030 rounded cells
Becomes embryonic disc, which will form embryo and
three or four extraembryonic membranes
Fourth extraembryonic membrane (chorion) is formed
by trophoblast
2016 Pearson Education, Inc.
Figure 28.3 Cleavage: From zygote to blastocyst.
4-cell stage
2 days
Zygote
(fertilized egg)
Zona
pellucida
Morula (a solid ball
of blastomeres)
3 days
Early blastocyst
(Morula hollows out,
fills with fluid, and
hatches from the
zona pellucida)
4 days
Degenerating
zona
pellucida
Sperm
Blastocyst
cavity
Uterine
tube
Fertilization
(sperm
meets and
enters egg)
Implanting blastocyst
(Consists of a sphere
of trophoblast cells and
an eccentric cell cluster
called the inner cell
mass) 7 days
Ovary
Oocyte
(egg)
Trophoblast
Ovulation
Uterus
Blastocyst
cavity
Endometrium
Inner cell
mass
Cavity of
uterus
2016 Pearson Education, Inc.
28.3 Implantation and Placentation
Implantation
Blastocyst floats for about 23 days
Nourished by uterine secretions
Implantation begins 67 days after ovulation
Trophoblast cells adhere to site with proper
receptors and chemical signals
Inflammatory-like response occurs in
endometrium
Uterine blood vessels become more permeable and
leaky; inflammatory cells invade area
2016 Pearson Education, Inc.
11
7/28/16
Figure 28.4a Implantation of the blastocyst.
Endometrium
Uterine endometrial
epithelium
Inner cell mass
Trophoblast
Blastocyst cavity
Lumen of uterus
2016 Pearson Education, Inc.
Implantation (cont.)
Trophoblast cells proliferate and form two
distinct layers
Cytotrophoblast (cellular trophoblast): inner
layer of cells
Syncytiotrophoblast (syncytial trophoblast):
cells in outer layer lose plasma membranes,
becoming multinuclear mass
Send out long protrusions that invade and digest
endometrium
2016 Pearson Education, Inc.
Implantation (cont.)
As endometrium is eroded, blastocyst burrows
into lining, surrounded by pool of leaked blood
Endometrial cells then cover and seal off
implanted blastocyst
2016 Pearson Education, Inc.
12
7/28/16
Figure 28.4b Implantation of the blastocyst.
Endometrial stroma
with blood vessels
and glands
Syncytiotrophoblast
Cytotrophoblast
Blastocyst cavity
Lumen of uterus
2016 Pearson Education, Inc.
Figure 28.4c Implantation of the blastocyst.
Endometrial stroma
with blood vessels
and glands
Syncytiotrophoblast
Cytotrophoblast
Lumen of uterus
2016 Pearson Education, Inc.
Implantation (cont.)
Implantation is usually completed by day 12
after ovulation (day 26 of menstrual cycle);
about same time menstruation would occur
Corpus luteum is maintained by hCG to prevent
menstruation
2016 Pearson Education, Inc.
13
7/28/16
Implantation (cont.)
Hormone human chorionic gonadotropin (hCG):
Secreted by trophoblast cells and later chorion
Prompts corpus luteum to continue secretion of
progesterone and estrogen
Promotes placental development via its autocrine
growth factor activity
hCG levels rise until end of month 2
Decline as placenta begins to secrete progesterone
and estrogen
Low values occur at 4 months and continue for rest
of pregnancy
2016 Pearson Education, Inc.
Implantation (cont.)
In cases where implantation fails to occur,
uterus becomes nonreceptive again
About two-thirds of all zygotes formed fail to
implant by end of first week or spontaneously
abort
An estimated 30% of implanted embryos later
miscarry because of genetic defects of embryo,
uterine malformation, or unknown problems
2016 Pearson Education, Inc.
Figure 28.5 Hormonal changes during pregnancy.
