Beruflich Dokumente
Kultur Dokumente
Out 2
Hochgeladen von
Andra KurniantoOriginaltitel
Copyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Out 2
Hochgeladen von
Andra KurniantoCopyright:
Verfügbare Formate
The Turkish Journal of Pediatrics 2011; 53: 67-74
Original
Transcutaneous measurement of bilirubin in Turkish newborns:
comparison with total serum bilirubin
Mnevver Kaynak-Trkmen1, S. Ayvaz Aydodu1, Cengiz Gkbulut2, idem Yenisey3,
mer Sz1, Bilin etinkaya-akmak1
Departments of 1Pediatrics and 3Biochemistry, Faculty of Medicine and 2Department of Pharmacology and Toxicology,
Faculty of Veterinary Medicine, Adnan Menderes University, Aydn, Turkey
SUMMARY: Kaynak-Trkmen M, Aydodu SA, Gkbulut C, Yenisey ,
Sz , etinkaya-akmak B. Transcutaneous measurement of bilirubin in
Turkish newborns: comparison with total serum bilirubin. Turk J Pediatr
2011; 53: 67-74.
Routine use of transcutaneous bilirubin (TcB) measurement in the newborn
nursery could reduce costs, readmission rates for hyperbilirubinemia and the
need for total serum bilirubin (TSB) measurements. The aim of this study
was to examine the correlation between TcB measurement, as performed
using BiliCheck, and TSB, measured with high-pressure liquid chromatography
(HPLC) and with standard laboratory methods, and to determine the TcB cutoff points with desirable sensitivity and specificity values for various clinically
relevant TSB levels by HPLC. Fifty-four infants of 30 weeks of gestational
age were enrolled in the study. Near simultaneous blood collection for TSB
analysis by three methods - bedside bilirubinometer, diazo method and HPLC
- and TcB measurement were performed. There was good correlation between
TcB and HPLC-bilirubin (B) (r=0.85), TSB by bilirubinometer and HPLC-B
(r=0.91) and TSB by diazo method and HPLC-B (r=0.91). The cut-off limits
providing a sensitivity of 100% for TcB measurements were TcB 9 mg/dl for
HPLC-B >17 mg/dl and TcB 8 mg/dl for HPLC-B >15 mg/dl and HPLC-B
>13 mg/dl. Despite having good correlation with HPLC, BiliCheck showed
worse performance than bilirubinometer and diazo method at various clinically
relevant cut-off values. Since BiliCheck required relatively lower thresholds
with false-positive results for having a sensitivity of 100%, it cannot be
recommended as a complete substitute for serum bilirubin measurements.
Key words: high performance liquid chromatography, jaundice, transcutaneous bilirubin
measurement.
The most frequent laboratory test performed in
the neonatal newborn nursery is total serum
bilirubin (TSB) measurement. Routine use of
transcutaneous bilirubin (TcB) measurement
in the newborn nursery could reduce costs,
readmission rates for hyperbilirubinemia, and
the need for TSB measurements1-4.
Since the early 1980s, a device dedicated
to bilirubin measurement was proposed 5 .
This device has various limitations. First, it
gives an index of jaundice, not the value of
serum bilirubin concentration. Moreover, race,
gestational age and body weight interfere with
the accuracy of the jaundice index6.
Measurement of bilirubin by various
transcutaneous techniques has been reported
from several studies with mixed results7-16.
Race, as assessed by skin color score, did not
affect the BiliCheck method9,17,18. In addition,
other potential confounding factors, such as
birth weight, gestational age, and postnatal age,
did not affect the BiliCheck device8,10,19.
The subject of this study is the BiliCheck
(BC; SpectRx Inc.), a TcB measuring device
that uses the entire spectrum of visible light
(380-760 nm) reflected from the skin. White
light is transmitted into the skin of the
newborn and the reflected light is used for
analysis. The mathematical isolation of the
68
Kaynak-Trkmen M, et al
The Turkish Journal of Pediatrics January-February 2011
Table I. Demographic Characteristics of the Newborn Infants Studied (n=54)
Characteristic
Gender
Female
Male
23 (43%)
31 (57%)
Birth weight (g) Mean SD (range)
2979656 (1550-4200)
Gestational age (week)
30-37
38-42
Postnatal age (day)
Mean SD (range)
light absorption of certain interfering factors
(hemoglobin, melanin, and dermal thickness)
allows the absorption of light caused by the
presence of bilirubin in the capillary vessels
and subcutaneous tissue to be isolated by
spectral subtraction.
Direct comparison between the TcB measurement and a single laboratory method depends
on the accuracy of the laboratory method.
Errors in the TSB might be interpreted as
errors in the TcB. Therefore, use of the gold
standard high-pressure liquid chromatography
bilirubin (HPLC-B) is necessary to serve as
the true reference value. Some investigators
have compared TcB measurements with HPLC
measurements8,9,20.
