Beruflich Dokumente
Kultur Dokumente
4.2 Notes
Hochgeladen von
Trinh Tat-Tran0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
6 Ansichten2 SeitenCHEM151 Notes
Originaltitel
4.2 notes
Copyright
© © All Rights Reserved
Verfügbare Formate
DOCX, PDF, TXT oder online auf Scribd lesen
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCHEM151 Notes
Copyright:
© All Rights Reserved
Verfügbare Formate
Als DOCX, PDF, TXT herunterladen oder online auf Scribd lesen
0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
6 Ansichten2 Seiten4.2 Notes
Hochgeladen von
Trinh Tat-TranCHEM151 Notes
Copyright:
© All Rights Reserved
Verfügbare Formate
Als DOCX, PDF, TXT herunterladen oder online auf Scribd lesen
Sie sind auf Seite 1von 2
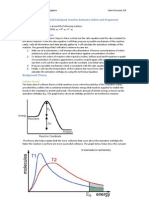
Understand Proportions
Probability of the forward process is much larger than the prob of backward
process (product-favored process)
Tracking Atoms
Need to balance the chemical equation
o Use stoichiometric coefficients to indicate minimum number of each
type of particle in the reactants and products
Balance C and H first, then O last
you can determine the amount of molar mass given the number of moles
Amount of Substances
A. Determine the amounts of reactants.
o First step is the balance the equation.
To calculate mass of reactant
B. Predicting amounts of products
o Called theoretical yield of the reaction
o Same as picture above, just switch product to reactant and vice versa
Find Limiting reactant: this indicate that 2 moles of water require 1 mole of
ethane. But the actual ratio is smaller (there is not enough H2O to react with
C2H6) so H2O is LR
If the actual ratio is smaller than stoichiometric ratio, the numerator
substance is LR. If greater. Then numerator is excess.
Das könnte Ihnen auch gefallen
- I. Introductory Concept: SHS - Physical Science (Stoichiometry)Dokument8 SeitenI. Introductory Concept: SHS - Physical Science (Stoichiometry)DYLANNoch keine Bewertungen
- I. Introductory Concept: SHS - Physical Science (Stoichiometry)Dokument8 SeitenI. Introductory Concept: SHS - Physical Science (Stoichiometry)DYLAN100% (1)
- Thermometric Titrimetry: International Series of Monographs in Analytical ChemistryVon EverandThermometric Titrimetry: International Series of Monographs in Analytical ChemistryNoch keine Bewertungen
- Narrative Report in Gas StoichiometryDokument13 SeitenNarrative Report in Gas Stoichiometryandrea romeroNoch keine Bewertungen
- Thermodynamic Models for Chemical Engineering: Design, Develop, Analyse and OptimizeVon EverandThermodynamic Models for Chemical Engineering: Design, Develop, Analyse and OptimizeNoch keine Bewertungen
- Emperical Formula NotesDokument5 SeitenEmperical Formula Notesah786ah3648Noch keine Bewertungen
- Stoichiometry and Balancing ReactionsDokument12 SeitenStoichiometry and Balancing Reactionsangeljin1207Noch keine Bewertungen
- Stoichiometry and Balancing ReactionsDokument11 SeitenStoichiometry and Balancing ReactionsEdizon De Andres JaoNoch keine Bewertungen
- Stoichiometry CalculationsDokument7 SeitenStoichiometry CalculationsDora Naj100% (1)
- StoichiometryDokument45 SeitenStoichiometryMay Lyn Rosal BerondoNoch keine Bewertungen
- Chemical Stoichiometry Calculations and Balancing EquationsDokument5 SeitenChemical Stoichiometry Calculations and Balancing EquationsNeharika PuriNoch keine Bewertungen