Relative blood levels
Human chorionic
gonadotropin
Estrogens
Progesterone
Ovulation
and fertilization
12
16
20
24
28
Gestation (weeks)
32
36
Birth
2016 Pearson Education, Inc.
14
7/28/16
Placentation
Formation of placenta, temporary organ that
originates from both embryonic and maternal
tissues
Embryonic portion of placenta includes:
Inner cell mass, which gives rise to layer of
extraembryonic mesoderm that lines inner surface
of trophoblast
Together these structures form chorion that then
develops fingerlike projections called chorionic villi
2016 Pearson Education, Inc.
Placentation (cont.)
Chorionic villi are then invaded by new blood vessels,
which extend to embryo as umbilical arteries and vein
Continuing erosion of endometrium produces large,
blood-filled lacunae (intervillous spaces) in stratum
functionalis
Villi lie in intervillous spaces totally immersed in
maternal blood
2016 Pearson Education, Inc.
Figure 28.6a Events of placentation, early embryonic development, and extraembryonic membrane formation.
Maternal
blood vessels
Proliferating
syncytiotrophoblast
Cytotrophoblast
Amniotic cavity
Bilayered
embryonic disc
Epiblast
Hypoblast
Endometrial
epithelium
Implanting 7-day blastocyst. The syncytiotrophoblast is eroding the endometrium. Cells of
the embryonic disc are now separated from the
amnion by a fluid-filled space.
2016 Pearson Education, Inc.
15
7/28/16
Figure 28.6b Events of placentation, early embryonic development, and extraembryonic membrane formation.
Endometrium
Maternal
blood vessels
Proliferating
syncytiotrophoblast
Amnion
Cytotrophoblast
Amniotic cavity
Yolk sac
Bilayered
embryonic disc
Epiblast
Hypoblast
Extraembryonic
mesoderm
Lumen of uterus
Chorion
being formed
12-day blastocyst. Implantation is complete. Extraembryonic
mesoderm is forming a discrete layer beneath the cytotrophoblast.
2016 Pearson Education, Inc.
Figure 28.6c Events of placentation, early embryonic development, and extraembryonic membrane formation.
Lacuna (intervillous
space) containing
maternal blood
Amniotic
cavity
Primary
germ layers
Chorionic villus
Ectoderm
Chorion
Mesoderm
Amnion
Endoderm
Yolk sac
Forming
umbilical
cord
Extraembryonic
mesoderm
Lumen of uterus
Allantois
Extraembryonic
coelom
16-day embryo. Cytotrophoblast and associated mesoderm have
become the chorion, and chorionic villi are elaborating. The embryo
exhibits all three germ layers, a yolk sac, and an allantois, which
forms the basis of the umbilical cord.
2016 Pearson Education, Inc.
Placentation (cont.)
Maternal portion of placenta includes:
Decidua basalis: stratum functionalis of endometrium
located between chorionic villi and stratum basalis of
endometrium
Decidua capsularis: part of endometrium at uterine
cavity face of implanted embryo
Portion of placenta that expands to accommodate
growing fetus
Villi in decidua capsularis degenerate as fetus grows,
while villi in decidua basalis increase in number and
branches
Together chorionic villi and decidua capsularis
make up placenta
2016 Pearson Education, Inc.
16
7/28/16
Figure 28.6d Events of placentation, early embryonic development, and extraembryonic membrane formation.
Decidua basalis
Maternal blood
Chorionic villus
Umbilical blood
vessels in
umbilical cord
Amnion
Amniotic cavity
Yolk sac
Extraembryonic
coelom
Chorion
Lumen
of uterus
Decidua
capsularis
4-week embryo. The decidua capsularis, decidua basalis, amnion, and
yolk sac are well formed. The chorionic villi lie in blood-filled intervillous
spaces within the endometrium. The embryo is nourished via the umbilical
vessels that connect it (through the umbilical cord) to the placenta.
2016 Pearson Education, Inc.
Figure 28.6e Events of placentation, early embryonic development, and extraembryonic membrane formation.
Placenta
Decidua basalis
Chorionic villi
Yolk sac
Amnion
Amniotic
cavity
Umbilical
cord
Decidua
capsularis
Extraembryonic
coelom
Uterus
Lumen of
uterus
13-week fetus.
2016 Pearson Education, Inc.
Placentation (cont.)