The objectives of the study were 1) to
determine whether TcB measurement, as
performed using BC, correlates with TSB
levels measured with HPLC and with standard
laboratory methods, and 2) to determine BC
cut-off points with desirable sensitivity and
specificity values for various clinically relevant
TSB levels by HPLC.
Material and Methods
The study was performed between December
2007 and October 2008 at Adnan Menderes
University Medical School in the well-baby
17 (32%)
37 (68%)
6.674.14 (3-19)
nurseries and the neonatal intensive care unit.
Fifty-four healthy infants of at least 30 weeks
of gestational age were enrolled in the study.
Patients who had known skin disorders, who
were receiving phototherapy, or who had
received exchange transfusions were excluded
from the study.
Transcutaneous bilirubin (TcB) was measured
using the BC (SpectRx, Inc; Norcross, GA),
which quantifies jaundice by multiwavelength
spectral analysis when applied to the skin. All
determinations were obtained from the infants
foreheads while they were in a quiet state.
A location was chosen free of any bruising,
local nevus, hemangioma, or melanotic patch.
Before each measurement, the device was
calibrated to a standard reference placed in
direct contact with the fiberoptic probe tip. To
obtain a measurement, the probe is positioned
on the skin of the infants forehead, and five
individual scans are taken to produce an average
measurement that is displayed in mg/dl or
mol/L. If an erroneous measurement is taken,
an error message is displayed and the scan
must be repeated.
Transcutaneous bilirubin (TcB) measurement
was performed 30 minutes or less before blood
collection for TSB assay. The blood samples for
TSB were collected by heel stick after warming
Table II. Mean and Standard Deviation of Total Serum Bilirubin Measurements by HPLC, BiliCheck
(BC), Bilirubinometer and Diazo Method
TSB
HPLC
BC
Bilirubinometer
Diazo
HPLC: High-pressure liquid chromatography.
Mean
11.79
9.80
11.30
13.85
SD (range) mg/dl
6.11 (0.85-29.37)
4.44 (1.20-18.40)
4.60 (1.10-24.00)
6.21 (0.50-33.21)
Volume 53 Number 1
Transcutaneous Bilirubin in Turkish Newborns
69
Table III. Correlation Coefficients Between Methods of Estimating or Measuring Bilirubin Level
Method
HPLC and TcB
HPLC and Bilirubinometer TSB
HPLC and Diazo TSB
Bilirubinometer TSB and TcB
Diazo TSB and TcB
r (95% CI)
0.85 (0.76-0.91)
0.91 (0.85-0.95)
0.91 (0.84-0.95)
0.90 (0.84-0.94)
0.83 (0.73-0.90)
Mean error mg/dl (95% CI)
1.845 (0.944-2.747)
0.351 (-0.392-1.094)
-2.340 (-2.956-1.511)
1.494 (0.949-2.040)
4.079 (3.110-5.047)
CI: Confidence interval. HPLC: High-pressure liquid chromatography. TcB: Transcutaneous bilirubin. TSB: Total serum
bilirubin.
of the heel and lancet puncture incision; blood
was collected by the drip method into heparin
containing capillary tubes. Venous samples for
TSB measurement were obtained if capillary
bilirubin level was >12 mg/dl or if it was
necessary for other medical reasons such as
screening test for congenital hypothyroidism at
4-6 postnatal days. TSB levels were determined
on heel stick (capillary) samples using bedside
BR 5000N Apel bilirubinometer and on venous
samples by a diazo method using Architect
c8000 automatic analyzer in the hospital
laboratory. In addition, a 0.5 to 1 ml sample
of plasma was frozen at -70C for subsequent
analysis of TSB by HPLC at the Research
and Development Center of the University.
Bilirubin concentrations were measured by
HPLC according to a previously described
method9. The HPLC researcher was blinded
to the results of the laboratory TSB and TcB
measurements.
Standard precautions were used to protect the
samples from exposure to light to prevent photo
conversion of bilirubin in the blood. The study
was approved by the Ethics Committee of the
Hospital, and informed consent was obtained
from the parents of each subject.
Correlation coefficients were calculated using
linear regression between each pair of methods
with all sample pairs included in the analysis.
Because linear correlation of two clinical
measurements can often be misleading, we
determined the limits of agreement that could
be applied to the whole population by the
statistical analysis described by Bland and
Altman21. The sensitivity and specificity of
TcB and TSB (bilirubinometer and diazo) to
predict HPLC-B accurately was estimated at a
range of values and plotted on receiver operator
characteristic (ROC) curves.
Results
The demographic characteristics of the infants
studied are shown in Table I. The study group
consisted of white newborn infants; 27.8% of
them had TSB 15 mg/dl.
