Beruflich Dokumente
Kultur Dokumente
Humanization Techniques
Hochgeladen von
Biotech Updtes0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
9 Ansichten1 SeiteHumanization is important for reducing the immunogenicity of monoclonal antibodies derived from xenogeneic sources (commonly rodent) and for improving their activation of the human immune system.
Copyright
© © All Rights Reserved
Verfügbare Formate
DOCX, PDF, TXT oder online auf Scribd lesen
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenHumanization is important for reducing the immunogenicity of monoclonal antibodies derived from xenogeneic sources (commonly rodent) and for improving their activation of the human immune system.
Copyright:
© All Rights Reserved
Verfügbare Formate
Als DOCX, PDF, TXT herunterladen oder online auf Scribd lesen
0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
9 Ansichten1 SeiteHumanization Techniques
Hochgeladen von
Biotech UpdtesHumanization is important for reducing the immunogenicity of monoclonal antibodies derived from xenogeneic sources (commonly rodent) and for improving their activation of the human immune system.
Copyright:
© All Rights Reserved
Verfügbare Formate
Als DOCX, PDF, TXT herunterladen oder online auf Scribd lesen
Sie sind auf Seite 1von 1
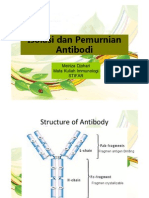
Humanization Techniques
Humanization is important for reducing the immunogenicity of monoclonal
antibodies derived from xenogeneic sources (commonly rodent) and for
improving their activation of the human immune system. Since the
development of the hybridoma technology, a large number of rodent
monoclonal antibodies with specificity for antigens of therapeutic interest
have been generated and characterized. Rodent antibodies are highly
immunogenic in humans, which limits their clinical applications, especially
when repeated administration is required. Importantly, they are rapidly
removed from circulation and can cause systemic inflammatory effects as
well. As a means of circumventing these problems, we have developed three
antibody humanization strategies that can preserve the specificity and affinity
of the antibody toward the antigen whereas significantly or completely
eliminate the immunogenicity of the antibody in humans. The first approach is
CDR grafting and the second is chain shuffling. These two methods are all
based on phage display of humanized scFv variants and selection of highaffinity humanized binders through bio-panning. The third method, humanized
IgG library screening, is somehow unique. We will make a library of humanized
whole IgG to be displayed on the surface of mammalian cells and then high
affinity binders will be sorted by FACS.
CDR Grafting & SDR Grafting
We have established a CDR (complementarity-determining region) grafting
platform, which is featured with randomization of a small set of framework
residues using phage display technology and computer modeling. In this
platform, six CDR loops comprising the antigen-binding site are grafted into
corresponding human framework regions. Unfortunately, simple grafting of
the rodent CDRs into human frameworks does not always reconstitute the
binding affinity and specificity of the original antibody because framework
residues are involved in antigen binding, either indirectly, by supporting the
conformation of the CDR loops, or directly, by contacting the antigen. For this
reason, we have developed a computer modeling method to randomize
certain framework residues in addition to CDR grafting. The grafted CDRs
together with the randomized residues are cloned into a phage display library
and the humanized antibodies with the best affinity are selected by screening
of the library. This approach allows the epitope specificity of the original
antibody to be retained. Of note, humanization by this approach is not 100%
since the CDR regions are still of a rodent origin.
To reduce the immunogenicity of CDR-grafted humanized antibodies, the
murine content in the CDR-grafted humanized antibodies is minimized
through SDR grafting. Within each CDR, there are more variable positions that
are directly involved in the interaction with antigen, i.e., specificitydetermining residues (SDRs), whereas there are more conserved residues that
maintain the conformations of CDRs loops. SDRs may be identified from the
3D structure of the antigen antibody complex and/or the mutational analysis
of the CDRs. An SDR-grafted humanized antibody is constructed by grafting
the SDRs and the residues maintaining the conformations of the CDRs onto
human template, and its immunogenic potential is evaluated by measuring
the reactivity to the sera from patients who had been immunized with the
parental antibody.
