Beruflich Dokumente
Kultur Dokumente
Encefalitis Equina y Sepsis Ingles
Hochgeladen von
Katerine BonillaCopyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Encefalitis Equina y Sepsis Ingles
Hochgeladen von
Katerine BonillaCopyright:
Verfügbare Formate
Journal of Clinical Virology 42 (2008) 418421
Case report
Eastern equine encephalitis leading to
multi-organ failure and sepsis
Anita J. Reddy a, , Christopher W. Woods b ,
Karen E. Welty-Wolf a,b
a
Division of Pulmonary, Allergy and Critical Care Medicine, Department of Medicine, Durham Veterans Administration
and Duke University Medical Centers, Durham, NC 27710, United States
b Division of Infectious Diseases, Department of Medicine, Durham Veterans Administration and
Duke University Medical Centers, Durham, NC 27710, United States
Received 6 March 2008; accepted 17 March 2008
Keywords: Eastern equine encephalitis; Multi-organ failure; Myocarditis; Neurologic dysfunction; Sepsis
1. Case presentation
A 51-year-old African American male presented to his
local physician in September with dysphagia and odynophagia to solids and cervical lymphadenopathy. A computed
tomographic (CT) scan of the neck was unremarkable, and
he was discharged with treatment for pneumonia with oral
antibiotics. He returned to the hospital a month later reporting cough productive of white sputum, shortness of breath
with mild activity, and an eight pound weight loss. A chest
X-ray (CXR) showed a new left lower lobe lung opacity and
cardiomegaly, and labs were notable for leukocytosis and
elevated myoglobin. Social history revealed that he was a
non-smoker and worked as a security officer. He had served
in the Army for 20 years, stationed in the Middle East, Southeast Asia and Japan. He was building his own home in a
woody lot but did not recall any tick bites. He was admitted
to the hospital and started on intravenous antibiotics for presumed community acquired pneumonia. Over the next few
days he had worsening odynophagia, persistent fevers, and
developed elevated cardiac troponin levels, prompting treatment for acute coronary syndrome and transfer to our facility
for further care.
Abbreviations: ARDS, acute respiratory distress syndrome; CDC, Centers for Disease Control; CT, computed tomography; CXR, chest radiograph;
EEE, Eastern equine encephalitis; MRI, magnetic resonance imaging.
Corresponding author at: 9500 Euclid Avenue, Desk A90, Cleveland
Clinic Health System, Cleveland, OH 44195, United States.
Tel.: +1 216 444 4506.
E-mail address: reddya3@ccf.org (A.J. Reddy).
1386-6532/$ see front matter 2008 Elsevier B.V. All rights reserved.
doi:10.1016/j.jcv.2008.03.008
After transfer he complained of sharp short-lived chest
pain without associated nausea, vomiting, diaphoresis, lower
extremity edema, orthopnea, or paroxysmal nocturnal dyspnea. He did report myalgias and muscle weakness which
had started at the same time as his odynophagia. On neurologic exam, he did not exhibit any motor weakness or any
other focal abnormalities. Fevers persisted despite negative
blood cultures, and over the following week he developed
transaminitis, acute renal failure, thrombocytopenia, anemia,
altered mental status, and worsening pulmonary infiltrates
with hypoxemic respiratory failure requiring mechanical ventilation. Laboratory data on arrival was also notable for
leukocytosis with a neutrophilic predominance, metabolic
acidosis, and elevated CPK and aldolase. Arterial blood gas
revealed pH 7.41, PCO2 32.4 Torr (2.2 kPa), PO2 76.5 Torr
(5.2 kPa) on 0.6 FiO2 . A lumbar puncture was performed and
cerebrospinal fluid revealed 82 red cells, 10 white cells with
55% monocytes, 21% lymphocytes, 19% bands, and elevated
protein of 89 and glucose 92. Blood, sputum, and CSF cultures and cryptococcal antigen were negative. Sedimentation
rate was elevated to 87, but rheumatologic studies were otherwise unremarkable. Chest radiograph and chest CT revealed
bilateral infiltrates consistent with acute lung injury.