The mean bilirubin concentrations in samples
measured by HPLC, BC, bilirubinometer, and
diazo are shown in Table II.
The correlations among bilirubin levels
measured by various methods are shown
in Table III. The correlation between TcB
and HPLC-B was very good (r=0.85; 95%
confidence interval (CI): 0.76-0.91). Mean error
(HPLC-B minus TcB) was 1.845 mg/dl. This
means that TcB measurement underestimated
true serum bilirubin as determined by HPLC.
Bilirubinometer and diazo TSB similarly had
high correlation with HPLC-B (r=0.91, 95%
CI: 0.91, respectively); however, contrary
to bilirubinometer TSB and TcB, diazo TSB
overestimated in comparison with HPLC-B
(mean error -2.340 mg/dl).
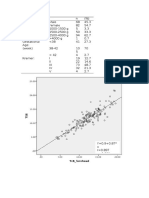
Regression plots of HPLCB versus TcB,
bilirubinometer TSB and diazo TSB are shown
in Figure 1 A-C.
Bland-Altman error plots for each pair are
shown with regression line in Figure 2 A-C.
At low bilirubin concentrations, TcB was higher
than HPLC-B, whereas at higher bilirubin
concentrations, TcB tended to be lower than
HPLCB.
Mean of the differences and 95% agreement
limits of the Bland-Altman bias plot are shown
in Table IV. While the mean differences between
HPLC-B and TcB (1.85 mg/dl) and HPLC-B
and bilirubinometer TSB (0.35 mg/dl) might
be considered clinically non-significant, 95%
agreement limits of the Bland-Altman bias plot
showed a relatively large range.
70
Kaynak-Trkmen M, et al
The Turkish Journal of Pediatrics January-February 2011
In Figure 3 A-C, the ROC curves are plotted.
As the sensitivity and specificity of the test
increases, the ROC curve will appear closer to
the upper left-hand corner of the plot. At 17
mg/dl, 15 mg/dl and 13 mg/dl cut-off points
of HPLC-B, bilirubinometer and diazo TSB
methods performed better than the BC. ROC
areas are shown in Table V.
The sensitivity, specificity, positive predictive
value, and negative predictive value of TcB,
bilirubinometer and diazo TSB methods in
relationship with HPLC-B at various clinically
relevant cut-off points are shown in Table
VI. At the higher levels of TSB, at which
phototherapy and exchange transfusion might
be considered, the bilirubinometer and diazo
methods performed better than the BC.
When the HPLC-B was set at 17 mg/dl,
use of a cut-off point of 15 mg/dl produced
different sensitivities and specificities: 100%
and 64% for diazo method, 80% and 98% for
bilirubinometer, and 70% and 98% for TcB.
In screening studies, it is desired that the
sensitivity of the diagnostic method is close
to 100%. The cut-off limits that provided a
sensitivity of 100% for TcB measurements were
TcB 9 mg/dl for HPLC-B >17 mg/dl, TcB
8 mg/dl for HPLC-B >15 mg/dl, and TcB
8 mg/dl for HPLC-B >13 mg/dl.
Discussion
Hyperbilirubinemia is the most common
diagnosis that leads to hospital readmission
of newborns within the first month of life.
Although the most jaundiced infants suffer no
lasting ill effects, acute bilirubin encephalopathy
(kernicterus) may appear at high bilirubin
levels. The possibility of using a noninvasive,
painless and reliable method to determine
the bilirubin level could be very important in
prevention of acute bilirubin encephalopathy.
Fig. 1A. Plot of HPLC-B versus TcB (n=54) with the
equation for the least squares best fit line. Linear
regression: HPLC-B= -0.12 + 1.20 *TcB; r 2 = 0.73.
Fig. 1B. Plot of HPLC -B versus bilirubinometer
TSB (n=54) with the equation for the least
squares best fit line. Linear regression: HPLC B= -2.27 + 1.23 *Bilirubinometer TSB; r 2 = 0.83.
Fig. 1C. Plot of HPLC-B versus diazo TSB (n=54) with
the equation for the least squares best fit line. Linear
regression: HPLC-B= -0.88 + 0.90 *Diazo TSB; r 2 = 0.82.
We evaluated the BiliCheck point-of-care device,
which performs transcutaneous measurement
of bilirubin by multiwavelength spectral
analysis. We compared results obtained with
the BiliCheck with bilirubin concentrations in
blood specimens measured by three methods:
bilirubinometer, diazo methods and HPLC.