Placenta is fully formed and functional by end of
month 3
Provides nutritive, respiratory, excretory, and
endocrine functions
Maternal and embryonic blood supplies normally
do not intermix
Embryonic placental barriers include:
Membranes of chorionic villi
Endothelium of embryonic capillaries
2016 Pearson Education, Inc.
17
7/28/16
Figure 28.7 Detailed anatomy of the vascular relationships in the mature decidua basalis.
Chorionic villi
Decidua
capsularis
Decidua basalis
Chorion
Amnion
Amniotic
fluid
Yolk sac
Placenta
Umbilical cord
Lumen of
uterus
Fetal portion
of placenta
(chorion)
Uterus
Maternal
Stratum
portion of
basalis of
placenta
endometrium
(decidua basalis)
Myometrium
Umbilical arteries
Mucous
plug
Umbilical vein
Amnion
Umbilical
cord
Maternal
veins
Connection
to yolk sac
Fetal venule
Fetal arteriole
Maternal
arteries
Maternal blood in lacuna
(intervillous space)
Chorionic villus containing
fetal capillaries
2016 Pearson Education, Inc.
Placentation (cont.)
If placental hormones are inadequate for any
reason, pregnancy is aborted
Throughout pregnancy, blood levels of
estrogens and progesterone increase
Prepare mammary glands for lactation
Placenta also secretes human placental
lactogen, human chorionic thyrotropin, and
relaxin
2016 Pearson Education, Inc.
28.4 Embryonic Development
During process of implantation, blastocyst
begins being converted into gastrula
Three germ layers, as well as extraembryonic
membranes, develop from gastrula
Inner cell mass divides into two layers: epiblast
and hypoblast
Subdivided inner cell mass is now called
embryonic disc
2016 Pearson Education, Inc.
18
7/28/16
Extraembryonic Membranes
Extraembryonic membranes form during first
23 weeks of development and include:
Amnion: epiblast cells form transparent sac filled
with amniotic fluid that envelopes embryo
Also called bag of waters, it provides buoyant
environment that protects embryo
Helps maintain constant homeostatic temperature
Allows freedom of movement; prevents parts from
fusing together
Initially, amniotic fluid comes from maternal blood;
later, fetal urine contributes to volume
2016 Pearson Education, Inc.
Extraembryonic Membranes (cont.)
Yolk sac: sac that hangs from ventral surface
of embryo
Forms part of digestive tube
Source of earliest blood cells and blood vessels
Allantois: small outpocketing at caudal end of
yolk sac
Structural base for umbilical cord
Becomes part of urinary bladder
Chorion: helps form placenta
Encloses embryonic body and all other membranes
2016 Pearson Education, Inc.
Figure 28.6a Events of placentation, early embryonic development, and extraembryonic membrane formation.
Maternal
blood vessels
Proliferating
syncytiotrophoblast
Cytotrophoblast
Amniotic cavity
Bilayered
embryonic disc
Epiblast
Hypoblast
Endometrial
epithelium
Implanting 7-day blastocyst. The syncytiotrophoblast is eroding the endometrium. Cells of
the embryonic disc are now separated from the
amnion by a fluid-filled space.
2016 Pearson Education, Inc.
19
7/28/16
Figure 28.6b Events of placentation, early embryonic development, and extraembryonic membrane formation.
Endometrium
Maternal
blood vessels
Proliferating
syncytiotrophoblast
Amnion
Cytotrophoblast
Amniotic cavity
Yolk sac
Bilayered
embryonic disc
Epiblast
Hypoblast
Extraembryonic
mesoderm
Lumen of uterus
Chorion
being formed
12-day blastocyst. Implantation is complete. Extraembryonic
mesoderm is forming a discrete layer beneath the cytotrophoblast.
2016 Pearson Education, Inc.
Figure 28.6c Events of placentation, early embryonic development, and extraembryonic membrane formation.
