Beruflich Dokumente
Kultur Dokumente
Temperature and Heat
Hochgeladen von
Mohammed Zaakir AllyCopyright
Verfügbare Formate
Dieses Dokument teilen
Dokument teilen oder einbetten
Stufen Sie dieses Dokument als nützlich ein?
Sind diese Inhalte unangemessen?
Dieses Dokument meldenCopyright:
Verfügbare Formate
Temperature and Heat
Hochgeladen von
Mohammed Zaakir AllyCopyright:
Verfügbare Formate
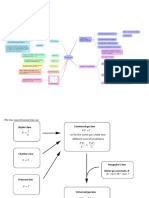
A physical property than changes with
temperature in a predictable way
Thermometric Property
Liquid (previously mercury, now alcohol)
expands up the gauge as temperature rises
Liquid in glass thermometer
Narrowing at the proximal end allows one to
remove the thermometer at any time
Based on Charles' Law: T proportionate
P in the tubing uncoiling or collapsing of
the Bourdon tube reflects on a gauge to
which it is connected
Heat
Temperature
Bourdon Thermometer
The amount of thermal energy a body contains
Average kinetic energy of the atoms/
molecules of a body
Capable of measuring high temperatures
accurately
Conduction
Platinum wire's resistance changes linearly
with temperature
Transfer of heat due to direct contact
between high Ek atoms/molecules of the
source and low Ek atoms/molecules of an
adjoining body
Platinum Resistor
Conduction causes high Ek molecules in the
air/liquid surrounding the source rising of
low density warm molecules and sinking of
high density cool molecules which surround
the body and maintain the temperature
gradient and rate of heat transfer
Cheap but not very sensitive
Passive Convection
Temperature-sensitive resistor
Metal oxide semiconductor's resistance with
temperature
Negative Thermal Conductivity (NTC)
Convection
Transfer of Heat
Motion of the surrounding air/liquid due to a fan
causes rate of cooling by constantly replacing
warmed surrounding molecules with cooler molecules
Active Convection
Measuring Temperature
Resistance is measured by a Wheatstone
Bridge Circuit
Thermistors
Disadvantages: Relationship is non-linear
compensated for by algorithms
Radiation
Electronic
Advantages: Cheap, small, accurate
Evaporation
Seebeck Effect: 2 dissimilar metals joined
together produce a voltage which fluctuates
linearly with T
Electromagnetic energy emitted due to kinetic
energy and motion of the atoms
Perspiration causes liquid formation on the
skin which then evaporates. Evaporation
requires Latent Energy which the molecules
acquire from the skin, resulting in cooling
A Black body absorbs all the electromagnetic
radiation it is exposed to
Thermocoupling
Previously used T1 and T2 as calibration, now
T1 is just calibrated to room air
It is the best absorber and best emitter
Small SHC so sensitive to small temperature
changes and very accurate
e - emissivity which is 1 for Black Bodies and
< 1 for non-Black Bodies
Thermopile converts the electromagnetic
radiation emitted by the TM to a temperature
reading
Temperature and Heat
Black Body Radiation
- S-B Constant
Emits energy according to the equation:
P = e..A.T (Stefan-Boltzmann Equation)
A - Surface Area of the BB
IR Tympanic Thermometer
TM temperature should be similar to
hypothalamus
T - Temperature
Must have a direct line of vision to TM or
artifact is produced by the ear canal walls
Temperature of Electromagnetic radiation 1/
The change in the internal energy of a system
is the difference between the energy added
and the work done
U = Q W
As very hot bodies pass through of the visible
spectrum, different colors can be seen.