Because of the patients persistent, unexplained fever,
myositis and myocarditis, and altered mental status, a
search for unusual infectious etiologies was undertaken.
Bronchoscopy with bronchoalveolar lavage was performed
and cultures for bacteria, fungi, viruses and opportunistic
organisms were negative. A muscle biopsy showed muscle fiber atrophy but no evidence of inflammation. Electron
A.J. Reddy et al. / Journal of Clinical Virology 42 (2008) 418421
419
was also severe neuronal loss and gliosis in the dorsal motor
nucleus of the vagus nerve, possibly representing an older,
inactive inflammatory process and would most probably be
associated with symptoms of autonomic or cranial nerve dysfunction.
2. Discussion
2.1. Eastern equine encephalitis
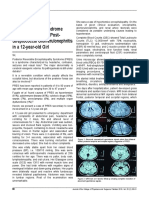
Fig. 1. Brain magnetic resonance imaging: T2 (A) and T1 (B) weighted
images. T2 prolongation in bilateral thalami, bilateral basal ganglia and right
posterior parietal cortex.
microscopy of muscle tissue demonstrated focal clearing of
the mitochondrial matrix, occasional mitochondrial inclusions, and decreased cristae consistent with sepsis or viral
myositis. Head CT without intravenous contrast was unremarkable but subsequent brain magnetic resonance imaging
(MRI) showed T2 prolongation in bilateral thalami and basal
ganglia, as well as the right posterior parietal cortex (Fig. 1).
Serologies for Eastern equine encephalitis (EEE) revealed a
positive EEE IgG at a titer of 1:128 and EEE IgM titer of
1:16. Other arboviral titers, including West Nile virus and
Western equine encephalitis serologies were negative. Confirmatory testing for EEE by plaque reduction neutralization
test (PRNT) revealed a titer of 1:640, consistent with acute
EEE infection.
The patients hospital course was complicated by sepsis and continued multi-organ failures of the lung, liver,
heart, brain and kidneys. The patient also exhibited signs
of neurologic dysfunction, including uncontrollable tachypnea despite adequate ventilation and labile blood pressure.
Profound ventilatory drive persisted despite liberal use of
sedatives and narcotics. Despite aggressive treatment the
patient failed to improve neurologically and passed away after
withdrawal of care.
Autopsy showed diffuse alveolar damage consistent with
acute respiratory distress syndrome (ARDS), passive congestion in the liver, esophageal erosion, tracheitis, and cystitis.
Neuropathology revealed marked encephalitis in the right
basal ganglia and modest inflammation in the pons. There
Eastern equine encephalitis is a mosquito-borne viral
disease which occurs mostly in the eastern half of the
United States. The EEE virus is a member of the alphavirus
genus and Togaviridae family1 and is transmitted to humans
through mosquitos, although viral replication and transmission cycles take place between birds and mosquitos.
Horses are the most frequent incidental hosts, and outbreaks of infection in horses commonly precede human
cases. The most common species of mosquito infected with
EEE is Culiseta melanura, but infected Anopheles sollicitans and A. vexans are the most likely vectors for infection
in mammals.2
EEE causes only about 1% of all cases of viral encephalitis in the United States each year, but due to mortality rates
of approximately 33%, it is considered one of the most serious mosquito-borne diseases. Individuals older than 50 and
younger than 15 are at the greatest risk of developing severe
disease. There have been approximately 220 confirmed cases
in the U.S. from 1964 to 2004, with most cases occurring
between June and October.1
Symptomatic EEE typically presents as a mild flu-like illness 310 days after being bitten by an infected mosquito.
There are no specific laboratory abnormalities characteristic
of the disease, although these patients may have leukocytosis and hyponatremia. EEE infection may also lead to rapid
progression of encephalitis and coma by spread to the central nervous system through the blood. Involvement of the
brain is manifested histologically by necrosis, neutrophilic
infiltrates and meningitis, as well as an acute vasculitis.