- Material Balances with Chemical ReactionsDokument30 SeitenMaterial Balances with Chemical ReactionsblessaNoch keine Bewertungen
- Iodination of Acetone KineticsDokument4 SeitenIodination of Acetone Kineticsinstrutech0% (2)
- q1 Module 10Dokument15 Seitenq1 Module 10Princess Angeles Andam100% (1)
- Chemical CalculationsDokument32 SeitenChemical CalculationssamNoch keine Bewertungen
- CH Cooh (A) + CH CH CH CH OH (B) (C) + H O (D) : Ch3Ch2Ch2Cooch2Ch3Dokument5 SeitenCH Cooh (A) + CH CH CH CH OH (B) (C) + H O (D) : Ch3Ch2Ch2Cooch2Ch3wanNoch keine Bewertungen
- Physical Science Week 11-12-1Dokument4 SeitenPhysical Science Week 11-12-1Drasszen SombilonNoch keine Bewertungen
- CHEM 2 - Module 4 StoichiometryDokument11 SeitenCHEM 2 - Module 4 StoichiometryMicah BlazaNoch keine Bewertungen
- Worksheet Limiting ReactantDokument8 SeitenWorksheet Limiting ReactantJoseph GutierrezNoch keine Bewertungen
- 3general Chemistry 1 2nd Quarter Week 3 RUCDokument6 Seiten3general Chemistry 1 2nd Quarter Week 3 RUCKyleNoch keine Bewertungen
- Balc 1004 NotesDokument103 SeitenBalc 1004 NotesJude ParasNoch keine Bewertungen
- CHEE 221: Chemical Processes and SystemsDokument22 SeitenCHEE 221: Chemical Processes and SystemsLinda Leon TomaNoch keine Bewertungen
- JEE Main Short Notes Mole Concept and Stoichiometry - pdf-15Dokument9 SeitenJEE Main Short Notes Mole Concept and Stoichiometry - pdf-15PUSHKAR SAININoch keine Bewertungen
- Material Balance 2022-PART1Dokument13 SeitenMaterial Balance 2022-PART1Harsh BopcheNoch keine Bewertungen
- University of Northeastern Philippines (UNEP) Iriga CityDokument9 SeitenUniversity of Northeastern Philippines (UNEP) Iriga CityNica GumbaNoch keine Bewertungen
- Material Balances With Chemical ReactionDokument19 SeitenMaterial Balances With Chemical Reactionjeas grejoyNoch keine Bewertungen
- Acetone KineticsDokument8 SeitenAcetone KineticsEddie ChuiNoch keine Bewertungen
- Neraca Massa ATKDokument49 SeitenNeraca Massa ATKMuhammad KholidinNoch keine Bewertungen
- General Guidelines For Balancing Simple Equations: StoichiometryDokument6 SeitenGeneral Guidelines For Balancing Simple Equations: StoichiometryJonel Mark CarandangNoch keine Bewertungen
- Grade 11 ChemistryDokument17 SeitenGrade 11 ChemistryKevin George100% (1)
- LE 005 007 General Chemistry 1 Continuation .Updated FinalDokument26 SeitenLE 005 007 General Chemistry 1 Continuation .Updated FinalShaman KingNoch keine Bewertungen
- A basic introduction to StoichiometryDokument5 SeitenA basic introduction to StoichiometrySara RaiNoch keine Bewertungen
- Balancing Chemical EquationsDokument5 SeitenBalancing Chemical Equationsseung mooNoch keine Bewertungen
- Reaction Kinetics-Ver 2Dokument4 SeitenReaction Kinetics-Ver 2Ivan Alberto NinaNoch keine Bewertungen
- StoichiometryDokument53 SeitenStoichiometryNoorSabaNoch keine Bewertungen
- STOCHIOMETRYDokument13 SeitenSTOCHIOMETRYHARI PRASATHNoch keine Bewertungen
- Chapter 8 - Section 1: Describing Chemical ReactionsDokument7 SeitenChapter 8 - Section 1: Describing Chemical ReactionsIsmail MedhatNoch keine Bewertungen
- 4 Chemical Equations and StoichiometryDokument1 Seite4 Chemical Equations and Stoichiometrynotyouravguplo876Noch keine Bewertungen
- Lecture 1 2024Dokument21 SeitenLecture 1 2024nonkululekomoya26Noch keine Bewertungen
- Mcats 2Dokument94 SeitenMcats 2fgbfxgvbNoch keine Bewertungen