Lacuna (intervillous
space) containing
maternal blood
Amniotic
cavity
Primary
germ layers
Chorionic villus
Ectoderm
Chorion
Mesoderm
Amnion
Endoderm
Yolk sac
Forming
umbilical
cord
Extraembryonic
mesoderm
Lumen of uterus
Allantois
Extraembryonic
coelom
16-day embryo. Cytotrophoblast and associated mesoderm have
become the chorion, and chorionic villi are elaborating. The embryo
exhibits all three germ layers, a yolk sac, and an allantois, which
forms the basis of the umbilical cord.
2016 Pearson Education, Inc.
Figure 28.6d Events of placentation, early embryonic development, and extraembryonic membrane formation.
Decidua basalis
Maternal blood
Chorionic villus
Umbilical blood
vessels in
umbilical cord
Amnion
Amniotic cavity
Yolk sac
Extraembryonic
coelom
Lumen
of uterus
Chorion
Decidua
capsularis
4-week embryo. The decidua capsularis, decidua basalis, amnion, and
yolk sac are well formed. The chorionic villi lie in blood-filled intervillous
spaces within the endometrium. The embryo is nourished via the umbilical
vessels that connect it (through the umbilical cord) to the placenta.
2016 Pearson Education, Inc.
20
7/28/16
Figure 28.6e Events of placentation, early embryonic development, and extraembryonic membrane formation.
Placenta
Decidua basalis
Chorionic villi
Yolk sac
Amnion
Amniotic
cavity
Umbilical
cord
Decidua
capsularis
Extraembryonic
coelom
Uterus
Lumen of
uterus
13-week fetus.
2016 Pearson Education, Inc.
Gastrulation: Germ Layer Formation
Gastrulation occurs during week 3, when
embryonic disc transforms into three-layered
embryo with three primary germ layers
present:
Ectoderm, mesoderm, and endoderm
Begins with appearance of primitive streak,
a raised dorsal groove that establishes
longitudinal axis of embryo
2016 Pearson Education, Inc.
Gastrulation: Germ Layer Formation (cont.)
Cells begin to migrate into groove
First cells that enter displace hypoblast of yolk
sac and form endoderm
Cells that follow push laterally, forming
mesoderm
Notochord: rod of mesodermal cells that serves as
first axial support of embryo
Formed from aggregation of mesoderm cells
Cells that remain on embryos dorsal surface
form ectoderm (formerly epiblast layer)
2016 Pearson Education, Inc.
21
7/28/16
Gastrulation: Germ Layer Formation (cont.)
Ectoderm, mesoderm, and endoderm are
primitive tissues from which all body organs
are derived
Epithelia cells found in:
Ectodermbecome nervous system and skin
epidermis
Endodermbecome epithelial linings of
digestive, respiratory, and urogenital systems
and associated glands
Mesenchyme cells are found in mesoderm
Mesodermbecomes everything else
2016 Pearson Education, Inc.
Figure 28.8 Formation of the three primary germ layers.
Amnion
Bilayered
embryonic disc
Head end of bilayered
embryonic disc
Yolk sac
Frontal
section
3-D view
Section
view in (e)
Primitive streak
Head end
Cut edge
of amnion
Epiblast
Yolk sac
(cut edge)