Necrosis generally involves the gray matter and spares the
spinal cord.3
EEE is difficult to diagnose due to its nonspecific symptoms and the Centers for Disease Control case definition
requires the presence of at least one clinical and one laboratory criteria.4 Confirmation requires clinical symptoms
(neuroinvasive or non-neuroinvasive) and specific serologic
findings or documentation of the virus in cerebrospinal fluid
or brain tissue (Table 1). Laboratory diagnosis includes IgM
testing of serum and cerebrospinal fluid and neutralizing antibody testing of acute and convalescent phase serum. Imaging
studies of the brain typically indicate involvement of the basal
ganglia, thalami, and brainstem.3,5
There is no specific treatment available for EEE and therapy is directed towards supportive care. Approximately half
of the patients who survive EEE have mild to severe perma-
420
A.J. Reddy et al. / Journal of Clinical Virology 42 (2008) 418421
Table 1
Centers for Disease Controls case definition for confirmed or probable EEE
Criteria (Denition of case requires at least one clinical and one
laboratory criteria)
CLINICAL
Neuroinvasive
Fever and one of the following (in the absence of a more likely
clinical explanation)
Acutely altered mental status (e.g., disorientation, obtundation, stupor,
or coma), or
Other acute signs of central or peripheral neurologic dysfunction (e.g.,
paresis or paralysis, nerve palsies, sensory deficits, abnormal reflexes,
generalized convulsions, or abnormal movements), or
Pleocytosis (increased white blood cell concentration in cerebrospinal
fluid [CSF]) associated with illness clinically compatible with meningitis
(e.g., headache or stiff neck)
Non-neuroinvasive
Presence of documented fever, and
Absence of neuroinvasive disease, and
Absence of more likely explanation for clinical illness
LABORATORY
Conrmed
Fourfold or greater change in virus-specific serum antibody titer, or
Isolation of virus from or demonstration of specific viral antigen or
genomic sequences in tissue, blood, CSF, or other body fluid, or
Virus-specific immunoglobulin M (IgM) antibodies demonstrated in
CSF by antibody-capture enzyme immunoassay (EIA), or
Virus-specific IgM antibodies demonstrated in serum by
antibody-capture EIA and confirmed by demonstration of virus-specific
serum immunoglobulin G (IgG) antibodies in the same or a later specimen
by another serologic assay (e.g., neutralization or hemagglutination
inhibition)
Probable
Stable (less than or equal to a twofold change) but elevated titer of
virus-specific serum antibodies, or
Virus-specific serum IgM antibodies detected by antibody-capture
EIA but with no available results of a confirmatory test for virus-specific
serum IgG antibodies in the same or a later specimen
inappropriate tachypnea. Viral encephalitis involving the
hypothalamus and brainstem has been shown in case reports
to be associated with Ondines curse and failure of automatic
control of ventilation,6 including central hyperventilation
syndromes. Viral infections involving the brain can also lead
to vagal impairment and systemic sympathetic upregulation
and subsequent change in receptor sensitivity of autonomic
reflexes.7 Another consideration could be dysfunction of the
autonomic centers in the thalamus or hypothalamus resulting in the condition termed paroxysmal autonomic instability
which can occur after various types of brain injury.8 Another
explanation for this patients tachypnea could be due to
damage or inflammation to the upper brainstem reticular
formation, resulting in central neurogenic hyperventilation
(CNH). Most cases of CNH in the literature are due to infiltrative processes or malignancy.9
One final characteristic of our case that has not been previously described with EEE is the prominent myocarditis,
which in our case was characterized by persistent elevation in cardiac markers, as well as atrial and ventricular
dysrhythmias. This was not attributable clinically to other
complicating events and was presumed due to his primary
viral infection. Although myocardial involvement is well
described in infection with other members of the Togavirus
family,10,11 it has not been reported with EEE in humans.
Notably, myositis is a reported, albeit unusual, feature of EEE
infection in other mammals,12 and it is possible that the previous absence in descriptions of the disease in humans is
related to the relative paucity of infections.
In summary, we report a patient with an unusual presentation of Eastern equine encephalitis, characterized by subacute
onset, clinical myocardial involvement, and abnormalities
in autonomic function and respiratory control complicating
supportive management.