- Physical Science-Module 8 Chemical ReactionsDokument57 SeitenPhysical Science-Module 8 Chemical ReactionsJoana CastilloNoch keine Bewertungen
- 200610chapter 16 Kinetics PDFDokument22 Seiten200610chapter 16 Kinetics PDFfearlessinchrist100Noch keine Bewertungen
- Rates of Chemical Reactions: The Iodination of AcetoneDokument9 SeitenRates of Chemical Reactions: The Iodination of AcetoneAbdur Rakib M Sarwar0% (1)
- Gen Chem Module 3 L3 &4Dokument10 SeitenGen Chem Module 3 L3 &4Hazel Cosep RicoNoch keine Bewertungen
- Chapter 16 KineticsDokument22 SeitenChapter 16 KineticsUdop CharlesNoch keine Bewertungen
- Fourth Chapter - Part 2Dokument20 SeitenFourth Chapter - Part 2toslim jahidNoch keine Bewertungen
- StoichDokument10 SeitenStoichAna LuisaNoch keine Bewertungen
- Homework - Molecular ChemistryDokument5 SeitenHomework - Molecular ChemistryJoana TolentinoNoch keine Bewertungen
- Kinetics of The Acid Catalysed Reaction Between Iodine and Propanone Final LolDokument23 SeitenKinetics of The Acid Catalysed Reaction Between Iodine and Propanone Final LolYann Perusset90% (10)
- A Balanced Chemical Equation Shows The Molar Amounts of Reactants That Will React Together To Produce Molar Amounts of ProductsDokument4 SeitenA Balanced Chemical Equation Shows The Molar Amounts of Reactants That Will React Together To Produce Molar Amounts of ProductsIan Khay CastroNoch keine Bewertungen
- Mass Relationships in Chemical ReactionsDokument21 SeitenMass Relationships in Chemical ReactionsVince DulayNoch keine Bewertungen
- Chemical Reactions and Reaction Stoichiometry: Lecture PresentationDokument71 SeitenChemical Reactions and Reaction Stoichiometry: Lecture PresentationJinNoch keine Bewertungen
- Stoichiometry moduleDokument16 SeitenStoichiometry moduleMai SasaNoch keine Bewertungen
- Stoichiometric 11Dokument23 SeitenStoichiometric 11Jeira Mei Casona DayonNoch keine Bewertungen
- Reactivity 2.1 - How Much-The Amount of Chemical ChangeDokument34 SeitenReactivity 2.1 - How Much-The Amount of Chemical ChangeLucia PesentiNoch keine Bewertungen
- General ChemistryDokument34 SeitenGeneral ChemistryTrixie HicaldeNoch keine Bewertungen
- Chem 1 Week 4 Stoichiometry CompilerDokument7 SeitenChem 1 Week 4 Stoichiometry CompilerMelcorr MontesclarosNoch keine Bewertungen
- HW 1 Trinny TatDokument2 SeitenHW 1 Trinny TatTrinh Tat-TranNoch keine Bewertungen
- 8a. Skeletal Lab-HPWDokument10 Seiten8a. Skeletal Lab-HPWTrinh Tat-TranNoch keine Bewertungen
- Bone Features Tables PDFDokument3 SeitenBone Features Tables PDFTrinh Tat-TranNoch keine Bewertungen
- Understand Stoichiometry, Limiting Reactants & Theoretical Yields in Chemical ReactionsDokument2 SeitenUnderstand Stoichiometry, Limiting Reactants & Theoretical Yields in Chemical ReactionsTrinh Tat-TranNoch keine Bewertungen
- Rib Vertebrae Articulation PDFDokument2 SeitenRib Vertebrae Articulation PDFTrinh Tat-TranNoch keine Bewertungen
- Characterizing Ionic NetworksDokument3 SeitenCharacterizing Ionic NetworksTrinh Tat-TranNoch keine Bewertungen
- Rib Vertebrae Articulation HandoutDokument1 SeiteRib Vertebrae Articulation HandoutTrinh Tat-TranNoch keine Bewertungen
- Lab 4 Pre-lab Worksheet Bones Anatomy IdentificationDokument1 SeiteLab 4 Pre-lab Worksheet Bones Anatomy IdentificationTrinh Tat-TranNoch keine Bewertungen