Rubaltelli et al.8 reported that both TcB and
laboratory TSB underestimated the HPLC-B
Volume 53 Number 1
Transcutaneous Bilirubin in Turkish Newborns
71
Table IV. Mean of the Differences and 95% Agreement Limits of Bland-Altman Bias Plot (mg/dl)
Bias
HPLC-B TcB
HPLC-B Diazo TSB
HPLC-B Bilirubinometer TSB
Diazo TSB TcB
Mean
1.85
-2.23
0.35
4.08
Lower bound
-4.63
-7.42
-4.98
-2.88
Upper bound
8.32
2.96
5.68
11.03
HPLC: High-pressure liquid chromatography. TcB: Transcutaneous bilirubin. TSB: Total serum bilirubin.
slightly. We found that TcB and bilirubinometer
TSB underestimated HPLC-B, whereas diazo
TSB overestimated HPLC -B. Engle and
associates 19 studied a Hispanic population
and found that BiliCheck measurements from
the forehead tended to underestimate TSB,
particularly when the TSB exceeded 10 mg/
dl. Ebbesen and colleagues11 found a good
correlation between TcB and total TSB in
Danish infants, although TcB was on average
lower than TSB. We also found that BiliCheck
tended to underestimate bilirubinometer TSB
and HPLC-B with increasing discrepancy at
higher TSB values.
Some investigators 8,9 have compared TcB
measurements with HPLC measurements
of serum bilirubin, while others12,15,22 have
compared the TcB measurements with standard
laboratory measurements of TSB. There were
good correlations in all studies and in our
study.
The sensitivity, specificity, positive predictive
value, and negative predictive value of TcB and
laboratory methods in relationship to HPLC
have been examined by several investigators.
Rubaltelli et al.8 reported that the use of a
cut-off point of 13 mg/dl for HPLC-B and
11 mg/dl for TcB and TSB produced similar
sensitivity and specificity (93% and 73% for
the TcB and 95% and 76% for TSB). In another
study, very high sensitivities were observed
only when relatively low TcB cut-off values
were used to predict TSB >10 mg/dl or >15
mg/dl19. In our study, use of cut-off points of
13 mg/dl for HPLC-B, 9 mg/dl for TcB, and
11 mg/dl for bilirubinometer TSB produced
high sensitivity (91% for TcB and 100% for
bilirubinometer).
In conclusion, despite having good correlation
with HPLC, BiliCheck showed worse
performance than bilirubinometer and diazo
methods at various clinically relevant HPLCB cut-off values. HPLC-B >17 mg/dl, >15
mg/dl and >13 mg/dl can be detected with
100% sensitivity with BiliCheck 9 mg/dl, 8
mg/dl, and 8 mg/dl, respectively. Given these
relatively lower thresholds with false-positive
results, BiliCheck cannot be recommended
as a complete substitute for serum bilirubin
measurements.
Table V. ROC areas at 17 mg/dl, 15 mg/dl, and 13 mg/dl cut-off points of HPLC-B
HPLC-B cut-off point
Method
ROC area
95% Confidence Interval
Lower Bound
Upper Bound
> 17 mg/dl
Diazo TSB
0.968
0.927
1.009
Bilirubinometer TSB
0.952
0.865
1.039
> 15 mg/dl
TcB
0.875
0.750
1.000
Diazo TSB
0.968
0.924
1.011
Bilirubinometer TSB
0.933
0.866
1.001
> 13 mg/dl
TcB
0.853
0.741
0.965
Diazo TSB
0.927
0.858
0.996
Bilirubinometer TSB
0.907
0.832
0.982
TcB
0.835
0.731
0.940
HPLC-B: High-pressure liquid chromatography-Bilirubin. ROC: Receiver operating characteristic. TSB: Total serum
bilirubin. TcB: Transcutaneous bilirubin.
72
Kaynak-Trkmen M, et al
Fig. 2A. Bland-Altman error plots for comparison of
HPLC-B and TcB. Linear regression: HPLC-B TcB =
-3.25 + 0.31 *Mean of HPLC-B and TcB; r 2 = 0.35.
Fig. 2B. Bland-Altman error plot for comparison of
HPLC-B and bilirubinometer TSB. Linear regression:
HPLC-B Bilirubinometer TSB = -2.08 + 0.36 *Mean
of HPLC -B and Bilirubinometer TSB; r 2 = 0.30.
Fig. 2C. Bland-Altman error plot for comparison of HPLC-B
and diazo TSB. Linear regression: HPLC-B Diazo TSB =
-2.19 + 0.00 *Mean of HPLC-B and Diazo TSB; r 2 = 0.00.
The Turkish Journal of Pediatrics January-February 2011
Fig.
TSB
Fig.
TSB
Fig.
TSB
3A. ROC curve for TcB, bilirubinometer and diazo
compared with HPLC-B at 17 mg/dl cut-off point.
3B. ROC curve for TcB, bilirubinometer and diazo
compared with HPLC-B at 15 mg/dl cut-off point.