Adapted from CDC website.4
nent neurologic damage. Studies have shown that patients
with an elevated white blood cell count in the cerebrospinal
fluid and severe hyponatremia had poorer outcomes. Patients
treated with anticonvulsants or steroids also had poorer outcomes that approached statistical significance.3
This patients course is particularly interesting in that his
subacute onset and presenting symptoms of odynophagia and
dysphagia were unusual. Sore throat has been previously
reported in acute presentation of EEE,5 though we did not
have early assessment of bulbar function to determine if his
dysphagia represented early neurologic involvement or vagus
nerve dysfunction.
In addition to the atypical presentation, our case illustrates
difficulties inherent in supporting critically ill patients with
infectious encephalitides. The pathologic examination of this
patients brain suggested involvement of areas responsible for
autonomic and respiratory control, and there was substantial clinical evidence of autonomic dysfunction, including
labile blood pressure and the patients uncontrollable and
References
Centers for Disease Control Eastern equine encephalitis fact sheet. Available online at: http://www.cdc.gov/ncidod/dvbid/arbor/eeefact.htm.
Accessed August 25, 2007.
Hirsch MS, Werner B. Case 17-2003: a 38-year-old woman with fever,
headache, and confusion. NEJM 2003;348(22):223947.
Deresiewicz RL, Thaler SJ, Hsu L, Zamani AA. Clinical and neuroradiographic manifestations of Eastern equine encephalitis. NEJM
1997;336(26):186774.
Centers for Disease Control Eastern equine encephalitis case definition. Available online at: www.cdc.gov/epo/dphsi/casedef/arboviral
current.htm. Accessed June 10, 2007.
Lury KM, Castillo M. Eastern equine encephalitis: CT and MRI findings in
one case. Emerg Rad 2004;11:468.
Giangaspero F, Schiavina M, Sturani C, Mondini S, Cirignotta F. Failure of
automatic control of ventilation (Ondines curse) associated with viral
encephalitis of the brainstem: a clinicopathologic study of one case. Clin
Neuropathol 1988;7(5):2347.
Yun AJ, Lee PY, Bazar KA. Modulation of autonomic balance by tumors
and viruses. Med Hypotheses 2004;63(2):34451.
Blackman JA, Patrick PD, Buck ML, Rust RS. Paroxysmal autonomic instability with dystonia after brain injury. Arch Neurol 2004;61:3218.
A.J. Reddy et al. / Journal of Clinical Virology 42 (2008) 418421
Tarulli AW, Lim C, Bui JD, Saper CB, Alexander MP. Central neurogenic
hyperventilation: a case report and discussion of pathophysiology. Arch
Neurol 2005;62:16324.
Monath TP, Kemp GE, Cropp CB, Chandler FW. Necrotizing myocarditis in mice infected with Western equine encephalitis virus: clinical,
electrocardiographic, and histopathologic correlations. J Infect Dis
1978;138:5966.
421
Harada T, Ohtaki E, Tobaru T, Kitahara K, Sumiyoshi T, Hosoda S. Rubellaassociated perimyocarditisa case report. Angiology 2002;53:727
32.
Elvinger F, Liggett AD, Tang KN, Harrison LR, Cole Jr JR, Baldwin CA, et
al. Eastern equine encephalitis virus infection in swine. J Am Vet Med
Assoc 1994;205:10146.