First Law
Piloerection
BMR
Heat cannot flow from a body with low
temperature to a body with high temperature
without work being done
Conservation of Heat
Shivering
heat production by inefficiency of muscle
contraction
A measure of the disorder in a system
Behaviour change incl voluntary muscle
contraction
The most likely state of the system is that with
the highest entropy
Entropy
Vasodilation - loss is then by electromagnetic
radiation (limited by reflective blankets)
Second Law
Increases with time
Regulation of Body Temperature
Elimination of Heat
S = Change in Entropy
Q = Change in energy
S = Q/T
Laws of Thermodynamics
Perspiration - evaporation causes cooling but
may be limited when humidity
Behavior change
T = Temperature
Respiration 10%
Absolute 0 (0K) in unattainable
Third Law
Evaporation 20%
Routes of Heat Loss
A process which does not require additional
energy but uses the internal energy already
existing in the system such that the change in
the system's internal energy (U) is 0
Convection 30%
Adiabatic Processes
Radiation 40%
A gas moving from high pressure to low
pressure results in a change in temperature,
mostly cooling
e.g. Gas being used from a cylinder
Differs from Gay-Lussac Law which refers to a
closed system at constant Volume
Joule-Thomson Effect
Das könnte Ihnen auch gefallen
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceVon EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceBewertung: 4 von 5 Sternen4/5 (895)
- NHLS Blood Form TemplateDokument1 SeiteNHLS Blood Form TemplateMohammed Zaakir AllyNoch keine Bewertungen
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeVon EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeBewertung: 4 von 5 Sternen4/5 (5794)
- NB: The 'Handful' Story Is My Own Simplified Way of Understanding This. It's Not Really A ThingDokument2 SeitenNB: The 'Handful' Story Is My Own Simplified Way of Understanding This. It's Not Really A ThingMohammed Zaakir AllyNoch keine Bewertungen
- The Yellow House: A Memoir (2019 National Book Award Winner)Von EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Bewertung: 4 von 5 Sternen4/5 (98)
- Summary of Anaesthetic MonitorsDokument3 SeitenSummary of Anaesthetic MonitorsMohammed Zaakir AllyNoch keine Bewertungen
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureVon EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureBewertung: 4.5 von 5 Sternen4.5/5 (474)
- Ethics-Jehovah S Witness PDFDokument2 SeitenEthics-Jehovah S Witness PDFMohammed Zaakir AllyNoch keine Bewertungen
- Shoe Dog: A Memoir by the Creator of NikeVon EverandShoe Dog: A Memoir by the Creator of NikeBewertung: 4.5 von 5 Sternen4.5/5 (537)
- Pertemuan - 12 MetopenDokument40 SeitenPertemuan - 12 MetopenulviaNoch keine Bewertungen
- The Little Book of Hygge: Danish Secrets to Happy LivingVon EverandThe Little Book of Hygge: Danish Secrets to Happy LivingBewertung: 3.5 von 5 Sternen3.5/5 (399)
- Azure Subscription and Service Limits, Quotas, and ConstraintsDokument54 SeitenAzure Subscription and Service Limits, Quotas, and ConstraintsSorinNoch keine Bewertungen
- Ibm v3700 Storeage PDFDokument694 SeitenIbm v3700 Storeage PDFJanakackvNoch keine Bewertungen
- On Fire: The (Burning) Case for a Green New DealVon EverandOn Fire: The (Burning) Case for a Green New DealBewertung: 4 von 5 Sternen4/5 (73)
- Instructions For The Safe Use Of: Web LashingsDokument2 SeitenInstructions For The Safe Use Of: Web LashingsVij Vaibhav VermaNoch keine Bewertungen
- Never Split the Difference: Negotiating As If Your Life Depended On ItVon EverandNever Split the Difference: Negotiating As If Your Life Depended On ItBewertung: 4.5 von 5 Sternen4.5/5 (838)
- Cosare V BroadcomDokument2 SeitenCosare V BroadcomapbueraNoch keine Bewertungen
- Grit: The Power of Passion and PerseveranceVon EverandGrit: The Power of Passion and PerseveranceBewertung: 4 von 5 Sternen4/5 (588)
- ReleaseNoteRSViewME 5 10 02Dokument12 SeitenReleaseNoteRSViewME 5 10 02Jose Luis Chavez LunaNoch keine Bewertungen
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryVon EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryBewertung: 3.5 von 5 Sternen3.5/5 (231)
- A2frc MetricDokument1 SeiteA2frc MetricSudar MyshaNoch keine Bewertungen