Right
14-15 days
Endoderm
Hypoblast
Left
Ectoderm
Primitive
streak
Tail end
Bilayered embryonic disc, superior view
16 days
Mesoderm
Endoderm
2016 Pearson Education, Inc.
22
Das könnte Ihnen auch gefallen
- Punctuation WorksheetsDokument10 SeitenPunctuation WorksheetsRehan Sadiq100% (2)
- Subordination, Non - Disturbance and Attornment AgreementDokument7 SeitenSubordination, Non - Disturbance and Attornment AgreementDavid CromwellNoch keine Bewertungen
- Dr. Ravichandran Doraiswamy Professor of AnatomyDokument70 SeitenDr. Ravichandran Doraiswamy Professor of AnatomySiva SrinivasanNoch keine Bewertungen
- Topic: Mechanisms of Fertilization:: Presented byDokument16 SeitenTopic: Mechanisms of Fertilization:: Presented byMajani RajbonshiNoch keine Bewertungen
- A BeginningDokument12 SeitenA BeginningAmey JatharNoch keine Bewertungen
- SpermatogenesisDokument33 SeitenSpermatogenesisSalman XahirNoch keine Bewertungen
- Fertilization & ImplantationDokument61 SeitenFertilization & ImplantationchidimmaNoch keine Bewertungen
- Writing Capstone Research Project For Senior High School A Modified Guide ManualDokument9 SeitenWriting Capstone Research Project For Senior High School A Modified Guide ManualIOER International Multidisciplinary Research Journal ( IIMRJ)Noch keine Bewertungen
- Subject-Verb AgreementDokument10 SeitenSubject-Verb AgreementLouie Jay Cañada AbarquezNoch keine Bewertungen
- Heat TreatmentDokument14 SeitenHeat TreatmentAkhilesh KumarNoch keine Bewertungen
- Manufacturing Finance With SAP ERP Financials: Subbu RamakrishnanDokument33 SeitenManufacturing Finance With SAP ERP Financials: Subbu RamakrishnanKhalifa Hassan100% (1)
- Biology Investigatory ProjectDokument30 SeitenBiology Investigatory ProjectSANDIPAN BISWAS80% (5)
- PURL Questions and AnswersDokument3 SeitenPURL Questions and AnswersSHAHAN VS100% (5)
- What Is FertilizationDokument8 SeitenWhat Is Fertilizationapi-303065931Noch keine Bewertungen
- Review of Fetal DevelopmentDokument71 SeitenReview of Fetal DevelopmentlisafelixNoch keine Bewertungen
- Spermatogenesis & Sperm Maturation : Dr. Amin JanDokument33 SeitenSpermatogenesis & Sperm Maturation : Dr. Amin JanSalman XahirNoch keine Bewertungen
- Chapter 1 Part 3 FertilizationDokument16 SeitenChapter 1 Part 3 FertilizationSyeda Fizza BatoolNoch keine Bewertungen
- Biol 216 2020 Topic 2 Development - Powerpoint 2.0 Fertilization - TaggedDokument24 SeitenBiol 216 2020 Topic 2 Development - Powerpoint 2.0 Fertilization - TaggedMichael HamoudiNoch keine Bewertungen
- Fertilization and Tubal FunctionsDokument35 SeitenFertilization and Tubal Functionsv_vijayakanth7656Noch keine Bewertungen
- Lesson4 FertilizationDokument33 SeitenLesson4 Fertilizationy041087Noch keine Bewertungen
- Developmental Biology: LS 311/ 411 External FertilizationDokument48 SeitenDevelopmental Biology: LS 311/ 411 External FertilizationSankalpa ChakrabortyNoch keine Bewertungen
- FertilizationDokument35 SeitenFertilizationAlpha GamingNoch keine Bewertungen
- Spermatogenesis and FertilizationDokument21 SeitenSpermatogenesis and FertilizationAyen FornollesNoch keine Bewertungen
- 4 FertilizationDokument59 Seiten4 FertilizationQaiser InayatNoch keine Bewertungen
- Lecture 2Dokument9 SeitenLecture 2SultanNoch keine Bewertungen
- AlakeshBarman Zoology PG 2ND FERTILIZATIONDokument28 SeitenAlakeshBarman Zoology PG 2ND FERTILIZATIONTrueAlpha 16Noch keine Bewertungen