Das könnte Ihnen auch gefallen
- Neurology Multiple Choice Questions With Explanations: Volume IIVon EverandNeurology Multiple Choice Questions With Explanations: Volume IIBewertung: 5 von 5 Sternen5/5 (2)
- KU SyllabusDokument181 SeitenKU Syllabuskumar_124Noch keine Bewertungen
- Acquired NeuropathiesDokument106 SeitenAcquired NeuropathiesDanny J. BrouillardNoch keine Bewertungen
- Anti-Factor B Antibodies and Acute Postinfectious GN in ChildrenDokument12 SeitenAnti-Factor B Antibodies and Acute Postinfectious GN in ChildrenCarlos DominguezNoch keine Bewertungen
- BI309 Practical 6Dokument8 SeitenBI309 Practical 6SanahKumar100% (1)
- Course Outline General Biology II: Course Code (S) and Mesrs Objectives Science (200.B0), Registered in 101-LCU-05Dokument12 SeitenCourse Outline General Biology II: Course Code (S) and Mesrs Objectives Science (200.B0), Registered in 101-LCU-05Nicole GuNoch keine Bewertungen
- 278 977 1 SMDokument2 Seiten278 977 1 SMMohamed MukhrizNoch keine Bewertungen
- A Review On Dengue and Treatments 13 23Dokument3 SeitenA Review On Dengue and Treatments 13 23Karina Mega WNoch keine Bewertungen
- Holanda Et Al - J Neurovirol 2022Dokument4 SeitenHolanda Et Al - J Neurovirol 2022jtd9h2xp5hNoch keine Bewertungen
- Infective EndocarditisDokument5 SeitenInfective Endocarditismustafa malvi100% (1)
- Guillain-Barre Syndrome or Is It?Dokument4 SeitenGuillain-Barre Syndrome or Is It?ThimiNoch keine Bewertungen
- Steroid Pulse Therapy in Herpes Simplex Encephalitis: Neurosciences July 2013Dokument3 SeitenSteroid Pulse Therapy in Herpes Simplex Encephalitis: Neurosciences July 2013Dwi KurniawanNoch keine Bewertungen
- Clinical Reasoning: A Young Man With Recurrent Paralysis, Revisable White Matter Lesions and Peripheral Neuropathy Word Count: 945Dokument13 SeitenClinical Reasoning: A Young Man With Recurrent Paralysis, Revisable White Matter Lesions and Peripheral Neuropathy Word Count: 945aileenzhongNoch keine Bewertungen
- Jurnal ChikungunyaDokument4 SeitenJurnal ChikungunyaFitri Nadia SilvaniNoch keine Bewertungen
- Acv AjemDokument3 SeitenAcv AjemYS NateNoch keine Bewertungen
- Advance Publication by J-STAGE: Japanese Journal of Infectious DiseasesDokument8 SeitenAdvance Publication by J-STAGE: Japanese Journal of Infectious DiseasesEdgar Azael GarciaNoch keine Bewertungen
- Journal of Medical Case ReportsDokument4 SeitenJournal of Medical Case ReportsSri Wahyuni YkNoch keine Bewertungen
- A Case of Male Sle: An Unusual PresentationDokument4 SeitenA Case of Male Sle: An Unusual PresentationIJAR JOURNALNoch keine Bewertungen
- Case Report: Vertigo As A Predominant Manifestation of NeurosarcoidosisDokument5 SeitenCase Report: Vertigo As A Predominant Manifestation of NeurosarcoidosisDjumadi AkbarNoch keine Bewertungen
- A 19 Year Old With Fever, Rash, and Conjunctivitis: A Connection With The HeartDokument3 SeitenA 19 Year Old With Fever, Rash, and Conjunctivitis: A Connection With The HeartLeberina TunjNoch keine Bewertungen
- Immune Reconstitution Inflammatory Syndrome and Cerebral ToxoplasmosisDokument2 SeitenImmune Reconstitution Inflammatory Syndrome and Cerebral ToxoplasmosisFirda PotterNoch keine Bewertungen
- Preprints202009 0544 v2Dokument10 SeitenPreprints202009 0544 v2matsuyamateoNoch keine Bewertungen