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaVon EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaBewertung: 4.5 von 5 Sternen4.5/5 (266)
- Order 49Dokument14 SeitenOrder 49NURADRIANA OMAR BAHSIRNoch keine Bewertungen
- Chinaware - Zen PDFDokument111 SeitenChinaware - Zen PDFMixo LogiNoch keine Bewertungen
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersVon EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersBewertung: 4.5 von 5 Sternen4.5/5 (344)
- X606 PDFDokument1 SeiteX606 PDFDany OrioliNoch keine Bewertungen
- The Emperor of All Maladies: A Biography of CancerVon EverandThe Emperor of All Maladies: A Biography of CancerBewertung: 4.5 von 5 Sternen4.5/5 (271)
- Siemens C321 Smart LockDokument2 SeitenSiemens C321 Smart LockBapharosNoch keine Bewertungen
- Risk, Return & Capital BudgetingDokument18 SeitenRisk, Return & Capital BudgetingMuhammad Akmal HussainNoch keine Bewertungen
- Team of Rivals: The Political Genius of Abraham LincolnVon EverandTeam of Rivals: The Political Genius of Abraham LincolnBewertung: 4.5 von 5 Sternen4.5/5 (234)
- Note 2958296 Pre-Implementation Steps: Create Table TypeDokument3 SeitenNote 2958296 Pre-Implementation Steps: Create Table Typevishnu900890Noch keine Bewertungen
- Midterm Quiz 01 - Adjusting Entries From Accrual To Provision For Uncollectible AccountsDokument3 SeitenMidterm Quiz 01 - Adjusting Entries From Accrual To Provision For Uncollectible AccountsGarp Barroca100% (1)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreVon EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreBewertung: 4 von 5 Sternen4/5 (1090)
- HRM OmantelDokument8 SeitenHRM OmantelSonia Braham100% (1)
- The Unwinding: An Inner History of the New AmericaVon EverandThe Unwinding: An Inner History of the New AmericaBewertung: 4 von 5 Sternen4/5 (45)
- Order To Cash Cycle Group 1Dokument4 SeitenOrder To Cash Cycle Group 1AswinAniNoch keine Bewertungen
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyVon EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyBewertung: 3.5 von 5 Sternen3.5/5 (2259)
- Negative Sequence Current in Wind Turbines Type 3 1637954804Dokument6 SeitenNegative Sequence Current in Wind Turbines Type 3 1637954804Chandra R. SirendenNoch keine Bewertungen
- Energia Eolica Nordex N90 2500 enDokument20 SeitenEnergia Eolica Nordex N90 2500 enNardo Antonio Llanos MatusNoch keine Bewertungen
- Bali Hai LawsuitDokument14 SeitenBali Hai LawsuitLas Vegas Review-JournalNoch keine Bewertungen
- LG+32LX330C Ga LG5CBDokument55 SeitenLG+32LX330C Ga LG5CBjampcarlosNoch keine Bewertungen
- EC312 Object Oriented ProgrammingDokument3 SeitenEC312 Object Oriented ProgrammingJazir HameedNoch keine Bewertungen
- Problems of Spun Concrete Piles Constructed in Soft Soil in HCMC and Mekong Delta - VietnamDokument6 SeitenProblems of Spun Concrete Piles Constructed in Soft Soil in HCMC and Mekong Delta - VietnamThaoNoch keine Bewertungen
- 04.CNOOC Engages With Canadian Stakeholders PDFDokument14 Seiten04.CNOOC Engages With Canadian Stakeholders PDFAdilNoch keine Bewertungen
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Von EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Bewertung: 4.5 von 5 Sternen4.5/5 (120)
- Steinway Case - CH 03Dokument5 SeitenSteinway Case - CH 03Twēéty TuiñkleNoch keine Bewertungen
- Optical Fibre CommunicationDokument60 SeitenOptical Fibre CommunicationN.ChanduNoch keine Bewertungen
- Firmware Upgrade To SP3 From SP2: 1. Download Necessary Drivers For The OMNIKEY 5427 CKDokument6 SeitenFirmware Upgrade To SP3 From SP2: 1. Download Necessary Drivers For The OMNIKEY 5427 CKFilip Andru MorNoch keine Bewertungen
- Release ACOS 4.1.4-GR1-P10 IssuesDokument241 SeitenRelease ACOS 4.1.4-GR1-P10 IssuesdanielatellaNoch keine Bewertungen
- Pavement Design - (Rigid Flexible) DPWHDokument25 SeitenPavement Design - (Rigid Flexible) DPWHrekcah ehtNoch keine Bewertungen
- Lecture 3 - Marriage and Marriage PaymentsDokument11 SeitenLecture 3 - Marriage and Marriage PaymentsGrace MguniNoch keine Bewertungen
- Panch ShilDokument118 SeitenPanch ShilSohel BangiNoch keine Bewertungen
- Her Body and Other Parties: StoriesVon EverandHer Body and Other Parties: StoriesBewertung: 4 von 5 Sternen4/5 (821)