Das könnte Ihnen auch gefallen
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeVon EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeBewertung: 4 von 5 Sternen4/5 (5795)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreVon EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreBewertung: 4 von 5 Sternen4/5 (1090)
- Never Split the Difference: Negotiating As If Your Life Depended On ItVon EverandNever Split the Difference: Negotiating As If Your Life Depended On ItBewertung: 4.5 von 5 Sternen4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceVon EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceBewertung: 4 von 5 Sternen4/5 (895)
- Grit: The Power of Passion and PerseveranceVon EverandGrit: The Power of Passion and PerseveranceBewertung: 4 von 5 Sternen4/5 (588)
- Shoe Dog: A Memoir by the Creator of NikeVon EverandShoe Dog: A Memoir by the Creator of NikeBewertung: 4.5 von 5 Sternen4.5/5 (537)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersVon EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersBewertung: 4.5 von 5 Sternen4.5/5 (345)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureVon EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureBewertung: 4.5 von 5 Sternen4.5/5 (474)
- Her Body and Other Parties: StoriesVon EverandHer Body and Other Parties: StoriesBewertung: 4 von 5 Sternen4/5 (821)
- The Emperor of All Maladies: A Biography of CancerVon EverandThe Emperor of All Maladies: A Biography of CancerBewertung: 4.5 von 5 Sternen4.5/5 (271)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Von EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Bewertung: 4.5 von 5 Sternen4.5/5 (121)
- The Little Book of Hygge: Danish Secrets to Happy LivingVon EverandThe Little Book of Hygge: Danish Secrets to Happy LivingBewertung: 3.5 von 5 Sternen3.5/5 (400)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyVon EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyBewertung: 3.5 von 5 Sternen3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)Von EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Bewertung: 4 von 5 Sternen4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaVon EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaBewertung: 4.5 von 5 Sternen4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryVon EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryBewertung: 3.5 von 5 Sternen3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnVon EverandTeam of Rivals: The Political Genius of Abraham LincolnBewertung: 4.5 von 5 Sternen4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealVon EverandOn Fire: The (Burning) Case for a Green New DealBewertung: 4 von 5 Sternen4/5 (74)
- The Unwinding: An Inner History of the New AmericaVon EverandThe Unwinding: An Inner History of the New AmericaBewertung: 4 von 5 Sternen4/5 (45)
- Advances in Immunoassay TechnologyDokument190 SeitenAdvances in Immunoassay TechnologySvarlgNoch keine Bewertungen
- GenBio2 PT 3 and 4Dokument5 SeitenGenBio2 PT 3 and 4Shaira Faye BarquioNoch keine Bewertungen
- Perspectives On Integrated ContinuousDokument6 SeitenPerspectives On Integrated ContinuousFrancineTramontinaNoch keine Bewertungen
- ELISA-AtoZ EDokument60 SeitenELISA-AtoZ EDr-Dalya ShakirNoch keine Bewertungen
- Endocrine Methodologies Chapter 4 9-1.2012 PDFDokument26 SeitenEndocrine Methodologies Chapter 4 9-1.2012 PDFLeora LiNoch keine Bewertungen