- Case Series and Case Reports: Pure Cerebellitis Due To Scrub Typhus: A Unique Case ReportDokument2 SeitenCase Series and Case Reports: Pure Cerebellitis Due To Scrub Typhus: A Unique Case ReportMaureen KoesnadiNoch keine Bewertungen
- Eastern Equine Encephalitis: A Classical Case: Connecticut Medicine October 2014Dokument4 SeitenEastern Equine Encephalitis: A Classical Case: Connecticut Medicine October 2014Chrislyn SanlaoNoch keine Bewertungen
- Infectious Dan Autoantibody Associated EncephalitisDokument13 SeitenInfectious Dan Autoantibody Associated EncephalitisAyudiah ParamitaNoch keine Bewertungen
- A Cohort Study To Assess The New WHO Japanese Encephalitis Surveillance StandardsDokument9 SeitenA Cohort Study To Assess The New WHO Japanese Encephalitis Surveillance StandardsarmankoassracunNoch keine Bewertungen
- Case Report: A Case of Herpes Simplex Virus-1 Encephalitis From A Medicolegal Point of ViewDokument4 SeitenCase Report: A Case of Herpes Simplex Virus-1 Encephalitis From A Medicolegal Point of Viewzefri suhendarNoch keine Bewertungen
- Acute Encephalitis Caused by Intrafamilial Transmission of Enterovirus 71 in AdultDokument7 SeitenAcute Encephalitis Caused by Intrafamilial Transmission of Enterovirus 71 in AdultAdhi SyukriNoch keine Bewertungen
- Neurology and Neurotherapy: ClinmedDokument4 SeitenNeurology and Neurotherapy: ClinmedChristian Hasudungan NainggolanNoch keine Bewertungen
- 72 - Progressive Multifocal Leukoencephalopathy in An Immunocompetent PatientDokument7 Seiten72 - Progressive Multifocal Leukoencephalopathy in An Immunocompetent PatientFaras ArinalNoch keine Bewertungen
- Case Report Tuberculosis of The Spine (Pott's Disease) Presenting As 'Compression Fractures'Dokument5 SeitenCase Report Tuberculosis of The Spine (Pott's Disease) Presenting As 'Compression Fractures'tari_margonoNoch keine Bewertungen
- Systemic AmiloidosisDokument4 SeitenSystemic AmiloidosisioannesturrisoricisNoch keine Bewertungen
- Journal Herpes Simplex EncephalitisDokument2 SeitenJournal Herpes Simplex EncephalitissavinaumarNoch keine Bewertungen
- Management of Infective Endocarditis: Valve DiseaseDokument17 SeitenManagement of Infective Endocarditis: Valve DiseaseLaura AmaliaNoch keine Bewertungen
- Toscana Virus Meningo-Encephalo-MyelitisDokument1 SeiteToscana Virus Meningo-Encephalo-MyelitisasmaNoch keine Bewertungen
- Case Report Herpes EncephalitisDokument3 SeitenCase Report Herpes EncephalitisAyu WindyaningrumNoch keine Bewertungen
- 1 s2.0 S1607551X09702748 MainDokument6 Seiten1 s2.0 S1607551X09702748 MainAbdul RaufNoch keine Bewertungen
- HIV Hemicorea CNS Histoplasmosis 2016Dokument3 SeitenHIV Hemicorea CNS Histoplasmosis 2016Juan Salazar PajaresNoch keine Bewertungen
- Progressive Multifocal Leukoencephalopathy - A Case: Ovidiu Rosca, Elena-Cecilia Rosca, Lucian NegrutiuDokument5 SeitenProgressive Multifocal Leukoencephalopathy - A Case: Ovidiu Rosca, Elena-Cecilia Rosca, Lucian Negrutiunoorhadi.n10Noch keine Bewertungen
- JurnalDokument9 SeitenJurnalMelita Amalia AyubaNoch keine Bewertungen
- Acute Disseminated Encephalomyelitis in Chicken Pox: Case ReportDokument2 SeitenAcute Disseminated Encephalomyelitis in Chicken Pox: Case ReportCorneliu VladNoch keine Bewertungen
- Adem LikeDokument4 SeitenAdem LikeRodrigo AriasNoch keine Bewertungen