- FertilizationDokument11 SeitenFertilization137 - ShubhamNoch keine Bewertungen
- The First Week of DevelopmentDokument17 SeitenThe First Week of Developmentandersonmack047Noch keine Bewertungen
- Alakeshbarman Zoology PG 2nd FertilizationDokument28 SeitenAlakeshbarman Zoology PG 2nd Fertilizationouafia liiamaniNoch keine Bewertungen
- Prof. Dr. Çiğdem ElmasDokument49 SeitenProf. Dr. Çiğdem ElmasMuhammet Fatih CantepeNoch keine Bewertungen
- 1st WeekDokument25 Seiten1st WeekNamra NoorNoch keine Bewertungen
- 5 - Fertilization Cleavage Implantation-Dr - GosaiDokument41 Seiten5 - Fertilization Cleavage Implantation-Dr - GosaiDr.B.B.GosaiNoch keine Bewertungen
- Cellular Mechanism of Sexual ReproductionDokument20 SeitenCellular Mechanism of Sexual ReproductionJessa BelleNoch keine Bewertungen
- Embryology - Fertilization: Paper 1 - Part BDokument5 SeitenEmbryology - Fertilization: Paper 1 - Part BViswa GiriNoch keine Bewertungen
- 02 FertilizationDokument6 Seiten02 Fertilizationhiba jasimNoch keine Bewertungen
- Ferti Liz AreDokument13 SeitenFerti Liz AreDontu MariaNoch keine Bewertungen
- Human FertilizationDokument3 SeitenHuman Fertilizationtamalbio7Noch keine Bewertungen
- Contraception S7 BDokument14 SeitenContraception S7 BArsh KaiwanNoch keine Bewertungen
- Acrosome ReactionDokument4 SeitenAcrosome ReactionKuldip DwivediNoch keine Bewertungen
- Phases of Embryonic DevelopmentDokument9 SeitenPhases of Embryonic DevelopmentIshu ChoudharyNoch keine Bewertungen
- Sexual Reproduction in HumansDokument66 SeitenSexual Reproduction in Humanssherisawilliams9Noch keine Bewertungen
- Fertilization & ImplantationDokument54 SeitenFertilization & ImplantationMarvelousNoch keine Bewertungen
- Gamete Fusion and The Prevention of PolyspermyDokument9 SeitenGamete Fusion and The Prevention of Polyspermyme and allNoch keine Bewertungen
- The Growing FetusDokument62 SeitenThe Growing Fetuscoosa liquorsNoch keine Bewertungen
- FertilizationDokument26 SeitenFertilizationStrangerNoch keine Bewertungen
- Physiology of Human Reproduction SystemDokument48 SeitenPhysiology of Human Reproduction SystemWivan Havilian DjohanNoch keine Bewertungen
- Polyspermy and How To Reduce ItDokument35 SeitenPolyspermy and How To Reduce ItbrachyotisNoch keine Bewertungen
- Module 5Dokument10 SeitenModule 5Phan MhiveNoch keine Bewertungen
- Fertilization - CCRGDokument27 SeitenFertilization - CCRGTushar AgrawalNoch keine Bewertungen
- SAQ Unit 2Dokument9 SeitenSAQ Unit 2Shilpa DuttaNoch keine Bewertungen
- Semen AnalysisDokument35 SeitenSemen AnalysisSalman KhanNoch keine Bewertungen
- GA&E 13 - Fertilization & ImplantationDokument49 SeitenGA&E 13 - Fertilization & ImplantationSu ZikaiNoch keine Bewertungen
- WWW - Studyguide.pk: Aspects of Human Reproduction Key ObjectivesDokument9 SeitenWWW - Studyguide.pk: Aspects of Human Reproduction Key ObjectivesP.P.KarthikeyanNoch keine Bewertungen
- Lec 3 Gametogenesis and FertilizationDokument43 SeitenLec 3 Gametogenesis and FertilizationDacks WangNoch keine Bewertungen
- Ferterlization and ImplantationDokument113 SeitenFerterlization and Implantationmoreen kipkemoiNoch keine Bewertungen
- Pregnancy and Lactation2Dokument66 SeitenPregnancy and Lactation2jonyNoch keine Bewertungen
- Reproduction PPT 6 PHYSIOLOGY OF PREGNANCYDokument98 SeitenReproduction PPT 6 PHYSIOLOGY OF PREGNANCYlisanames.23Noch keine Bewertungen