- Fast and Automated Characterization of Monoclonal Antibody Minor Variants From Cell Cultures by Combined Protein A and Multidimensional LC:MS MethodologiesDokument8 SeitenFast and Automated Characterization of Monoclonal Antibody Minor Variants From Cell Cultures by Combined Protein A and Multidimensional LC:MS Methodologiesyun baiNoch keine Bewertungen
- Detection of Ruminant Meat and Bone Meal in Feeds by Sandwich ELISA With Monoclonal AntibodiesDokument5 SeitenDetection of Ruminant Meat and Bone Meal in Feeds by Sandwich ELISA With Monoclonal AntibodiesCarlos Gene QuirozNoch keine Bewertungen
- Ebook PDF Cell Culture Bioprocess Engineering Second Edition 2nd Edition PDFDokument41 SeitenEbook PDF Cell Culture Bioprocess Engineering Second Edition 2nd Edition PDFjohn.vail635100% (32)
- Moyle Et Al-2019-Experimental DermatologyDokument13 SeitenMoyle Et Al-2019-Experimental DermatologyIlham WahyuNoch keine Bewertungen
- AntibodiesDokument39 SeitenAntibodiespilot abdi baariNoch keine Bewertungen
- Scope and Importance of BiotechDokument43 SeitenScope and Importance of BiotechSubhi MishraNoch keine Bewertungen
- Ciclo Estral em CamundongosDokument6 SeitenCiclo Estral em CamundongosAluana Santana CarlosNoch keine Bewertungen
- Homologous or Heterologous Booster of Inactivated Vaccine Reduces Sars-Cov-2 Omicron Variant Escape From Neutralizing AntibodiesDokument12 SeitenHomologous or Heterologous Booster of Inactivated Vaccine Reduces Sars-Cov-2 Omicron Variant Escape From Neutralizing AntibodiesSariSyahruniNoch keine Bewertungen
- Targeted Therapy in CancerDokument51 SeitenTargeted Therapy in CancerSatya WangsaNoch keine Bewertungen
- Interferences in Protein Electrophoresis Mahajan SlidesDokument21 SeitenInterferences in Protein Electrophoresis Mahajan SlidesDarshana JuvekarNoch keine Bewertungen
- Microspheres As A Novel Drug Delivery Sysytem - A ReviewDokument9 SeitenMicrospheres As A Novel Drug Delivery Sysytem - A ReviewkasoutNoch keine Bewertungen
- Bahan Kuliah Pemurnian AntibodiDokument40 SeitenBahan Kuliah Pemurnian Antibodidesy rahmanisyaNoch keine Bewertungen
- 9700 w18 QP 21 PDFDokument16 Seiten9700 w18 QP 21 PDFNoor HannyNoch keine Bewertungen
- Clinical Pharmacology, Bispecipic AntibodiesDokument17 SeitenClinical Pharmacology, Bispecipic AntibodiesimasNoch keine Bewertungen
- Erythroblastic SynartesisDokument11 SeitenErythroblastic SynartesisKata TölgyesiNoch keine Bewertungen
- GMP GlosseyDokument94 SeitenGMP Glosseymona khNoch keine Bewertungen
- IMMUNOPROPHYLAXISDokument30 SeitenIMMUNOPROPHYLAXISAli TARARNoch keine Bewertungen
- 1505chromatan Bpi Europe Presentation Final April 2018 1Dokument48 Seiten1505chromatan Bpi Europe Presentation Final April 2018 1sushaantb400Noch keine Bewertungen
- Biotecnologia Pais VascoDokument47 SeitenBiotecnologia Pais Vascogapam_2Noch keine Bewertungen
- 1.6-7 Monoclonal Antibody Production WorksheetDokument2 Seiten1.6-7 Monoclonal Antibody Production WorksheetColdblood 3000Noch keine Bewertungen
- No Natural Home: Placing The Promise of BiopharmingDokument333 SeitenNo Natural Home: Placing The Promise of BiopharmingrjmilneNoch keine Bewertungen
- Atlas de Marcadores Tumorales - DakoDokument120 SeitenAtlas de Marcadores Tumorales - Dakodavlab76Noch keine Bewertungen
- Cambridge International AS & A Level: BIOLOGY 9700/21Dokument16 SeitenCambridge International AS & A Level: BIOLOGY 9700/21Malak ShokryNoch keine Bewertungen
- Biotechnology Analytical Development Director in CT Resume Shirish DhumeDokument5 SeitenBiotechnology Analytical Development Director in CT Resume Shirish DhumeSharishDhumeNoch keine Bewertungen
- Drug Discovery Historical Prescpective PDFDokument5 SeitenDrug Discovery Historical Prescpective PDFKarthi ShanmugamNoch keine Bewertungen