- Unusual Cause of Fever in A 35-Year-Old Man: ECP YuenDokument4 SeitenUnusual Cause of Fever in A 35-Year-Old Man: ECP YuenhlNoch keine Bewertungen
- Urinary Tract Infection Presenting With Nonspecific Complaints and Normal Urinalysis in 84-Year-Old WomanDokument3 SeitenUrinary Tract Infection Presenting With Nonspecific Complaints and Normal Urinalysis in 84-Year-Old WomanAmeldaNoch keine Bewertungen
- Meningoencephalitis and New Onset of Seizures in A Patient With Normal Brain CT and Multiple Lesions On MRIDokument3 SeitenMeningoencephalitis and New Onset of Seizures in A Patient With Normal Brain CT and Multiple Lesions On MRITryas YulithaNoch keine Bewertungen
- Covid-Stroke 1 PDFDokument18 SeitenCovid-Stroke 1 PDFMelya UmbulNoch keine Bewertungen
- The Dangers of PRESDokument5 SeitenThe Dangers of PRESShahnaaz ShahNoch keine Bewertungen
- Clinical Presentation: by Dr. Raffiq AbbasDokument36 SeitenClinical Presentation: by Dr. Raffiq AbbasKarthick UnleashNoch keine Bewertungen
- Elena HepDokument2 SeitenElena HepMirela IoanaNoch keine Bewertungen
- Adult-Onset Still's Disease Mimicking Acute Rheumatic Fever: Akut Romatizmal Ateş'i Taklit Eden Erişkin Still HastalığıDokument3 SeitenAdult-Onset Still's Disease Mimicking Acute Rheumatic Fever: Akut Romatizmal Ateş'i Taklit Eden Erişkin Still HastalığıNanda AungaungNoch keine Bewertungen
- Acute Viral Encephalitis - NEJMDokument17 SeitenAcute Viral Encephalitis - NEJMFredghcNoch keine Bewertungen
- 1471 2334 12 240 PDFDokument4 Seiten1471 2334 12 240 PDFBreno PontesNoch keine Bewertungen
- Cardiac Leptospirosis: Iralphuaborque Md14thbatch Hds DocharlabardaDokument15 SeitenCardiac Leptospirosis: Iralphuaborque Md14thbatch Hds DocharlabardaJr. CesingNoch keine Bewertungen
- Eritema Nodoso: Causas Más Prevalentes en Pacientes Que Se Hospitalizan para Estudio, y Recomendaciones para El DiagnósticoDokument7 SeitenEritema Nodoso: Causas Más Prevalentes en Pacientes Que Se Hospitalizan para Estudio, y Recomendaciones para El DiagnósticoCamilo PerillaNoch keine Bewertungen
- Osmotic Demyelination Syndrome.Dokument5 SeitenOsmotic Demyelination Syndrome.Nontaphon PiyawattanamethaNoch keine Bewertungen
- JMV 25888-2Dokument8 SeitenJMV 25888-2Cynthia Torres-GonzálezNoch keine Bewertungen
- Mediterranean Spotted Fever With Encephalitis: Case ReportDokument5 SeitenMediterranean Spotted Fever With Encephalitis: Case Reportracut_khansatraNoch keine Bewertungen
- HiperesosinofiliaDokument3 SeitenHiperesosinofiliaAlifa Hasya NadhiraNoch keine Bewertungen
- Lupus Primary CareDokument22 SeitenLupus Primary Carewoopdeedoo903Noch keine Bewertungen
- Laporan Kasus-Acute Embolic Stroke As The Sole Presentation of Infective Endocarditis in Mitral Valve ProlapseDokument2 SeitenLaporan Kasus-Acute Embolic Stroke As The Sole Presentation of Infective Endocarditis in Mitral Valve ProlapseSari Novianty SilitongaNoch keine Bewertungen
- Encefalitis Autoinmune 2011 PDFDokument11 SeitenEncefalitis Autoinmune 2011 PDFDiana Vanessa SuarezNoch keine Bewertungen
- CaseDokument24 SeitenCasecintaNoch keine Bewertungen
- Enterovirus ImagingDokument7 SeitenEnterovirus ImagingMomeneo NeoNoch keine Bewertungen