- Conception 2021Dokument15 SeitenConception 2021Dhinesh ManoharanNoch keine Bewertungen
- FertilizationDokument8 SeitenFertilizationAmy WuNoch keine Bewertungen
- Chapter 7 FertilizationDokument44 SeitenChapter 7 Fertilizationyacla1Noch keine Bewertungen
- Fertilization, Pregnancy, and LactationDokument57 SeitenFertilization, Pregnancy, and Lactationmega anggunNoch keine Bewertungen
- Applications of Biology 9700 - Nos - As - 5Dokument9 SeitenApplications of Biology 9700 - Nos - As - 5Nana_Banana_94Noch keine Bewertungen
- 6i. Male Reproduction - OnPRC Module 2-Student HandoutDokument12 Seiten6i. Male Reproduction - OnPRC Module 2-Student HandoutAkash ShawNoch keine Bewertungen
- Fertilization Overview: Sperm PenetrationDokument13 SeitenFertilization Overview: Sperm PenetrationAmira FahruddinNoch keine Bewertungen
- 100Dokument12 Seiten100Rajat K. SNoch keine Bewertungen
- JICA Helmya DCC Building FFDokument4 SeitenJICA Helmya DCC Building FFMuhammad ElbarbaryNoch keine Bewertungen
- Assignment 1 SolutionDokument11 SeitenAssignment 1 SolutionKash TorabiNoch keine Bewertungen
- Usp3 ComDokument5 SeitenUsp3 ComMike MelgaNoch keine Bewertungen
- Engineering Data: Wireway SelectionDokument3 SeitenEngineering Data: Wireway SelectionFidel Castrzzo BaeNoch keine Bewertungen
- Logical Database Design ModelingDokument2 SeitenLogical Database Design ModelingGio Agudo100% (1)
- Tata Motors - Strategic ManagementDokument16 SeitenTata Motors - Strategic ManagementVaishakh MenonNoch keine Bewertungen
- 1623 Asm2Dokument21 Seiten1623 Asm2Duc Anh nguyenNoch keine Bewertungen
- Pte Lastest QuestionsDokument202 SeitenPte Lastest QuestionsIelts Guru ReviewNoch keine Bewertungen
- Radiology PearlsDokument2 SeitenRadiology PearlsSalman Rashid100% (2)
- Paper Cutting 6Dokument71 SeitenPaper Cutting 6Vidya AdsuleNoch keine Bewertungen
- LeaP Math G7 Week 8 Q3Dokument10 SeitenLeaP Math G7 Week 8 Q3Reymart PalaganasNoch keine Bewertungen
- BAFINAR - Quiz 2 ColarDokument3 SeitenBAFINAR - Quiz 2 ColarRonalyn ColarNoch keine Bewertungen
- Simulado InglesDokument6 SeitenSimulado InglesWandercleyson da SilvaNoch keine Bewertungen
- Ecological Building: Term Project For ME 599Dokument32 SeitenEcological Building: Term Project For ME 599Junaid AnwarNoch keine Bewertungen
- Lab5.ipynb - ColaboratoryDokument8 SeitenLab5.ipynb - ColaboratoryMin YNoch keine Bewertungen
- 3 - RA-Erecting and Dismantling of Scaffolds (WAH) (Recovered)Dokument6 Seiten3 - RA-Erecting and Dismantling of Scaffolds (WAH) (Recovered)hsem Al EimaraNoch keine Bewertungen
- Australia Visa RequirementsDokument1 SeiteAustralia Visa RequirementsJoana DetomasNoch keine Bewertungen
- Foundstone Hacme Bank User and Solution Guide v2.0Dokument60 SeitenFoundstone Hacme Bank User and Solution Guide v2.0Yeison MorenoNoch keine Bewertungen
- Richard Dennis Sonterra Capital Vs Cba Nab Anz Macquarie Gov - Uscourts.nysd.461685.1.0-1Dokument87 SeitenRichard Dennis Sonterra Capital Vs Cba Nab Anz Macquarie Gov - Uscourts.nysd.461685.1.0-1Maverick MinitriesNoch keine Bewertungen
- 44Dokument2 Seiten44menakadevieceNoch keine Bewertungen
- Orchid Group of Companies Company ProfileDokument3 SeitenOrchid Group of Companies Company ProfileAngelica Nicole TamayoNoch keine Bewertungen
- SalivaDokument42 SeitenSalivaAtharva KambleNoch keine Bewertungen
- BronchiolitisDokument5 SeitenBronchiolitisreshianeNoch keine Bewertungen