3C. ROC curve for TcB, bilirubinometer and diazo
compared with HPLC-B at 13 mg/dl cut-off point.
Volume 53 Number 1
Transcutaneous Bilirubin in Turkish Newborns
73
Table VI. The Sensitivity, Specificity, Positive Predictive Value, and Negative Predictive Value of BC and
Laboratory Methods in Relationship to HPLC
Method
BC
HPLC>
Test
Sensitivity
(%)
Specificity
(%)
Positive
predictive
value (%)
Negative
predictive
value (%)
17
17
15
13
9
15
13
11
10
8
13
11
9
8
17
16
15
15
14
13
12
17
15
13
11
15
13
11
13
12
11
50
70
70
100
53
60
73
87
100
45
64
91
100
100
100
100
100
100
100
100
60
80
90
100
60
87
100
73
82
100
98
98
91
45
100
95
72
64
41
97
75
56
50
84
75
64
72
62
66
53
100
98
75
48
100
82
54
88
75
66
83
88
64
29
100
82
50
48
39
91
64
59
58
59
48
38
58
50
67
59
100
89
45
30
100
65
45
80
69
67
90
93
93
100
85
86
88
93
100
72
75
90
100
100
100
100
100
100
100
15
13
Diazo
17
15
13
Bilirubinometer
17
15
13
100
92
96
97
100
87
94
100
82
86
100
BC: BiliCheck. HPLC: High-pressure liquid chromatography.
Acknowledgement
We are grateful to Edward F. Bell, MD, from the
Iowa Childrens Hospital for his corrections and
suggestions on the manuscript. This study was
supported by a grant (TPF07068) from Adnan
Menderes University Research Foundation.
REFERENCES
1. Maisels MJ, Kring E. Transcutaneous bilirubinometry
decreases the need for serum bilirubin measurements
and saves money. Pediatrics 1997; 99: 599-601.
2. Stevenson DK, Wong RJ, Vreman HJ. Reduction in
hospital readmission rates for hyperbilirubinemia
is associated with use of transcutaneous bilirubin
measurements. Clin Chem 2005; 51: 481-482.
3.
Petersen JR, Okorodudu AO, Mohammad AA, Fernando
A, Shattuck KE. Association of transcutaneous bilirubin
testing in hospital with decreased readmission rate for
hyperbilirubinemia. Clin Chem 2005; 51: 540-544.
4. Wong CM, van Dijk PJ, Laing IA. A comparison of
transcutaneous bilirubinometers: SpectRx BiliCheck
versus Minolta AirShields. Arch Dis Child Fetal
Neonatal Ed 2002; 87: F137-140.
5. Yamanouchi I, Yamauchi Y, Igarashi I. Transcutaneous
bilirubinometry: preliminary studies of noninvasive
transcutaneous bilirubin meter in the Okayama
National Hospital. Pediatrics 1980; 65: 195-202.
6. Ya m a u c h i Y, Ya m a n o u c h i I . Tr a n s c u t a n e o u s
bilirubinometry: effect of postnatal age. Acta Paediatr
Jpn 1991; 33: 663-667.
7. Yamauchi Y, Yamanouchi I, Igarashi I. Transcutaneous
bilirubinometry: bilirubin kinetics of the skin and
serum during and after phototherapy. Biol Neonate
1989; 56: 263-269.
74
Kaynak-Trkmen M, et al
8. Rubaltelli FF, Gourley GR, Loskamp N, et al.
Transcutaneous bilirubin measurement: a multicenter
evaluation of a new device. Pediatrics 2001; 107:
1264-1272.
9. Bhutani VK, Gourley GR, Adler S, Kreamer B,
Dalin C, Johnson LH. Noninvasive measurement of
total serum bilirubin in a multiracial predischarge
newborn population to assess the risk of severe
hyperbilirubinemia. Pediatrics 2000; 106: E17.
10. Maisels JM, Enrique M. Ostrea EM, Touch S, Clune
SE, Cepeda E. Evaluation of a new transcutaneous
bilirubinometer. Pediatrics 2004; 113: 1628-1635.
11. Ebbesen F, Rasmussen LM, Wimberley PD. A new
transcutaneous bilirubinometer, BiliCheck, used in the
neonatal intensive care unit and the maternity ward.
Acta Paediatr 2002; 91: 203-211.
12. Engle WD, Jackson GL, Stehel EK, Sendelbach DM,
Manning MD. Evaluation of a transcutaneous jaundice
meter following hospital discharge in term and nearterm neonates. J Perinatol 2005; 25: 486-490.
13. Szabo P, Wolf M, Bucher HU, Fauchre JC, Haensse D,
Arlettaz R. Detection of hyperbilirubinemia in jaundiced
full term neonates by eye or by bilirubinometer. Eur
J Pediatr 2004; 163: 722-727.