- Posterior Reversible Encephalopathy Syndrome Secondary To Acute Post-Streptococcal Glomerulonephritis in A 12-Year-Old GirlDokument2 SeitenPosterior Reversible Encephalopathy Syndrome Secondary To Acute Post-Streptococcal Glomerulonephritis in A 12-Year-Old GirlwawaningNoch keine Bewertungen
- Biology GlossaryDokument93 SeitenBiology Glossaryjudo1964beginsNoch keine Bewertungen
- Immunoglobulins - Structure and Function Definition: Immunoglobulins (Ig)Dokument9 SeitenImmunoglobulins - Structure and Function Definition: Immunoglobulins (Ig)Valdez Francis ZaccheauNoch keine Bewertungen
- Hypersensitivity: - When The Immune System OverreactsDokument37 SeitenHypersensitivity: - When The Immune System Overreactsapi-19916399Noch keine Bewertungen
- 2012 - Prosiding Seminar Internasional UNSYAHDokument5 Seiten2012 - Prosiding Seminar Internasional UNSYAHSayu Putu Yuni ParyatiNoch keine Bewertungen
- Disease and Immunity P1Dokument8 SeitenDisease and Immunity P1Soliman MNoch keine Bewertungen
- Is FinalsDokument78 SeitenIs FinalsMarissa CordovaNoch keine Bewertungen
- Slots 25-26Dokument13 SeitenSlots 25-26Thúy An NguyễnNoch keine Bewertungen
- Veeda - Vaccine Final BrochureDokument8 SeitenVeeda - Vaccine Final BrochureSanket SawantNoch keine Bewertungen
- Vaccines Depopulation PDFDokument11 SeitenVaccines Depopulation PDFSidNoch keine Bewertungen
- The Many Faces of Monoclonal GammopathiesDokument44 SeitenThe Many Faces of Monoclonal GammopathiesimagigatoNoch keine Bewertungen
- Leukemia 2009 - CMV EBVDokument6 SeitenLeukemia 2009 - CMV EBVmgounari7293Noch keine Bewertungen
- Working With MicrospheresDokument20 SeitenWorking With MicrospheresSreeman MypatiNoch keine Bewertungen
- CC 11 3611Dokument16 SeitenCC 11 3611Sergeat18BNoch keine Bewertungen
- Zoologyall SemsDokument64 SeitenZoologyall SemsCh GovardhanNoch keine Bewertungen
- Immunology Exam Q S With AnswersDokument28 SeitenImmunology Exam Q S With AnswersIceBearNoch keine Bewertungen
- Biochem Midterm ReviewerDokument19 SeitenBiochem Midterm ReviewerERIKA ROSE ALEJONoch keine Bewertungen
- Efficacy of Differentiated Instruction On Photosynthesis and Cell Respiration Using Google ClassroomDokument7 SeitenEfficacy of Differentiated Instruction On Photosynthesis and Cell Respiration Using Google Classroomjordy ramosNoch keine Bewertungen
- Immunology Mcqs-4: InstructionsDokument22 SeitenImmunology Mcqs-4: InstructionsadehkordiNoch keine Bewertungen
- Evaluation of Allergenicity of Genetically Modified FoodsDokument30 SeitenEvaluation of Allergenicity of Genetically Modified FoodsChirag EducationNoch keine Bewertungen
- Immunology in Haematology (Part 1)Dokument48 SeitenImmunology in Haematology (Part 1)kiedd_04100% (4)
- Questions ExplanationDokument20 SeitenQuestions ExplanationnomintmNoch keine Bewertungen
- Practice Exam - Qats PDFDokument53 SeitenPractice Exam - Qats PDFsdfbgshfgNoch keine Bewertungen
- IPHA PatentDokument55 SeitenIPHA PatentCharles GrossNoch keine Bewertungen
- The Bodys Defence - MechanismDokument74 SeitenThe Bodys Defence - Mechanismagarwaljenish2005Noch keine Bewertungen
- Immunology Mcqs-IV (Gate Helpline)Dokument4 SeitenImmunology Mcqs-IV (Gate Helpline)Santhosh Kalash50% (2)
- Modern Blood Banking and Transfusion Practices 6th Edition Harmening Test BankDokument9 SeitenModern Blood Banking and Transfusion Practices 6th Edition Harmening Test Bankxaviaalexandrawp86i100% (28)