Beruflich Dokumente
Kultur Dokumente
Prepspm Chemical E 2
Hochgeladen von
Supia Nazma0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
6 Ansichten1 Seitequestion
Copyright
© © All Rights Reserved
Verfügbare Formate
DOCX, PDF, TXT oder online auf Scribd lesen
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenquestion
Copyright:
© All Rights Reserved
Verfügbare Formate
Als DOCX, PDF, TXT herunterladen oder online auf Scribd lesen
0 Bewertungen0% fanden dieses Dokument nützlich (0 Abstimmungen)
6 Ansichten1 SeitePrepspm Chemical E 2
Hochgeladen von
Supia Nazmaquestion
Copyright:
© All Rights Reserved
Verfügbare Formate
Als DOCX, PDF, TXT herunterladen oder online auf Scribd lesen
Sie sind auf Seite 1von 1
1. (a) Iodine monochloride , ICl dissociates at 25 oC to I2 and Cl2.
The equilibrium constant , Kp
for the dissociation is 2.2 x 10-3.
ICl (g)
I2 (g) + Cl2 (g)
Calculate Kp and Kc for the following reaction at temperature 25oC :
I2 (g) + Cl2 (g)
2ICl (g)
[7 marks]
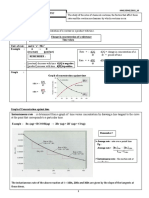
(b) At a temperature of 400 oC and pressure of 3.0 x 107 Pa, the hydrogen gas and nitrogen
gas are mixed in the mole ratio of 3: 1. Under these conditions, 61% of nitrogen is
converted to ammnonia.
N2(g) + 3H2 (g)
2NH3 (g)
Calculate the percentage of ammonia produced in an equilibrium mixture and calculate
the equilibrium constant, Kp for the reaction above.
[13 marks]
Das könnte Ihnen auch gefallen
- Shoe Dog: A Memoir by the Creator of NikeVon EverandShoe Dog: A Memoir by the Creator of NikeBewertung: 4.5 von 5 Sternen4.5/5 (537)
- Chapter 1: Matter 1.1 Atoms and Molecules: Packed in A Small NucleusDokument35 SeitenChapter 1: Matter 1.1 Atoms and Molecules: Packed in A Small NucleusSupia NazmaNoch keine Bewertungen
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeVon EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeBewertung: 4 von 5 Sternen4/5 (5794)
- Quiz PHASE EQUILIBRIA (Set 3)Dokument4 SeitenQuiz PHASE EQUILIBRIA (Set 3)Supia NazmaNoch keine Bewertungen
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceVon EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceBewertung: 4 von 5 Sternen4/5 (895)
- Collision Theory States That For A Reaction To OccurDokument9 SeitenCollision Theory States That For A Reaction To OccurSupia NazmaNoch keine Bewertungen
- The Yellow House: A Memoir (2019 National Book Award Winner)Von EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Bewertung: 4 von 5 Sternen4/5 (98)
- 2 Metallic BondsDokument13 Seiten2 Metallic BondsSupia NazmaNoch keine Bewertungen
- Grit: The Power of Passion and PerseveranceVon EverandGrit: The Power of Passion and PerseveranceBewertung: 4 von 5 Sternen4/5 (588)
- Instantaneous Rate: Is Determined From A Graph of Time Versus Concentration by Drawing A Line Tangent To The CurveDokument13 SeitenInstantaneous Rate: Is Determined From A Graph of Time Versus Concentration by Drawing A Line Tangent To The CurveSupia NazmaNoch keine Bewertungen
- The Little Book of Hygge: Danish Secrets to Happy LivingVon EverandThe Little Book of Hygge: Danish Secrets to Happy LivingBewertung: 3.5 von 5 Sternen3.5/5 (400)
- Extra Exercises - Measurement of ConcentrationDokument1 SeiteExtra Exercises - Measurement of ConcentrationSupia NazmaNoch keine Bewertungen
- The Emperor of All Maladies: A Biography of CancerVon EverandThe Emperor of All Maladies: A Biography of CancerBewertung: 4.5 von 5 Sternen4.5/5 (271)
- Quiz 1.1 2021 LectureDokument4 SeitenQuiz 1.1 2021 LectureSupia NazmaNoch keine Bewertungen
- Never Split the Difference: Negotiating As If Your Life Depended On ItVon EverandNever Split the Difference: Negotiating As If Your Life Depended On ItBewertung: 4.5 von 5 Sternen4.5/5 (838)
- Quiz States of Matter (Set 4)Dokument4 SeitenQuiz States of Matter (Set 4)Supia NazmaNoch keine Bewertungen