14. Knupfer M, Pulzer F, Gebauer C, Robel-Tillig E,
Vogtmann C. Transcutaneous bilirubinometry in
preterm infants. Acta Paediatr 2001; 90: 899-903.
15. Slusher T, Ishaya A, Angyo A, et al. Transcutaneous
bilirubin measurements and serum total bilirubin
levels in indigenous African infants. Pediatrics 2004;
113: 6.
The Turkish Journal of Pediatrics January-February 2011
16. Tan KL, Dong F. Transcutaneous bilirubinometry during
and after phototherapy. Acta Paediatr 2003; 92: 327331.
17. Robertson A, Kazmierczak S, Vos P. Improved
transcutaneous bilirubinometry: comparison of
SpectR(X) BiliCheck and Minolta Jaundice Meter JM102 for estimating total serum bilirubin in a normal
newborn population. J Perinatol 2002; 22: 12-14.
18. Karon BS, Teske A, Santrach PJ, Cook WJ. Evaluation of
the BiliChek noninvasive bilirubin analyzer for prediction
of serum bilirubin and risk of hyperbilirubinemia. Am
J Clin Pathol 2008; 130: 976-982.
19. Engle DW, Gregory LJ, Sendelbach D, Manning D,
Frawler WH. Assessment of a transcutaneous device
in the evaluation of neonatal hyperbilirubinemia in a
primarily Hispanic population. Pediatrics 2002; 110:
61-67.
20. Kazmierczak SC, Robertson AF, Briley KP, Kreamer
B, Gourley GR. Transcutaneous measurement of
bilirubin in newborns: comparison with an automated
Jendrassik-Grof Procedure and HPLC. Clin Chem 2004;
50: 433-435.
21. Bland JM, Altman DG. Statistical methods for
assessing agreement between two methods of clinical
measurement. Lancet 1986; 1: 307-310.
22. Carceller AM, Delvin E, Gonthier M, Grgoire
MC, Cousineau J, Alexandrov L. Evaluation of
transcutaneous bilirubin measurement and agreement
with bilirubinemia. Ann Biol Clin 2006; 64: 575579.
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
Das könnte Ihnen auch gefallen
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryVon EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryBewertung: 3.5 von 5 Sternen3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Von EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Bewertung: 4.5 von 5 Sternen4.5/5 (121)
- Grit: The Power of Passion and PerseveranceVon EverandGrit: The Power of Passion and PerseveranceBewertung: 4 von 5 Sternen4/5 (588)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaVon EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaBewertung: 4.5 von 5 Sternen4.5/5 (266)
- The Little Book of Hygge: Danish Secrets to Happy LivingVon EverandThe Little Book of Hygge: Danish Secrets to Happy LivingBewertung: 3.5 von 5 Sternen3.5/5 (400)
- Never Split the Difference: Negotiating As If Your Life Depended On ItVon EverandNever Split the Difference: Negotiating As If Your Life Depended On ItBewertung: 4.5 von 5 Sternen4.5/5 (838)
- Shoe Dog: A Memoir by the Creator of NikeVon EverandShoe Dog: A Memoir by the Creator of NikeBewertung: 4.5 von 5 Sternen4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerVon EverandThe Emperor of All Maladies: A Biography of CancerBewertung: 4.5 von 5 Sternen4.5/5 (271)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeVon EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeBewertung: 4 von 5 Sternen4/5 (5794)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyVon EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyBewertung: 3.5 von 5 Sternen3.5/5 (2259)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersVon EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersBewertung: 4.5 von 5 Sternen4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnVon EverandTeam of Rivals: The Political Genius of Abraham LincolnBewertung: 4.5 von 5 Sternen4.5/5 (234)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreVon EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreBewertung: 4 von 5 Sternen4/5 (1090)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceVon EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceBewertung: 4 von 5 Sternen4/5 (895)