- Lab 4 Pre-Lab Assignment PDFDokument1 SeiteLab 4 Pre-Lab Assignment PDFTrinh Tat-TranNoch keine Bewertungen
- Vertebrae, Ribs and Sternum Features Table PDFDokument1 SeiteVertebrae, Ribs and Sternum Features Table PDFTrinh Tat-TranNoch keine Bewertungen
- Bond Strength (Bond Dissociation Energy) Energy Needed To SeparateDokument4 SeitenBond Strength (Bond Dissociation Energy) Energy Needed To SeparateTrinh Tat-TranNoch keine Bewertungen
- 2.4 NotesDokument2 Seiten2.4 NotesTrinh Tat-TranNoch keine Bewertungen
- Modeling Chemical ReactionDokument3 SeitenModeling Chemical ReactionTrinh Tat-TranNoch keine Bewertungen
- 4.3 NotesDokument1 Seite4.3 NotesTrinh Tat-TranNoch keine Bewertungen
- 2.1 NotesDokument2 Seiten2.1 NotesTrinh Tat-TranNoch keine Bewertungen
- Scales of Polymers and ProteinsDokument2 SeitenScales of Polymers and ProteinsTrinh Tat-TranNoch keine Bewertungen
- 1.2 NotesDokument4 Seiten1.2 NotesTrinh Tat-TranNoch keine Bewertungen
- Top, Below, Right Then LeftDokument4 SeitenTop, Below, Right Then LeftTrinh Tat-TranNoch keine Bewertungen
- Polarizability, Molec Polar, Bond Polar, Results in Net Force BTW DifferentDokument3 SeitenPolarizability, Molec Polar, Bond Polar, Results in Net Force BTW DifferentTrinh Tat-TranNoch keine Bewertungen
- 1.3 NotesDokument1 Seite1.3 NotesTrinh Tat-TranNoch keine Bewertungen
- Scales of Polymers and ProteinsDokument2 SeitenScales of Polymers and ProteinsTrinh Tat-TranNoch keine Bewertungen
- Modeling Chemical ReactionDokument3 SeitenModeling Chemical ReactionTrinh Tat-TranNoch keine Bewertungen
- Characterizing Ionic NetworksDokument3 SeitenCharacterizing Ionic NetworksTrinh Tat-TranNoch keine Bewertungen
- 4.3 NotesDokument1 Seite4.3 NotesTrinh Tat-TranNoch keine Bewertungen
- Bond Strength (Bond Dissociation Energy) Energy Needed To SeparateDokument4 SeitenBond Strength (Bond Dissociation Energy) Energy Needed To SeparateTrinh Tat-TranNoch keine Bewertungen
- Polarizability, Molec Polar, Bond Polar, Results in Net Force BTW DifferentDokument3 SeitenPolarizability, Molec Polar, Bond Polar, Results in Net Force BTW DifferentTrinh Tat-TranNoch keine Bewertungen
- 2.4 NotesDokument2 Seiten2.4 NotesTrinh Tat-TranNoch keine Bewertungen
- Top, Below, Right Then LeftDokument4 SeitenTop, Below, Right Then LeftTrinh Tat-TranNoch keine Bewertungen
- 2.1 NotesDokument2 Seiten2.1 NotesTrinh Tat-TranNoch keine Bewertungen
- Sully: The Untold Story Behind the Miracle on the HudsonVon EverandSully: The Untold Story Behind the Miracle on the HudsonBewertung: 4 von 5 Sternen4/5 (103)
- The Fabric of Civilization: How Textiles Made the WorldVon EverandThe Fabric of Civilization: How Textiles Made the WorldBewertung: 4.5 von 5 Sternen4.5/5 (57)
- Packing for Mars: The Curious Science of Life in the VoidVon EverandPacking for Mars: The Curious Science of Life in the VoidBewertung: 4 von 5 Sternen4/5 (1395)
- The Beekeeper's Lament: How One Man and Half a Billion Honey Bees Help Feed AmericaVon EverandThe Beekeeper's Lament: How One Man and Half a Billion Honey Bees Help Feed AmericaNoch keine Bewertungen
- The Weather Machine: A Journey Inside the ForecastVon EverandThe Weather Machine: A Journey Inside the ForecastBewertung: 3.5 von 5 Sternen3.5/5 (31)
- Highest Duty: My Search for What Really MattersVon EverandHighest Duty: My Search for What Really MattersNoch keine Bewertungen