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyVon EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyBewertung: 3.5 von 5 Sternen3.5/5 (2259)
- Quiz PHASE EQUILIBRIA (Set 2)Dokument4 SeitenQuiz PHASE EQUILIBRIA (Set 2)Supia NazmaNoch keine Bewertungen
- On Fire: The (Burning) Case for a Green New DealVon EverandOn Fire: The (Burning) Case for a Green New DealBewertung: 4 von 5 Sternen4/5 (74)
- Exercise Born HaberDokument17 SeitenExercise Born HaberSupia NazmaNoch keine Bewertungen
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureVon EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureBewertung: 4.5 von 5 Sternen4.5/5 (474)
- Exercise Born HaberDokument17 SeitenExercise Born HaberSupia NazmaNoch keine Bewertungen
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryVon EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryBewertung: 3.5 von 5 Sternen3.5/5 (231)
- Quiz C6 Set 4Dokument2 SeitenQuiz C6 Set 4Supia NazmaNoch keine Bewertungen
- Team of Rivals: The Political Genius of Abraham LincolnVon EverandTeam of Rivals: The Political Genius of Abraham LincolnBewertung: 4.5 von 5 Sternen4.5/5 (234)
- Quiz C6 Set 3Dokument1 SeiteQuiz C6 Set 3Supia NazmaNoch keine Bewertungen
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaVon EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaBewertung: 4.5 von 5 Sternen4.5/5 (266)
- Quiz C6 Set 2Dokument2 SeitenQuiz C6 Set 2Supia NazmaNoch keine Bewertungen
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersVon EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersBewertung: 4.5 von 5 Sternen4.5/5 (345)
- Quiz C6 Set 1Dokument2 SeitenQuiz C6 Set 1Supia NazmaNoch keine Bewertungen
- Gases (B)Dokument115 SeitenGases (B)Supia NazmaNoch keine Bewertungen
- The Unwinding: An Inner History of the New AmericaVon EverandThe Unwinding: An Inner History of the New AmericaBewertung: 4 von 5 Sternen4/5 (45)
- Quiz C5 STATES OF MATTER (Set 5)Dokument2 SeitenQuiz C5 STATES OF MATTER (Set 5)Supia NazmaNoch keine Bewertungen
- Topic 14.0: Haloalkanes (Alkyl Halides)Dokument12 SeitenTopic 14.0: Haloalkanes (Alkyl Halides)Supia NazmaNoch keine Bewertungen
- Gaya Komunikasi Ketua Unit (Ku) Kimia Dan Kepuasan Kerja Pensyarah Kimia Di Kolej Matrikulasi SelangorDokument12 SeitenGaya Komunikasi Ketua Unit (Ku) Kimia Dan Kepuasan Kerja Pensyarah Kimia Di Kolej Matrikulasi SelangorSupia NazmaNoch keine Bewertungen
- Tutorial 1.1 (PG 1-2)Dokument3 SeitenTutorial 1.1 (PG 1-2)Supia NazmaNoch keine Bewertungen
- Set 1 Lampiran 1C - PelajarDokument1 SeiteSet 1 Lampiran 1C - PelajarSupia NazmaNoch keine Bewertungen
- CHC NH Cooh H H CH C CH: Organic Compound That Contains Both An Amino Group, - NH2 and A Carboxyl Group, - COOHDokument6 SeitenCHC NH Cooh H H CH C CH: Organic Compound That Contains Both An Amino Group, - NH2 and A Carboxyl Group, - COOHSupia NazmaNoch keine Bewertungen
- Worksheet 1Dokument6 SeitenWorksheet 1Supia NazmaNoch keine Bewertungen
- Topic 14.0: Haloalkanes (Alkyl Halides)Dokument12 SeitenTopic 14.0: Haloalkanes (Alkyl Halides)Supia NazmaNoch keine Bewertungen
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreVon EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreBewertung: 4 von 5 Sternen4/5 (1090)
- CARBOXYLIC ACIDS Nomenclature StudentDokument23 SeitenCARBOXYLIC ACIDS Nomenclature StudentSupia NazmaNoch keine Bewertungen
- 19 (B)Dokument4 Seiten19 (B)Supia NazmaNoch keine Bewertungen
- Chapter 4.4-Intermolecular ForcesDokument3 SeitenChapter 4.4-Intermolecular ForcesSupia NazmaNoch keine Bewertungen
- 7.0 Ionic Equilibria (Students)Dokument187 Seiten7.0 Ionic Equilibria (Students)Supia Nazma100% (1)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Von EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Bewertung: 4.5 von 5 Sternen4.5/5 (121)
- Her Body and Other Parties: StoriesVon EverandHer Body and Other Parties: StoriesBewertung: 4 von 5 Sternen4/5 (821)