- Her Body and Other Parties: StoriesVon EverandHer Body and Other Parties: StoriesBewertung: 4 von 5 Sternen4/5 (821)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureVon EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureBewertung: 4.5 von 5 Sternen4.5/5 (474)
- The Unwinding: An Inner History of the New AmericaVon EverandThe Unwinding: An Inner History of the New AmericaBewertung: 4 von 5 Sternen4/5 (45)
- 2016 Pediatric HydrocephalusDokument15 Seiten2016 Pediatric HydrocephalusYudit Arenita100% (1)
- The Yellow House: A Memoir (2019 National Book Award Winner)Von EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Bewertung: 4 von 5 Sternen4/5 (98)
- On Fire: The (Burning) Case for a Green New DealVon EverandOn Fire: The (Burning) Case for a Green New DealBewertung: 4 von 5 Sternen4/5 (73)
- Peds QL 13-18 THDokument2 SeitenPeds QL 13-18 THAndra KurniantoNoch keine Bewertungen
- Peds QL 13-18 THDokument2 SeitenPeds QL 13-18 THAndra KurniantoNoch keine Bewertungen
- (Mahesh Chandra) Objective Cardiology PDFDokument609 Seiten(Mahesh Chandra) Objective Cardiology PDFfajarNoch keine Bewertungen
- Adl IadlDokument20 SeitenAdl IadlKrisna Eka Yudha100% (1)
- Physical Activity Questionnaire For AdolescentDokument6 SeitenPhysical Activity Questionnaire For AdolescentAndra Kurnianto0% (2)
- The Finnish Open Dialogue Approach To PDFDokument8 SeitenThe Finnish Open Dialogue Approach To PDFJoão Vitor Moreira MaiaNoch keine Bewertungen
- SAMPLE FDAR CHARTING PainDokument1 SeiteSAMPLE FDAR CHARTING Painjpm100% (1)
- Skin Care PowerpointDokument52 SeitenSkin Care Powerpointisapatrick812667% (3)
- Diabetes MellitusDokument41 SeitenDiabetes MellitusYuttapol PimpisonNoch keine Bewertungen
- Suggamadex Remifentanyl PalonosetronDokument28 SeitenSuggamadex Remifentanyl PalonosetronSai TejeswiNoch keine Bewertungen
- Kurva Pertumbuhan Anak WhoDokument30 SeitenKurva Pertumbuhan Anak WhoAlia Salvira100% (1)
- Kurva Pertumbuhan Anak WhoDokument30 SeitenKurva Pertumbuhan Anak WhoAlia Salvira100% (1)
- Pulmonary EmbolismDokument27 SeitenPulmonary EmbolismEfren Ezekiel AlbiosNoch keine Bewertungen
- Caring For Older AdultsDokument5 SeitenCaring For Older AdultsOsama Elsayed AhmedNoch keine Bewertungen
- Williams' Basic Nutrition & Diet Therapy: Food Habits and Cultural PatternsDokument25 SeitenWilliams' Basic Nutrition & Diet Therapy: Food Habits and Cultural PatternsAndra KurniantoNoch keine Bewertungen
- (Sesi-2) Journal ReviewDokument7 Seiten(Sesi-2) Journal ReviewAndra KurniantoNoch keine Bewertungen
- Dr. Dr. Eddy Fadlyana, SpA (K), MKesDokument42 SeitenDr. Dr. Eddy Fadlyana, SpA (K), MKesAndra KurniantoNoch keine Bewertungen
- Common Growth Disorders in Pediatric With Chronic DiseaseDokument32 SeitenCommon Growth Disorders in Pediatric With Chronic DiseaseAndra KurniantoNoch keine Bewertungen
- (Sesi-2) Journal ReviewDokument7 Seiten(Sesi-2) Journal ReviewAndra KurniantoNoch keine Bewertungen
- Case Report Renal Cell Carcinoma Metastasis To The Breast: A Rare PresentationDokument9 SeitenCase Report Renal Cell Carcinoma Metastasis To The Breast: A Rare PresentationAndra KurniantoNoch keine Bewertungen
- A Rare Case of Retroperitoneal and Mesenteric LymphangiomatosisDokument4 SeitenA Rare Case of Retroperitoneal and Mesenteric LymphangiomatosisAndra KurniantoNoch keine Bewertungen
- Hypodense Cerebral Venous Sinus Thrombosis On Unenhanced CT: A Potential Pitfall. of A Case and Review of The LiteratureDokument4 SeitenHypodense Cerebral Venous Sinus Thrombosis On Unenhanced CT: A Potential Pitfall. of A Case and Review of The LiteratureAndra KurniantoNoch keine Bewertungen
- Pancreatic Acinar Cell Carcinoma - Literature Review and Case of A 56-Year-Old Man Presenting With Abdominal PainDokument5 SeitenPancreatic Acinar Cell Carcinoma - Literature Review and Case of A 56-Year-Old Man Presenting With Abdominal PainAndra KurniantoNoch keine Bewertungen