- Hero Found: The Greatest POW Escape of the Vietnam WarVon EverandHero Found: The Greatest POW Escape of the Vietnam WarBewertung: 4 von 5 Sternen4/5 (19)
- Faster: How a Jewish Driver, an American Heiress, and a Legendary Car Beat Hitler's BestVon EverandFaster: How a Jewish Driver, an American Heiress, and a Legendary Car Beat Hitler's BestBewertung: 4 von 5 Sternen4/5 (28)
- Transformed: Moving to the Product Operating ModelVon EverandTransformed: Moving to the Product Operating ModelBewertung: 4 von 5 Sternen4/5 (1)
- The End of Craving: Recovering the Lost Wisdom of Eating WellVon EverandThe End of Craving: Recovering the Lost Wisdom of Eating WellBewertung: 4.5 von 5 Sternen4.5/5 (80)
- 35 Miles From Shore: The Ditching and Rescue of ALM Flight 980Von Everand35 Miles From Shore: The Ditching and Rescue of ALM Flight 980Bewertung: 4 von 5 Sternen4/5 (21)
- A Place of My Own: The Architecture of DaydreamsVon EverandA Place of My Own: The Architecture of DaydreamsBewertung: 4 von 5 Sternen4/5 (241)
- The Future of Geography: How the Competition in Space Will Change Our WorldVon EverandThe Future of Geography: How the Competition in Space Will Change Our WorldBewertung: 4.5 von 5 Sternen4.5/5 (4)
- Dirt to Soil: One Family’s Journey into Regenerative AgricultureVon EverandDirt to Soil: One Family’s Journey into Regenerative AgricultureBewertung: 5 von 5 Sternen5/5 (124)
- Pale Blue Dot: A Vision of the Human Future in SpaceVon EverandPale Blue Dot: A Vision of the Human Future in SpaceBewertung: 4.5 von 5 Sternen4.5/5 (586)
- Across the Airless Wilds: The Lunar Rover and the Triumph of the Final Moon LandingsVon EverandAcross the Airless Wilds: The Lunar Rover and the Triumph of the Final Moon LandingsNoch keine Bewertungen
- Data-ism: The Revolution Transforming Decision Making, Consumer Behavior, and Almost Everything ElseVon EverandData-ism: The Revolution Transforming Decision Making, Consumer Behavior, and Almost Everything ElseBewertung: 3.5 von 5 Sternen3.5/5 (12)
- Recording Unhinged: Creative and Unconventional Music Recording TechniquesVon EverandRecording Unhinged: Creative and Unconventional Music Recording TechniquesNoch keine Bewertungen
- Einstein's Fridge: How the Difference Between Hot and Cold Explains the UniverseVon EverandEinstein's Fridge: How the Difference Between Hot and Cold Explains the UniverseBewertung: 4.5 von 5 Sternen4.5/5 (50)
- Reality+: Virtual Worlds and the Problems of PhilosophyVon EverandReality+: Virtual Worlds and the Problems of PhilosophyBewertung: 4 von 5 Sternen4/5 (24)
- The Technology Trap: Capital, Labor, and Power in the Age of AutomationVon EverandThe Technology Trap: Capital, Labor, and Power in the Age of AutomationBewertung: 4.5 von 5 Sternen4.5/5 (46)
- Broken Money: Why Our Financial System is Failing Us and How We Can Make it BetterVon EverandBroken Money: Why Our Financial System is Failing Us and How We Can Make it BetterBewertung: 5 von 5 Sternen5/5 (3)
- Fallout: The Hiroshima Cover-up and the Reporter Who Revealed It to the WorldVon EverandFallout: The Hiroshima Cover-up and the Reporter Who Revealed It to the WorldBewertung: 4.5 von 5 Sternen4.5/5 (82)
- The Path Between the Seas: The Creation of the Panama Canal, 1870-1914Von EverandThe Path Between the Seas: The Creation of the Panama Canal, 1870-1914Bewertung: 4.5 von 5 Sternen4.5/5 (124)