- Primary Ewing Sarcoma of The Adrenal Gland: A Rare Cause of Abdominal MassDokument6 SeitenPrimary Ewing Sarcoma of The Adrenal Gland: A Rare Cause of Abdominal MassAndra KurniantoNoch keine Bewertungen
- Effects of Nutrition On Brain Development in Humans PDFDokument5 SeitenEffects of Nutrition On Brain Development in Humans PDFAndra KurniantoNoch keine Bewertungen
- Fatty Acid Profile of Betok Fish Oil (Anabas Testudineus)Dokument8 SeitenFatty Acid Profile of Betok Fish Oil (Anabas Testudineus)Andra KurniantoNoch keine Bewertungen
- Head Ultrasound Anatomy 2013 (Compatibility Mode)Dokument38 SeitenHead Ultrasound Anatomy 2013 (Compatibility Mode)Andra KurniantoNoch keine Bewertungen
- Poster Andrak Apcp 2018 Fix 2Dokument1 SeitePoster Andrak Apcp 2018 Fix 2Andra KurniantoNoch keine Bewertungen
- Grafik CDCDokument10 SeitenGrafik CDCArief Budi LesmanaNoch keine Bewertungen
- CharacteristicsDokument3 SeitenCharacteristicsAndra KurniantoNoch keine Bewertungen
- ReferenceDokument3 SeitenReferenceAndra KurniantoNoch keine Bewertungen
- Bilirubin MetabolismDokument2 SeitenBilirubin MetabolismAndra KurniantoNoch keine Bewertungen
- Lambang RSMHDokument1 SeiteLambang RSMHAndra KurniantoNoch keine Bewertungen
- Lambang RSMHDokument1 SeiteLambang RSMHAndra KurniantoNoch keine Bewertungen
- Biology of The Neonate 2004 85, 1 ProquestDokument5 SeitenBiology of The Neonate 2004 85, 1 ProquestAndra KurniantoNoch keine Bewertungen
- Head Ultrasound in Neonate 2013 (Compatibility Mode)Dokument39 SeitenHead Ultrasound in Neonate 2013 (Compatibility Mode)Andra KurniantoNoch keine Bewertungen
- Presentation 1Dokument2 SeitenPresentation 1Andra KurniantoNoch keine Bewertungen
- Transcutaneous Bilirubin Measurement: A Multicenter Evaluation of A New DeviceDokument10 SeitenTranscutaneous Bilirubin Measurement: A Multicenter Evaluation of A New DeviceAndra KurniantoNoch keine Bewertungen
- Post Partum HemorrhageDokument40 SeitenPost Partum HemorrhageGita GirsangNoch keine Bewertungen
- Medel SonataDokument54 SeitenMedel Sonatamaclab macNoch keine Bewertungen
- WAC Apollo Hospitals - JahjaDokument2 SeitenWAC Apollo Hospitals - JahjaJahja Aja0% (1)
- Air Liquide 2015 Annual Report enDokument60 SeitenAir Liquide 2015 Annual Report enCar Și PolicarNoch keine Bewertungen
- Eem Education Session3Dokument21 SeitenEem Education Session3Gama AntaresNoch keine Bewertungen
- Latisha Kelly Resume AssignmentDokument3 SeitenLatisha Kelly Resume Assignmentapi-324927307Noch keine Bewertungen
- Pcol MnemonicsDokument12 SeitenPcol MnemonicsJustine Rubillete Mendoza MarianoNoch keine Bewertungen
- Keuler - Sensorimotor ObsessionsDokument5 SeitenKeuler - Sensorimotor Obsessionsbferrreira2371100% (1)
- Catalog Tracoe 2014 PDFDokument94 SeitenCatalog Tracoe 2014 PDFLiudmila RailescuNoch keine Bewertungen
- Ob2rle Sas 1Dokument13 SeitenOb2rle Sas 1Meow MeowNoch keine Bewertungen
- Care For Elderly PDFDokument10 SeitenCare For Elderly PDFDzulfiqarNoch keine Bewertungen
- Yesterday's Tomorrow - Suvika PDFDokument223 SeitenYesterday's Tomorrow - Suvika PDFSindhu Jhaveri100% (2)
- Cefadroxil Journal ABSTRACTDokument3 SeitenCefadroxil Journal ABSTRACTAyu Kusuma NingrumNoch keine Bewertungen
- Checklist For Review of Floor Plans InfirmaryDokument3 SeitenChecklist For Review of Floor Plans Infirmaryjherica baltazarNoch keine Bewertungen
- Acute and Chronic Gastritis Due To Helicobacter PyloriDokument8 SeitenAcute and Chronic Gastritis Due To Helicobacter PyloriCarla HolandNoch keine Bewertungen
- 2011 07 PHARMA Prescription WritingDokument8 Seiten2011 07 PHARMA Prescription WritingdtimtimanNoch keine Bewertungen
- Va SwotDokument3 SeitenVa Swotapi-280277788Noch keine Bewertungen
- Leptospirosis, Typhoid and Other FeversDokument37 SeitenLeptospirosis, Typhoid and Other FeverskarageeNoch keine Bewertungen
- NCP Proper TahbsoDokument3 SeitenNCP Proper TahbsoMiriam EstradaNoch keine Bewertungen
- AmoebiasisDokument33 SeitenAmoebiasisMike Serge Razafi0% (1)